Introduction
Evidence from clinical and epidemiological studies suggested that traumatic stress in childhood and adulthood is related to a wide range of somatic disorders and particularly cardiovascular disease (CVD) (Goodwin and Stein, Reference Goodwin and Stein2004; Baker et al., Reference Baker, Norris, Jones and Murphy2009). For example, in their study using data from the US national comorbidity survey, Goodwin and Stein found that childhood sexual abuse was associated with a significantly increased risk for different cardiac diseases (Goodwin and Stein, Reference Goodwin and Stein2004). Other researchers reported that exposure to combat trauma, natural disasters, and sexual or physical assault was associated with various somatic disorders including CVD (Boscarino, Reference Boscarino1997; Gallo et al., Reference Gallo, Roesch, Fortmann, Carnethon, Penedo, Perreira, Birnbaum-Weitzman, Wassertheil-Smoller, Castañeda, Talavera, Sotres-Alvarez, Daviglus, Schneiderman and Isasi2014).
Post-traumatic stress disorder (PTSD), one of the most common and severe psychological sequelae of trauma exposure, was found to be associated with increased risk for CVD: Spitzer et al. investigated the relationship of trauma exposure, PTSD, and physical illnesses in the general population and found increased rates of renal diseases, stroke, angina pectoris, and heart failure in traumatized persons (Spitzer et al., Reference Spitzer, Barnow, Völzke, John, Freyberger and Grabe2009). Those individuals positive for PTSD showed even higher odds ratios (OR) for angina pectoris and heart failure and were additionally at higher risks of peripheral artery disease than traumatized individuals without PTSD. Boscarino found positive associations of mortality from early-age heart disease with the level of post-traumatic symptoms in a large sample of male Vietnam veterans (Boscarino, Reference Boscarino2008).
Results from clinical and experimental studies indicated that dysregulation of different neuroendocrine systems is involved in the relation between trauma, PTSD, and somatic health outcomes.
Altered activity of the hypothalamic–pituitary–adrenal (HPA-) axis as well as the sympathoadreno-medullary (SAM-) system has been found in persons with a history of trauma and current PTSD. Heim et al. reviewed a series of studies investigating endocrine alterations in individuals with a history of childhood trauma and found increased pituitary–adrenal and autonomic responses to mild stress (Heim et al., Reference Heim, Newport, Mletzko, Miller and Nemeroff2008). Moreover, an evidence showed that increased stress reactivity in response to childhood trauma is further enhanced when additional traumas occurred during adulthood (Heim et al., Reference Heim, Newport, Wagner, Wilcox, Miller and Nemeroff2002). In PTSD, cortisol levels were found to be reduced under baseline conditions [for review (Meewisse et al., Reference Meewisse, Reitsma, Vries, Gersons and Olff2007)] and increased in the presence of psychological stress [for review (de Kloet et al., Reference De Kloet, Vermetten, Geuze, Kavelaars, Heijnen and Westenberg2006)].
There is a close relation of the renin–angiotensin–aldosterone-system (RAAS) with the HPA-axis and the SAM-system in stress response (Groeschel and Braam, Reference Groeschel and Braam2011). The RAAS is the key regulatory system for blood-pressure levels. Stress-associated sympathetic activation leads to an increased release of renin and, subsequently, to increased levels of Angiotensin II (ANG II) (Yang et al., Reference Yang, Xi, Wan, Wang and Bi1994), the primary effector of the RAAS. Under the conditions of acute stress, enhanced ANG II concentrations mediate the release of glucocorticoids and aldosterone from the adrenal cortex (Armando et al., Reference Armando, Carranza, Nishimura, Hoe, Barontini, Terrón, Falcón-Neri, Ito, Juorio and Saavedra2001). In contrast, aldosterone release was found to be reduced in the presence of chronic stress due to downregulated adrenal ANG II receptors (Aguilera, Reference Aguilera1993). Also, the activity of the aldosterone synthetase, the rate-limiting enzyme in aldosterone metabolism, was found to be inhibited under chronic stress conditions (Aguilera, Reference Aguilera1993). Finally, adrenocorticotropic hormone (ACTH), which is a potent stimulating factor for the release of cortisol as well as aldosterone from the adrenal cortex, is regulated by a negative feedback circuit: Stress-induced increase in cortisol levels results in the suppression of ACTH output, and subsequently, in reduced cortisol and aldosterone release (Miller et al., Reference Miller, Chen and Zhou2007).
Only few studies investigated RAAS activity in relation to chronic stress in humans: Häfner et al. reported on elevated renin and aldosterone levels in depressed individuals living alone (Häfner et al., Reference Häfner, Baumert, Emeny, Lacruz, Bidlingmaier, Reincke, Kuenzel, Holle, Rupprecht and Ladwig2012). Terock et al. found associations of chronic psychosocial stress imposed by ‘living alone’ with elevated renin, but not aldosterone levels in a community-based sample (Terock et al., Reference Terock, Hannemann, Janowitz, Völzke, Nauck, Freyberger, Wallaschofski and Grabe2017).
The RAAS has been linked with effects on a wide range of organ systems including the brain. Consistent with these findings, therapeutic and protective effects of angiotensin-converting enzyme inhibitors (ACE-I) and ANG II receptor blockers (ARB) on different brain functions including stress reduction have been found. Specifically, Khoury et al. examined the relation between prescription of different antihypertensive drugs and symptoms of PTSD (Khoury et al., Reference Khoury, Marvar, Gillespie, Wingo, Schwartz, Bradley, Kramer and Ressler2012). The authors found that intake of ACE-I and ARBs, but not of other antihypertensive drugs, was associated with reduced PTSD symptoms, indicating that the RAAS may take in a critical role in the regulation of stress response in PTSD patients. These findings were recently replicated and extended in a study by Nylocks et al. who demonstrated that the protective effects of ACE-I and ARBs on PTSD were mitigated by a common polymorphism of the ACE gene (Nylocks et al., Reference Nylocks, Michopoulos, Rothbaum, Almli, Gillespie, Wingo, Schwartz, Habib, Gamwell, Marvar, Bradley and Ressler2015). In another study, inhibition of type I angiotensin receptors with a common ACE-I enhanced extinction of fear memory in mouse models of PTSD-like symptoms (Marvar et al., Reference Marvar, Goodman, Fuchs, Choi, Banerjee and Ressler2014).
In summary, there is a substantial evidence for altered HPA- and SAM-activity associated with trauma and PTSD, while the role of the RAAS is unclear. Using data from a large general population sample, we sought to investigate the levels of renin and aldosterone in relation to the number of traumatic events and the occurrence of PTSD adjusting for behavioral and metabolic risk factors as well as life-time diagnosis of depression and antihypertensive medication. Based on the previous studies showing differential effects of chronic v. acute stress on renin and aldosterone levels, we hypothesized that (i) exposure to traumatic events would be associated with increased renin, but not aldosterone levels, that the number of traumatic events is associated with renin levels (ii) and that (iii) individuals suffering from PTSD show higher levels of renin, but not aldosterone, compared with traumatized participants without PTSD and to non-traumatized individuals.
Methods and materials
General population sample
Data were obtained from the first follow-up of the Study of Health in Pomerania (SHIP), a population-based cohort study from the general population (Völzke et al., Reference Völzke, Alte, Schmidt, Radke, Lorbeer, Friedrich, Aumann, Lau, Piontek, Born, Havemann, Ittermann, Schipf, Haring, Baumeister, Wallaschofski, Nauck, Frick, Arnold, Jünger, Mayerle, Kraft, Lerch, Dörr, Reffelmann, Empen, Felix, Obst, Koch, Gläser, Ewert, Fietze, Penzel, Dören, Rathmann, Haerting, Hannemann, Röpcke, Schminke, Jürgens, Tost, Rettig, Kors, Ungerer, Hegenscheid, Kühn, Kühn, Hosten, Puls, Henke, Gloger, Teumer, Homuth, Völker, Schwahn, Holtfreter, Polzer, Kohlmann, Grabe, Rosskopf, Kroemer, Kocher, Biffar, John and Hoffmann2011). A multi-stage sampling scheme was adopted from the World Health Organization's Monica Project, Germany. The target population was comprised of adult German residents in northeast Germany living in three cities and 29 communities, with a total population of 212 157. A two-stage stratified cluster sample of adults aged 20–79 years had been drawn from local population registration files. The net sample comprised 6267 eligible persons, of which 4308 persons participated at baseline SHIP-0 between 1997 and 2001. The first follow-up examination (SHIP-1) was conducted 5 years after baseline and included 3300 individuals aged 25–88 years.
The investigations were carried out in accordance with the Declaration of Helsinki, including written informed consent of all participants. The survey and study methods of both the studies were approved by the institutional review boards of the University of Greifswald.
Analytic sample
After exclusion of all SHIP-1 participants with missing information on exposure, outcome, or confounding variables, with extremely high renin (above 1000 ng/l) or aldosterone (above 500 ng/l) concentrations and all pregnant women, our analytic sample comprised 3092 participants. Figure 1 provides a detailed overview over the selection process and composition of our analytic sample.
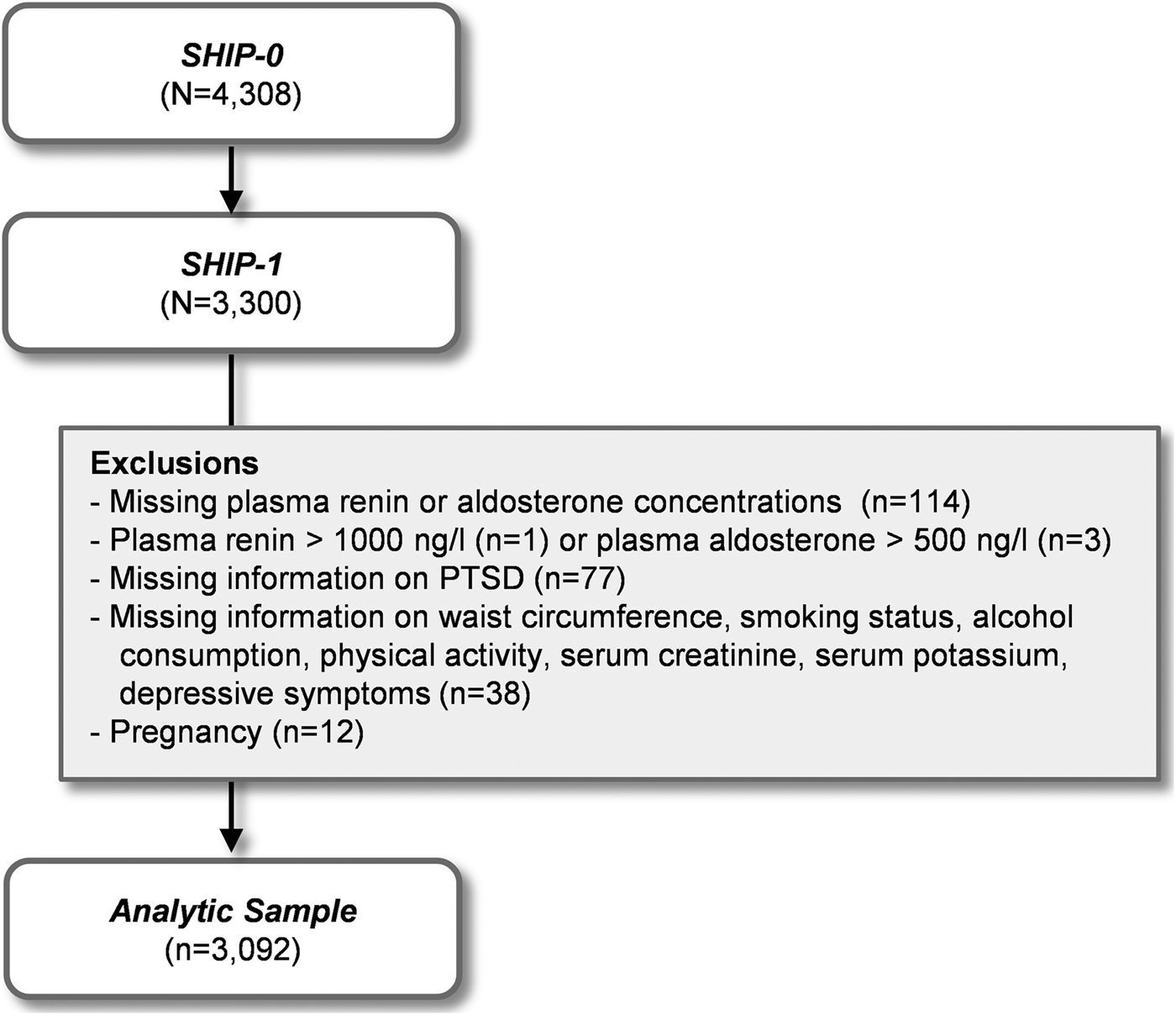
Fig. 1. Selection of the study population.
Interview and examination
Sociodemographic characteristics including behavioral risk factors were obtained in a computer-assisted face-to-face interview. Moreover, participants were asked to bring all medication taken in the last 7 days prior to the examination. The drugs were categorized according to the anatomical therapeutic chemical (ATC) classification code. Antihypertensives (ATC C02), diuretics (ATC C03), β-blockers (ATC C07), calcium channel blockers (ATC C08), ACE-I (ATC C09A, C09B), and ARBs (ATC C09C, C09D) were classified as altering renin or aldosterone concentrations. Participants also underwent a routine medical examination including measurement of waist circumference. Smoking and physical activities were evaluated based on self-report. Smoking was categorized into the categories ‘non-smoker’, ‘smoker’, and ‘ex-smoker’. Participants who reported regular or irregular smoking during the last 4 weeks were defined as ‘smoker’, those who reported former smoking as ‘ex-smoker’, and all other persons as ‘non-smoker’. Participants were defined as ‘physically active’ if they answered affirmatively to the question of whether they performed any physical exercise. All other persons were defined as ‘physically inactive’. Heart rate, systolic, and diastolic blood pressures were measured three times on the right arm of seated persons, using a digital blood-pressure monitor (HEM-705CP, Omron Corporation, Tokyo, Japan). The mean of the second and third measurements was used for statistical analyses. Hypertension was defined as systolic blood pressure ⩾140 mm Hg or diastolic blood pressure ⩾90 mm Hg or self-reported intake of antihypertensive medication.
Assessment of trauma, PTSD, and depressive symptoms
The PTSD module of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) was applied to assess the occurrence of PTSD in participants. The PTSD module is a frequently used instrument in clinical practice (Elhai et al., Reference Elhai, Gray, Kashdan and Franklin2005). At the beginning, participants were asked if they had been exposed to one of the following events which are enlisted as traumatic events in the DSM-IV: combat or war-zone experience, physical assault, rape, childhood sexual abuse, natural disaster, life-threatening illness, serious or nearly fatal accident, imprisonment and/or torture, sudden and unexpected death of a loved one, as well as witnessing or learning about traumas to others. The module was terminated if the participants responded with ‘no’ to each of these questions. If a participant affirmed exposure to more than one traumatic event, the person was asked to identify the most distressing experience and to relate to this event when answering the subsequent questions. The PTSD symptoms according to the DSM-IV were then continuously asked in the interview. If the respondent did not pass the required diagnostic threshold (e.g. at least one re-experiencing symptom), the interview was also terminated. We also assessed the number of traumatic events in order to estimate the severity of traumatic stress.
Co-existing depressive symptoms were assessed using the corresponding screening questions of the Composite International Diagnostic Screener for mental disorders (CID-S) (Wittchen et al., Reference Wittchen, Höfler, Gander, Pfister, Storz, Üstün, Müller and Kessler1999). The CID-S is a 12-item self-report scale evaluating the core symptoms of mental disorders according to the DSM-IV. Two questions covering the dimensions ‘depressed mood’ and ‘loss of interest’ are included in order to screen for lifetime major depression. Participants were classified as having a depressive syndrome if at least one positive answer is given.
Laboratory analyses
Trained and certified medical staff collected non-fasting venous blood samples from the SHIP-1 participants following a standardized protocol. Plasma renin concentrations and plasma aldosterone concentrations were measured in EDTA plasma by radioimmunometric procedures (renin, Renin III generation, Cisbio Bioassay, Bagnols-sur-Cèze Cedex, France; aldosterone, Coat-A-Count Aldosterone, Siemens Healthcare Diagnostics, Eschborn, Germany) as previously described (Hannemann, Reference Hannemann2012). The aldosterone-to-renin ratio was calculated from both measures as an index of autonomous aldosterone production. The serum potassium concentration was determined with indirect potentiometry with ion-selective electrodes using a Dimension RxL (Siemens Healthcare Diagnostics, Eschborn, Germany). Serum creatinine concentrations were determined with the Jaffé method (Siemens Dimension RxL; Siemens Healthcare Diagnostics, Eschborn, Germany). The estimated glomerular filtration rate (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease formula.
Statistical analyses
Selected sociodemographic and health-related characteristics were compared between participants without trauma, with trauma but without PTSD, and with PTSD using measures of descriptive statistics. Categorical variables are presented as proportions, and the asymmetrically distributed continuous variables are presented as medians with 1st–3rd quartiles as recommended by the STROBE initiative (Vandenbroucke et al., Reference Vandenbroucke, Von Elm, Altman, Gøtzsche, Mulrow, Pocock, Poole, Schlesselman, Egger and Initiative2007). Group differences were tested for statistical significance with χ2 (categorical data) or Kruskal–Wallis tests (continuous data). Two-tailed p values <0.05 were considered statistically significant.
We then assessed whether statistically significant group differences in adjusted mean renin or aldosterone concentrations or the aldosterone-to-renin ratio exist between persons without trauma, with trauma but without PTSD, and with PTSD by analysis of covariance (ANCOVA) [hypotheses (i, iii)]. The effect size was determined by the calculation of η 2. Post-hoc tests followed by a Bonferroni adjustment of p values for multiple testing were then applied to assess pairwise differences between the three groups. These and all following models were adjusted for age, sex, waist circumference, alcohol consumption, physical activity, serum creatinine and potassium concentrations, depressive symptoms, and intake of medication that alters renin or aldosterone concentrations. Renin and aldosterone concentrations as well as aldosterone-to-renin ratios were log-transformed before being entered in the respective models.
Multivariable logistic regression models were performed to assess whether the number of traumatic events was associated with PTSD (dependent variable). In our analytic sample, the number of traumata ranged between zero and seven (zero traumata n = 1399; one trauma n = 1060; two traumata n = 380; three traumata n = 155; four traumata n = 66; five traumata n = 25; six traumata n = 4; seven traumata n = 3). As the number of individuals with more than four traumatic events was low, we collapsed these categories to four or more traumata (n = 98). Finally, we calculated multivariable linear regression models to assess the associations between the number of traumata and renin or aldosterone concentrations or aldosterone-to-renin ratios [hypotheses (ii)]. Moreover, we examined whether the association between PTSD and renin or aldosterone or the aldosterone-to-renin ratio is altered after additional adjustment for the number of traumatic events. Effect size was estimated by Cohen's f 2 in the regression analyses.
In a sensitivity analysis, we repeated all calculation after exclusion of participants taking medication that alters renin or aldosterone concentrations.
All statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Descriptive statistics
Sociodemographic and health-related characteristics according to the three subgroups ‘without trauma’, ‘trauma without PTSD’, ‘PTSD’ are given in Table 1. In all, 1693 of 3092 individuals reported at least one traumatic event (54.8%). Out of these 1693 participants, 65 fulfilled criteria for current PTSD (3.8%). Individuals who had been exposed to traumata were significantly older (χ2 = 197.77, df = 1, p < 0.01), showed a higher waist circumference (χ2 = 27.96, df = 1, p < 0.01), and were more often living alone (χ2 = 31.16, df = 1, p < 0.01). Traumatic stress with or without PTSD was highly related to increased reporting of depressive symptoms (χ2 = 51.61, df = 1, p < 0.01). We observed that persons with PTSD had significantly higher renin than those without trauma (χ2 = 16.94, df = 1, p < 0.01) and those with trauma but without PTSD (χ2 = 8.72, df = 1, p < 0.01), while there were no group differences in aldosterone concentrations (χ2 = 2.74, df = 1, p = 0.10 for ‘PTSD’ v. ‘without trauma’ and χ2 = 2.53, df = 1, p = 0.11 for ‘PTSD’ v. ‘with trauma but without PTSD’).
Table 1. Characteristics of the study population

Adjusted mean renin and aldosterone concentrations according to trauma and PTSD status
The subsequently performed ANCOVA supported the above findings (Fig. 2). Significant group differences were observed for renin concentrations (F = 9.48, df = 2, p < 0.01, η2 = 0.11) and the aldosterone-to-renin ratio (F = 3.60, df = 2, p = 0.03, η2 = 0.06) but not for aldosterone (F = 1.23, df = 2, p = 0.29, η2 = 0.06). Post-hoc tests for pairwise group differences revealed that adjusted mean renin concentrations were significantly higher in traumatized persons than in those without trauma (t = 2.67, df = 1, p = 0.02) and even higher in individuals with PTSD (t = 3.13, df = 1, p < 0.01), while there were no significant differences in aldosterone concentrations between the three groups (t = 1.03, df = 1, p = 0.91 for ‘without trauma’ v. ‘trauma without PTSD’; t = 1.35, df = 1, p = 0.53 for ‘without trauma’ v. ‘PTSD’; t = 1.06, df = 1, p = 0.86 for ‘trauma without PTSD’ v. ‘PTSD’). Thereby, our hypotheses (i) and (iii) were confirmed. In addition, the aldosterone-to-renin ratio was lower in participants suffering from PTSD (t = −2.41, df = 1, p = 0.05) than in those without trauma.

Fig. 2. Adjusted means with 95% confidence intervals for the plasma renin concentration (PRC), the plasma aldosterone concentration (PAC), and the aldosterone-to-renin ratio (ARR) according to trauma and post-traumatic stress disorder (PTSD) status. Adjustment for sex, age, waist circumference, smoking status, alcohol consumption, physical activity, serum creatinine and potassium concentrations, depressive symptoms, and intake of medication that alters renin or aldosterone concentrations.
Associations between the number of traumatic events and PTSD
The proportion of persons with PTSD increased with growing trauma load, from 2.26% in persons with one trauma up to 11.2% in individuals with four or more traumata. Logistic regression analyses confirmed an association between trauma load and PTSD. In individuals with at least one trauma, each additional trauma increased the odds for PTSD (OR 1.63, 95% CI 1.30–2.03, χ2 = 9.33, df = 1, p < 0.01). Compared with persons with one trauma, those with two trauma had an OR of 2.59 (95% CI 1.37–4.90, χ2 = 8.62, df = 1, p < 0.01) for PTSD, those with three traumata had an OR of 2.95 (95% CI 1.19–6.82, χ2 = 5.99, df = 1, p = 0.01) and those with four or more traumata had an OR of 5.65 (95% CI 2.31–13.35, χ2 = 15.21, df = 1, p < 0.01).
Associations between the number of traumatic events and renin or aldosterone concentrations
Multivariable linear regression analyses revealed dose–response relationships between the number of traumatic events and renin as well as the aldosterone-to-renin ratio in our analytic sample (Table 2 and Supplemental Fig. S1) whereby our hypothesis (ii) was confirmed. When the number of traumata was entered as continuous variable in the models, we found a significant positive association for renin (β = 0.065, t = 0.35, df = 1, p < 0.001) and an inverse association for the aldosterone-to-renin ratio (β = −0.061, t = −3.26, df = 1, p < 0.01), but no association with aldosterone (β = 0.004, t = 4.10, df = 1, p = 0.72). Additional linear regression analyses were performed to estimate the effects of the specific number of traumatic events on renin and aldosterone levels. We found that, compared with the reference group of persons without trauma, those with a history of two traumas showed significantly higher renin levels (β = 0.16, t = 3.10, df = 1, p < 0.01) and those who had been exposed to four or more traumatic events had even higher levels of renin (β = 0.457, t = 3.67, df = 1, p < 0.001). Comparable associations for aldosterone were not observed.
Table 2. Associations between the number of traumata and the log-transformed plasma renin concentration (PRC), plasma aldosterone concentration (PAC), or aldosterone-to-renin ratio (ARR)

Results from fully adjusted linear regression models.
Associations between PTSD and renin or aldosterone concentrations
Finally, we assessed whether the association between PTSD and renin is altered by the trauma load (Table 3). The low-to-moderate association of PTSD with renin concentrations was slightly attenuated but remained significant (β = 0.316 v. β = 0.382, p < 0.01 v. p < 0.01, f 2 = 0.12 v. f 2 = 0.13) after additional adjustment for the number of traumatic events. Thereby, PTSD was associated with distinctive alterations of RAAS activity independent from the trauma load.
Table 3. Associations between post-traumatic stress disorder (PTSD) and the log-transformed plasma renin concentration (PRC), plasma aldosterone concentration (PAC), or aldosterone-to-renin ratio (ARR)

Results from fully adjusted linear regression models.
Sensitivity analysis
After exclusion of all participants taking medication that alters renin or aldosterone concentrations, a population of 1862 individuals, including 38 with PTSD, resulted. We observed a tendency toward increasing renin concentrations with the number of traumata or PTSD but the results missed statistical significance (Supplemental Tables S1–S3 and Supplemental Fig. S2).
Discussion
To our best knowledge, this is the first study systematically investigating the activity of the RAAS in relation to the number of traumatic events and current PTSD. It adds to the previous studies that primarily focused on specific traumas and their relation with other neuroendocrine systems. Our findings suggest that chronic stress imposed by traumatization and PTSD relates to the activation of the RAAS with increased renin levels, while we did not find evidence for altered aldosterone levels. More specifically, we found elevated renin levels in traumatized persons compared with non-traumatized participants and a dose–response relationship between the number of traumas and renin concentrations. These findings suggest that traumatic stress has long-lasting and cumulative effects on the RAAS. Particularly persons who had been exposed to four or more traumatic events were affected by a marked increase in plasma renin concentrations, indicating that the subgroup of severely traumatized persons is at specific risk for a dysregulation of the RAAS.
These results add to and extend previous findings showing that trauma causes long-term dysregulation in the HPA-axis and the autonomic nervous system (Plotsky and Meaney, Reference Plotsky and Meaney1993; Heim et al., Reference Heim, Newport, Heit, Graham, Wilcox, Bonsall, Miller and Nemeroff2000, Reference Heim, Newport, Bonsall, Miller and Nemeroff2003). Moreover, they contribute to explain previous results showing dose–response relationships between childhood and adulthood adversities and various physical and mental diseases (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, Koss and Marks1998; Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry, Dube and Giles2006). In persons with PTSD, renin levels were considerably higher than in traumatized individuals without PTSD and were still significantly elevated when controlled for the trauma load. These findings support the concept of PTSD as a mental health condition with specific neuroendocrine characteristics and are in line with the results from animal models showing enhanced plasma renin activity in response to acute as well as chronic psychological and physiological stress (Jindra and Kvetnanskỳ, Reference Jindra and Kvetnanskỳ1982; Aguilera et al., Reference Aguilera, Kiss and Sunar-Akbasak1995). The autonomic nervous system is the main effector for stress-induced renin release, and enhanced activity of the autonomic nervous system in PTSD patients at baseline as well as in response to acute stress was found in previous studies (Cohen et al., Reference Cohen, Kotler, Matar, Kaplan, Loewenthal, Miodownik and Cassuto1998, Reference Cohen, Benjamin, Geva, Matar, Kaplan and Kotler2000). Stress-induced renin release is mediated by stimulating renal β-adrenergic receptors. There is an evidence showing that blockade of renal β-receptors by administration of propranolol diminishes the increased renin activity in response to stress (Golin et al., Reference Golin, Gotoh, Said and Ganong1988). Given that treatment with propranolol immediately after trauma exposure has repeatedly been found to reduce symptoms of PTSD, our results support the concept of renin to take in a critical role in the pathophysiology of PTSD (Pitman et al., Reference Pitman, Sanders, Zusman, Healy, Cheema, Lasko, Cahill and Orr2002; Vaiva et al., Reference Vaiva, Ducrocq, Jezequel, Averland, Lestavel, Brunet and Marmar2003).
In contrast, no statistically significant changes of aldosterone concentrations in traumatized persons with and without PTSD were found, which is in line with the results from previous studies investigating the effects of chronic stress on RAAS activity in animals and humans (Aguilera et al., Reference Aguilera, Kiss and Sunar-Akbasak1995; Terock et al., Reference Terock, Hannemann, Janowitz, Völzke, Nauck, Freyberger, Wallaschofski and Grabe2017). Possible mechanisms explaining the lack of aldosterone increase include the reduction of adrenal ANG II receptors, a reduced activity of the aldosterone synthetase as well as suppressed ACTH release due to a negative feedback circuit in the presence of chronic stress (Aguilera, Reference Aguilera1993; Miller et al., Reference Miller, Chen and Zhou2007). Results from previous studies indicated that increased activity of the RAAS may not only be a result of chronic stress imposed by exposure to trauma or PTSD, but could also be directly involved in the development of stress-related disorders. For example, animal studies showed that blockade of brain AT1 receptors ameliorates stress-related behavior, anxiety, and brain inflammation (Saavedra et al., Reference Saavedra, Sánchez-Lemus and Benicky2011). More specifically, AT1 receptors on corticotropin-releasing factor neurons were found to contribute to the expression of conditioned fear in mice (Hurt et al., Reference Hurt, Garrett, Keifer, Linares, Couling, Speth, Ressler and Marvar2015). These findings were supported by the study of Khoury et al. showing that intake of ARB and ACE-I but not of other antihypertensive drugs were associated with reduced PTSD symptoms in persons who had been exposed to traumatic events (Khoury et al., Reference Khoury, Marvar, Gillespie, Wingo, Schwartz, Bradley, Kramer and Ressler2012). In line with the concept of neuroendocrine alterations preceding the development of PTSD, some authors reported that decreased cortisol levels and increased autonomic nervous system activity at the time of the traumatic event were associated with a higher risk for the later development of PTSD [for review: (Yehuda et al., Reference Yehuda, McFarlane and Shalev1998)]. In this context, one could hypothesize that increased levels of renin and subsequent elevations of ANG II secondary to traumatic stress may contribute to an increased vulnerability for developing PTSD.
In line with the previous findings (Spitzer et al., Reference Spitzer, Barnow, Völzke, John, Freyberger and Grabe2009), we found significantly lower renal function in traumatized persons. Given that hypertension, which is closely related to trauma and PTSD, is an important risk factor for renal disease, it can be speculated that increased renin, a potent effector for blood pressure, contributes to explain the association between trauma and renal disease. However, as impaired renal function is restricted to traumatized persons without PTSD in our sample, the underlying mechanism may be more complex.
While our study has some strengths including the large general population sample and the controlling for different potential behavioral and metabolic confounders, there are several limitations which need to be acknowledged: First, our results are based on cross-sectional data which generally do not allow for causal conclusions. While the causal relationship between exposure to trauma and the increase of renin levels seems relatively clear, the link between RAAS dysregulation and PTSD may be more complex. Longitudinal studies are needed to investigate the direction of causality between RAAS activation and PTSD.
Second, in order to sustain the statistical power of our sample, we decided not to exclude participants taking antihypertensive drugs in the main analyses but to adjust the analyses for the intake of such medication. While results of the sensitivity analyses pointed in the same direction, the statistical uncertainty increased markedly.
Third, trauma was assessed based on self-reported retrospective information. Although the PTSD module of the DSM-IV is well-established and widely used in clinical practice and research, there has been some discussion about the accuracy of the obtained information. For example, participants may prefer not to completely disclose certain experiences or have difficulties in recalling particularly painful aspects of their early memory (Edwards et al., Reference Edwards, Anda, Nordenberg, Felitti, Williamson and Wright2001). Results from longitudinal studies indicated that traumatized participants tend to underestimate the actual occurrence particularly of childhood traumatic events (Williams, Reference Williams1995). Considering these findings, the relationship between trauma and RAAS activity may be even stronger than found in our analyses.
Fourth, the lifetime diagnosis of major depressive disorders was made based on only two questions from the CID-S. Although the original publication showed satisfactory psychometric properties, a sensitivity of 62.6% for depressive disorders suggests that a significant number of MDD cases may have been missed in our study. However, more detailed analysis in the original investigation showed that primarily fairly short depressive episodes were not detected by the CID-S, suggesting a rather weak interference with our results.
Finally, only levels of renin and aldosterone, but not of ANG II were available to us. When planning the SHIP study, diagnosis of primary aldosteronism was of particular interest to some researchers and the aldosterone-to-renin ratio is an established measure for screening for primary aldosteronism. However, given the strong functional relationship of renin and ANG II, we believe that renin levels may be used as an adequate proxy for circulating ANG II concentrations.
Despite these limitations, our study demonstrating enhanced renin levels in relation to trauma and PTSD extends existing findings on neuroendocrine alterations in traumatized persons. In analogy to the HPA-axis, our findings suggest that trauma and PTSD impact the RAAS. Given the well-established relationship of RAAS activity with physical disorders and particularly CVD, these findings contribute to elucidate the complex interaction between traumatic stress, PTSD, and physical health. Additional research is needed to investigate the role of a dysregulated RAAS on the physical health of traumatized individuals. Moreover, longitudinal studies should examine a putatively predictive effect of elevated renin on the onset of PTSD.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001496
Acknowledgements
The authors wish to thank Professor Michael Lucht for his valuable comments and suggestions to improve on the quality of this article.
Conflicts of interest
None.
Financial support
SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Deutsche Forschungsgemeinschaft (GR1912/5-1) Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Instand e.V. provided partial grant support for the determination of plasma samples in SHIP.







