The peripheral intravenous catheter/cannula (PIVC) is the most commonly used vascular access device in healthcare today.Reference Alexandrou, Ray-Barruel and Carr 1 Generally, patients admitted to hospital have a PIVC due to clinical needs, the need for prescribed intravenous therapy and/or medicines, the need for procedures, or the need for diagnostics such as a computerized tomography scanning. Premature failure of the PIVC after insertion reveals undesirable rates of failure, with 30%–50% postinsertion failure (PIF) before the completion of therapy.Reference Carr, Glynn, Dineen and Kropmans 2 – Reference Marsh, Webster, Larsen, Cooke, Mihala and Rickard 5 Most strategies are targeted to reduce infection; however, although infection is the most harmful to patients, it is also the least likely to occur.Reference Rickard, Webster and Wallis 6 Highly prevalent forms of PIF include infiltration/extravasation; occlusion; dislodgement (accidental-securement device failure or purposeful removal); and phlebitis/thrombophlebitis due to pharmacological, mechanical, and infective causes.Reference Wallis, McGrail and Webster 4
Some PIF risk factors are modifiable, such as the use of tissue adhesive after a successful PIVC insertion to avoid accidental dislodgement.Reference Bugden, Shean and Scott 7 Despite such advances, patients are commonly exposed to repeated PIVC insertions when appropriately placed and functional PIVCs are not achieved.Reference Chopra, Flanders and Saint 8 Consequently, alternative devices such as midlines, peripherally inserted central catheters, or central venous catheters may be required. Focusing on the emergency department (ED) environment where many patients are exposed to their first hospital PIVC insertion could achieve implementation of improvement strategies at the source of this clinical issue. We investigated insertion-related risk factors and predictors for PIF in patients admitted to hospital through the ED. Because the reason for removal (including PIF) is poorly recorded in the medical record in routine practice,Reference Carr, Rippey and Moore 9 we prospectively followed admitted patients who had had a PIVC inserted in 2 EDs.
Methods
We published the protocol and methods for this study as secondary outcomes in an open access journal.Reference Carr, Rippey and Cooke 10 Our study has been registered with the Australian and New Zealand Trials Registry (ANZCTRN12615000588594). We used the STROBE checklist to assist with the reporting of this observational study.Reference Elm, von, Altman and Egger 11
Study design and setting
A prospective multicenter observational cohort of admitted patients from ED who had their PIVC insertion observed by trained data collectors were followed up to identify whether PIF occurred. The researchers were predominantly present during the day shift on weekdays but were intermittently present on weekends. They were not present during night shifts. The study was performed in Sir Charles Gairdner Hospital and Fiona Stanley Hospital, 2 large academically affiliated hospitals in Perth, Western Australia. The former is a 650-bed hospital with approximately 65,000 ED patients assessed annually; the latter is a 783-bed hospital with ~80,000 adult ED presentations annually.
Data collection variables of interest
We collected data from June 2015 to May 2016 using a case report form with a content validity index score >0.78 (reflecting good content validityReference Polit and Beck 12 ) and an initial Fleiss κ of 0.90 (suggesting almost perfect agreement when used for data collectionReference McHugh 13 ). As defined in our published protocol,Reference Carr, Rippey and Cooke 10 patient variables included gender, age, skin condition (good, fair, poor),Reference Marsh, Mihala, Ray-Barruel, Webster, Wallis and Rickard 14 skin shade using the Fitzpatrick skin shade index,Reference Fitzpatrick 15 the venous international assessment scale (number of visible veins),Reference de la Torre-Montero, Montealegre-Sanz and Faraldo-Cabana 16 vein palpability (with and without a tourniquet, and never palpable), and insertion site (back of hand, wrist, forearm, ante cubital fossa upper arm). Clinical presentations associated with the following characteristics were recorded: confusion, sepsis, chemotherapy, diabetes, and difficult intravenous access. We collected the patient’s Australasian Triage Scale (ATS) score of 1 to 5: patients with an ATS score of 1 are patients with immediately life threatening events, and patients with an ATS of 5 are less urgent.Reference Considine, LeVasseur and Villanueva 17 Clinician variables included role (phlebotomist, nurse, medical student, intern, registered medical officer, registrar, consultant); insertion experience (as a numerical category (0 to >1,000 PIVCs inserted); number of needle redirections; whether PIVC was inserted on the first attempt; and the use of ultrasound. Data on the PIVC gauge 14–22g, type of add-on device (J-Loop, 3-way stopcock, or needlefree connector), the indication for the PIVC (eg, contrast CT scan, crystalloid and/or colloid intravenous fluids), and intravenous medications (eg, antibiotics, steroids, analgesia, antipyretics, cardiac medication) were also collected. Additionally, we observed any infection prevention breach defined as a compromise of an aseptic nontouch technique (ANTT) during the PIVC insertion.Reference Beaumont, Wyland and Lee 18 We defined PIF per our protocol: PIVC removal owing to failure defined as a composite of the following: infiltration, occlusion, pain and/or peripheral intravenous assessment score >2 (the hospital’s assessment tool for PIVC phlebitis), or dislodgement (ie, accidental-securement device failure or purposeful removal).
Sample
A convenience sample of ED presentations who were subsequently admitted a hospital ward was used to measure rates of PIVC insertion failure.
Statistical analysis
The primary outcome was to establish the reasons for removal of the PIVC to identify rates of PIF and dwell times. Summary statistics for all variables of interest were performed, including means and standard deviations (SD) for normally distributed continuous variables, medians, and interquartile ranges (IQRs) for nonnormally distributed continuous variables and counts (N) and percentages (%) for categorical variables. Cox proportional hazards regression was used to model the time from PIVC insertion to failure, where the PIVCs that did not fail are censored at their date of removal. Forward stepwise model selection was performed where all variables (as described in the Data collection variables of interest section) were considered for inclusion, and the significance level for variable entry and exit was set at 5%. The proportional hazards assumption was tested by including the time-dependent covariates in the model and checking for significance. This assumption was met as no variables were found to depend on time (all P>.9). Hazard ratios (HR), 95% confidence intervals (CIs), and P values were calculated. Data were analyzed using the R environment for statistical computing (version 3.4.4). 19
Ethical approval
Full ethical approval for this study was obtained from Sir Charles Gairdner Hospital, Human Research Ethics (office ref. HR 2015-149) and reciprocated at Fiona Stanley Hospital, and Griffith University. This approval included a waiver of consent for the inclusion of the patients receiving a PIVC.
Results
General characteristics
Having observed the insertion of their PIVC in the ED, we followed 391 patients to measure dwell time and reason(s) for removal. Table 1 displays characteristics of variables associated with PIF for all 391 patients with and without PIVC failure. Supplementary Table S1 displays baseline clinical characteristics of all patient, clinician, environment, product, technology, and infusate factors with summary statistics influencing PIVC failure.
Table 1 Summary of Baseline Characteristics Broken Down by Peripheral Intravenous Catheter Failure
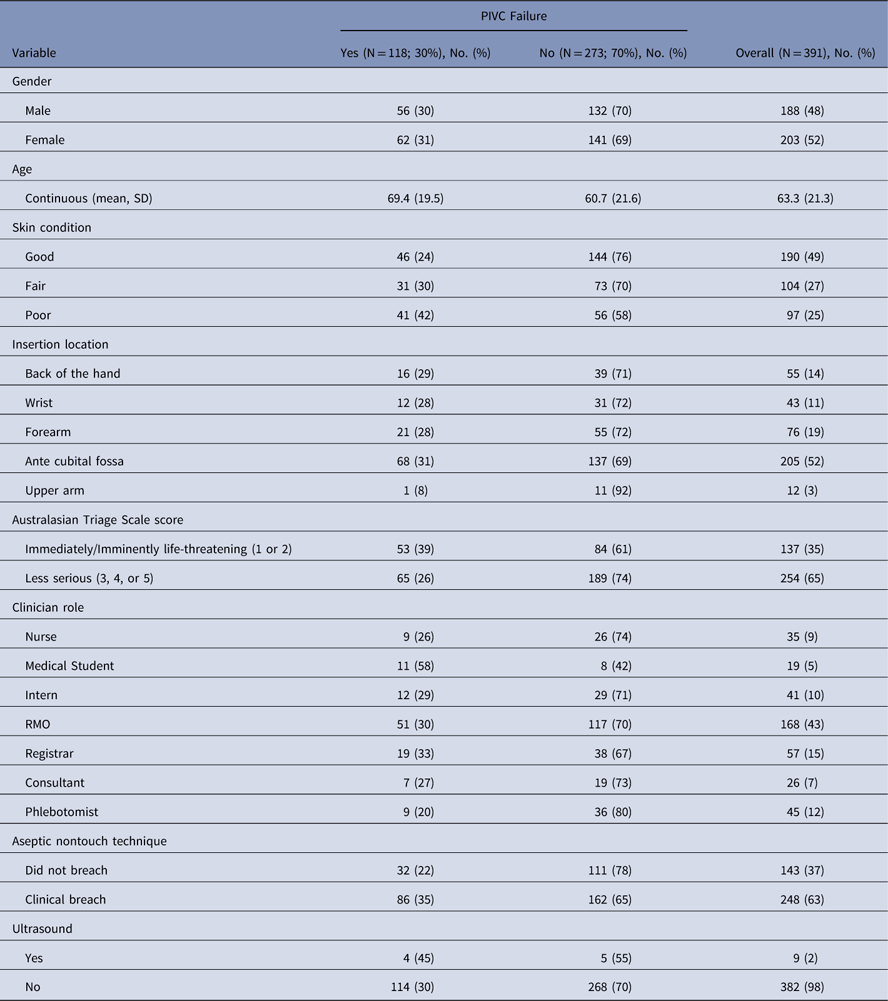
Note. PIVC, peripheral intravenous catheter/cannula; SD, standard deviation; RMO, registered medical officer.
The characteristics of our sample included female gender (N=203; 52%) and mean age of 63.3 years (SD, 21.3 years). Also, 137 patients (35%) had an Australasian Triage Scale score of 1 or 2, that is, these patients were in an immediately or imminently life-threatening condition. The percentage of PIVCs not used for any intravenous therapy, intravenous medications and intravenous contrast scans was 25%. The first attempt insertion success rate in this admitted cohort was 79%.
The median hospital length of stay for 385 patients (6 observations missing due to transfer to others healthcare facilities) was 2 days (IQR, 1–5 days), and the median PIVC dwell time was 28.5 hours (IQR, 17.4–50.8 hours). The rate of PIVC failure was 30% (N=118), and patients with PIVC failure had a median PIVC dwell time of 24.1 hours (IQR, 11.7–50.8 hours). Patients without PIVC failure had a median PIVC dwell time of 29.9 hours (IQR, 20.7–49.8 hours) (Table 2). Infiltration and occlusion accounted for 15% of all reasons for removal (Table 3) with zero PIVC related blood stream infections identified.
Table 2 PIVC Dwell Time (Hours) for the Entire Cohort as well as Those With and Without PIVC Failure

Note. PIVC, peripheral intravenous catheter/cannula.
Table 3 Reasons for PIVC Failure and Removal for Entire Cohort

Note. PIVC, peripheral intravenous catheter/cannula; PIF, postinsertion failure; PVAS, peripheral vascular access score; VAD, vascular access device.
Analysis results
The patient variables significantly related to PIVC failure in the final multivariate model were patient age (P<.0001), triage category (P=.0003), and insertion site (P=.0337). Specifically, as age increased, individuals were more likely to have PIVC PIF (for a 1-year increase in age (HR, 1.02; 95% CI, 1.01–1.03)), and those with ATS scores of 1 or 2 (immediately or imminently life-threatening condition) were more likely to have PIVC PIF (HR, 2.04; 95% CI, 1.39–3.01) than patients with ATS scores of 3–5, that is, needing less urgent care. A patient with a PIVC inserted into the ante cubical fossa or the back of the hand was significantly more likely to have PIVC PIF than a patient with a PIVC inserted in the upper arm (HR, 11.3; 95% CI, 1.39–91.1 and HR, 12.8; 95% CI, 1.47–110.3, respectively).
The clinician variable significantly related to PIVC failure in the final multivariate model was clinician role (P=.0095). PIVCs inserted by clinical personnel in certain roles were significantly less likely to fail than PIVCs inserted by medical students: registrars (HR, 0.31; 95% CI, 0.14–0.68); registered medical officers, (HR, 0.37; 95% CI, 0.19–0.72); interns (HR, 0.21; 95% CI, 0.09–0.49); and phlebotomists (HR 0.27, 95% CI, 0.11–0.67). Whether the clinician used or did not use an aseptic nontouch technique approach defined as clinical breach was significant (P=.032) in the final multivariate model. PIVCs inserted without any observed compromise of clinical breach (eg, using an aseptic nontouch technique) were associated with less PIF than those PIVCs inserted while compromising an aseptic nontouch approach (HR, 0.63; 95% CI, 0.42–0.96). Furthermore, patients requiring an ultrasound-guided PIVC (USG-PIVC) insertion were significantly (P=.0011) more likely to have PIVC failure than patients not requiring an ultrasound (HR, 6.52; 95% CI, 2.11–20.1). Table 4 lists all the multivariate results from analyzing time to PIVC failure.
Table 4 Multivariate Results From Analyzing Time to PIVC Failure

Note. HR, hazard ratio; CI, confidence interval; RMO, registered medical officer.
Discussion
In this study, almost 1 in 3 PIVCs (30%) failed due to a complication. This rate is only slightly lower than that of another study reporting 34% failure,Reference Wallis, McGrail and Webster 4 and it is in agreement with recent findings from multiple studies that PIF is a highly prevalent problem in healthcare.Reference Helm, Klausner, Klemperer, Flint and Huang 3 , Reference Marsh, Webster, Larsen, Cooke, Mihala and Rickard 5 It has been established that first-time insertion success rates are varied and need to improve.Reference Carr, Higgins, Cooke, Rippey and Rickard 20 Additionally, these results provide rates of PIF that are a cause for concern and will inform any planned quality initiatives or further interventional studies. For example, in the older population, increasing age was significantly associated with PIF. Patients with higher ATS scores who required immediate and urgent ED care were significantly more likely to have PIVC PIF. This finding is not surprising given the large number of intravenous interventions that occur in this patient cohort, and perhaps more haste is taken in placement in these patients, which results in this higher PIF rate. The other significant patient finding from the multivariate model was that PIVCs inserted into the ante cubital fossa region failed significantly more often than those inserted into PIVCs placed in the forearm or upper arm. However, only a small number of upper-arm insertions were included in this study, so these interpretations must be viewed with caution. Given that >50% of PIVCs are inserted in the ante cubital fossa region, better decision making is needed with regard to site of insertion.Reference Carr, Rippey, Budgeon, Cooke, Higgins and Rickard 21 Although it was beyond the scope of this study, this finding does suggest that PIF is associated with other mechanistic causes and is certainly worthy of further investigation.Reference Piper, Carr, Kelsey, Bulmer, Keogh and Doyle 22
The PIVCs inserted by medical students appear to be a clinician factor associated with PIF. This finding may be relevant to this group’s first clinical exposure to PIVC. However, this finding provides a potential opportunity for greater proctoring, use of simulation and mentoring regarding PIVC-related procedures. Additionally, it may promote better decision making regarding venous site selection, PIVC size, and the use and care of add-on devices. An issue underlying PIVC PIF stems from the variety of clinicians from different disciplines who insert and access them, which points to a lack of standardized approach to education, insertion practice, device care and management, from within disciplines and between individual practitioners. Without clinical agreement and an acceptance of common standards of assessment, insertion, and maintenance, PIVC care will remain dependent on individual clinicians from a variety of disciplines with different experience levels and different practices performing less than acceptable care.
This study found USG-PIVC to be associated with significantly higher PIF. Although we acknowledge the small number of these catheters studied, previous studies have also identified more postinsertion failure when ultrasound-guided approaches are used.Reference Fields, Dean and Todman 23 This finding may be due to the higher-risk group in which ultrasound is more commonly used, or to the longer-length PIVC that is used. In this study, all the USG-PIVC inserted were 48 mm in length. Clearly, given the cost associated with advanced insertion techniques such as using ultrasound, USG-PIVCs should survive longer. In our study, USG-PIVCs had a median dwell time of 31.9 hours (range, 2–42 hours).
Infiltration and occlusion were the most common form of PIF observed in this study; they occurred in 47% of PIVCs. Evidence-based infiltration and occlusion prevention strategies remain scarce. Because current PIVC assessment tools specifically focus on phlebitis, rather than other complications, a clinically credible PIVC assessment tool covering all aspects of failure is needed. In this study, 63% of all insertions involved a clinical breach, which is concerning because hospitals spend considerable money implementing concepts such as aseptic nontouch technique to prevent infections.
Our study has some limitations, and we must interpret these results with caution. We used a convenience sampling method due to limited funding and resources. With regard to infiltration, it was not feasible to identify the rates and speed of infusion, which is likely to be an important considerationReference Piper, Carr, Kelsey, Bulmer, Keogh and Doyle 22 nor the number of PIVC flushes used, nor whether the PIVCs were used for blood sampling. In terms of occlusion, we did not perform USG assessment of the site to determine whether if thrombus was a predictor of occlusion and or infiltration. We did not power our study to accept or reject any hypothesis, we primarily wanted to identify causes of PIF prospectively. Therefore, our data may be vulnerable to type 2 error. We sought to determine the rates of PIF for use in future studies. Finally, this study identifies an association, and in no way represents cause and effect that an interventional design would seek to identify.
We have used a generalizable sample, and our failure rates are in line with previous studies. Our results of 30% succumbing to PIF echoes recent reports of similar failure due to largely modifiable reasons.Reference Marsh, Webster, Larsen, Cooke, Mihala and Rickard 5 To our knowledge, this is the first study to report that PIF is greatest when the PIVC is inserted by medical students in the ED as opposed to 6 other clinician types who provide insertion. Future strategies could develop a specific PIVC educational intervention for all staff inserting PIVCs and perhaps specifically for medical students to assess the validity of this result. This study is one of the first to identify prospectively that PIVCs inserted with aseptic nontouch technique are associated with less PIF. Furthermore, where it is clinically achievable, the ante cubital fossa region and back of the hand should be avoided in favor of the upper arm and/or forearm veins for PIVC placement. Finally, targeted educational and workforce strategies should be considered for patients with ATS scores of 1 and 2 and for those undergoing USGPIVC who experience PIF.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.190
Acknowledgments
The authors wish to thank the patients who presented to the ED and allowed us to observe their PIVC insertions. We are also extremely grateful to the clinicians of both Sir Charles Gairdner Hospital and Fiona Stanley Hospital who consented to allow us to observe their procedures. We are also grateful to the staffs of both EDs. We are grateful to Ms Shannon Nell, RN, who assisted with observational data collection, to Ms Mel Sarti, CN, for follow up data collection, and to Ms Pip Bain, CN, and Ms Lisa Douglas Smith, CN, for data entry.
Financial support
We acknowledge Associate Professor Karen Bradley and the WA Nursing and Midwifery Health Department for an academic support grant to complete this work.
Conflicts of interest
Peter J Carr is an academic researcher and has received speaker’s bureau payment from CareFusion in 2013 and from Becton Dickinson (BD) in 2014 for lectures on vascular access. He received a grant from CareFusion (facilitated by his institution at the time) to attend a scientific meeting on vascular access in the United States in 2012. Marie L Cooke is an academic researcher. Griffith University (not Prof Cooke) has also received unrestricted, educational grant from Baxter to support the development of educational materials on PIVC insertion, maintenance, and removal. Griffith University (not Professor Cooke) has also received unrestricted, grant-in-aid donations from manufacturers of IV catheters and related equipment (Becton Dickinson, Centurion, Entrotech). Claire M Rickard is an academic researcher and speaker in the field of vascular access. Griffith University (not Prof Rickard) has received payments from manufacturers of intravenous (IV) catheters and related equipment for her to give educational lectures or expert opinion on products (3M, Bard, BBraun, BD, Carefusion, Mayo, ResQDevices, Smiths Medical). Griffith University (not Prof Rickard) has also received unrestricted, grant-in-aid donations from manufacturers of IV catheters and related equipment (3M, Adhezion, Angiodynamics, Bard, Baxter, BD, Centurion, Carefusion, Cook, Entrotech, Flomedical, Medtronic, Smiths Medical and Teleflex) to (1) support Prof Rickard’s independent research and (2) to support travel costs for research staff and students to present their independent research at conferences. Manufacturers had no involvement in study design, execution, data handling, publication preparation or approval. All other authors report no conflicts of interest relevant to this article.






