INTRODUCTION
Trypanosoma cruzi, a flagellated protozoan that may infect any mammalian species and cause Chagas disease, is a major public health problem in Latin America with rising importance in developed countries due to international migration (Tibayrenc, Reference Tibayrenc, Edwin and Spear2009; Tarleton et al. Reference Tarleton, Gürtler, Urbina, Ramsey and Viotti2014). Trypanosoma cruzi presents a complex population genetic structure classified into six discrete typing units (DTUs) ranging from TcI to TcVI (Miles et al. Reference Miles, Llewellyn, Lewis, Yeo, Baleela, Fitzpatrick, Gaunt and Mauricio2009; Tibayrenc, Reference Tibayrenc, Edwin and Spear2009; Zingales et al. Reference Zingales, Andrade, Briones, Campbell, Chiari, Fernandes, Guhl, Lages-Silva, Macedo, Machado, Miles, Romanha, Sturm, Tibayrenc and Schijman2009), and the recently described DTU denominated Tcbat (Marcili et al. Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009a ). All DTUs may produce human Chagas disease, and show a heterogeneous distribution depending on geographic area, biome, mammalian host species and triatomine species involved (Zingales et al. Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012). Thus TcII, TcV and TcVI are closely associated with anthropogenic environments, although TcII had also been identified in wild mammals from several biomes (Lima et al. Reference Lima, das C. Xavier, Maldonado, Roque, Vicente and Jansen2014); TcIII and TcIV with sylvatic transmission cycles, and TcI circulates in sylvatic and domestic cycles depending on geographic area (Yeo et al. Reference Yeo, Acosta, Llewellyn, Sánchez, Adamson, Miles, López, González, Patterson, Gaunt, Rojas De Arias and Miles2005; Miles et al. Reference Miles, Llewellyn, Lewis, Yeo, Baleela, Fitzpatrick, Gaunt and Mauricio2009; Zingales et al. Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012). A major question of epidemiological relevance is whether both types of transmission cycles of T. cruzi are connected or independent. Identifying the circulating DTUs and the structure of sylvatic transmission cycles is also important to trace the origins of (re)emerging cases in areas under vector or disease surveillance (Miles et al. Reference Miles, Llewellyn, Lewis, Yeo, Baleela, Fitzpatrick, Gaunt and Mauricio2009).
The epidemiological role of many host species of T. cruzi is still unresolved and may vary with landscape features (Noireau et al. Reference Noireau, Diosque and Jansen2009; Jansen et al. Reference Jansen, Xavier and Roque2015). Some mammalian host species are more exposed to T. cruzi, especially those that are nest-dwelling or use hollow trees, burrows and caves such as opossums, armadillos, rodents and bats (Noireau et al. Reference Noireau, Diosque and Jansen2009). Opossums and armadillos are considered the most ancient hosts of T. cruzi (Noireau et al. Reference Noireau, Diosque and Jansen2009). Rodents are frequently involved in sylvatic and (peri)domestic transmission cycles (Noireau et al. Reference Noireau, Diosque and Jansen2009; Orozco et al. Reference Orozco, Piccinali, Mora, Enriquez, Cardinal and Gürtler2014; Jansen et al. Reference Jansen, Xavier and Roque2015). Synanthropic rats and mice may act as a link between sylvatic and domestic cycles (Gürtler and Cardinal, Reference Gürtler and Cardinal2015).
Bats (Chiroptera) are reservoir hosts of numerous infectious agents, many of which are zoonotic (Calisher et al. Reference Calisher, Childs, Field, Holmes and Schountz2006). Some of their ecological characteristics make them especially suitable for pathogen emergence or re-emergence: high densities; gregarious behaviour; use of multi-species refuges and spaces close to human dwellings, and high dispersal capacity (Calisher et al. Reference Calisher, Childs, Field, Holmes and Schountz2006; Luis et al. Reference Luis, Hayman, O'Shea, Cryan, Gilbert, Pulliam, Mills, Timonin, Willis, Cunningham, Fooks, Rupprecht, Wood and Webb2013). These distinctive attributes enhance the transmission of pathogens between wild and domestic animals or humans in a context of increasing habitat fragmentation and loss (Molyneux et al. Reference Molyneux, Ostfeld, Bernstein and Chivian2008). Trypanosoma cruzi frequently infects bats in the wild and may have derived from bat trypanosomes (Hamilton et al. Reference Hamilton, Teixeira and Stevens2012). As part of the Southern Cone Initiative for the interruption of vector- and blood-borne transmission of human T. cruzi infection, Misiones Province (Northeastern Argentina) was declared free of vector-borne transmission through T. infestans in 2011 (PAHO, 2012). The local host distribution of T. cruzi in sylvatic transmission cycles, including reservoir host species, vectors and DTUs implicated, remain unknown. To address this gap in knowledge, we assessed the occurrence of T. cruzi infection in sylvatic mammals from a well-defined area in Misiones; identified the circulating DTUs; assessed the hosts’ infectiousness to the vector, and searched for triatomines in sites where the infected mammals had been caught.
MATERIALS AND METHODS
Study area
Field work was conducted in Capital (27°24′S55°55′W) and Candelaria (27°22′S 55°34′W) Departments in Misiones Province, Argentina (Fig. 1). The study area belongs to the Southern Cone Mesopotamian savannah ecoregion. Originally composed of grasslands, marshes, woodlands and gallery forests (Viglizzo et al. Reference Viglizzo, Frank and Carreño2006), the region has undergone major environmental changes and currently is characterized by cattle ranches, tree and “yerba mate” (Ilex paraguariensis) plantations. The climate is humid subtropical, with an average annual rainfall of 1500 mm uniformly distributed across the year. Annual temperatures averaged 22 °C. The study sites suffered an outbreak of sylvatic yellow fever affecting Alouatta caraya monkeys in 2009.
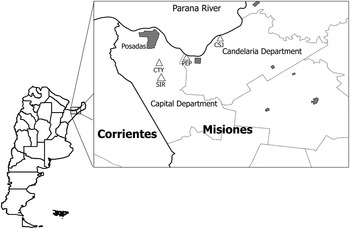
Fig. 1. Location of study sites in Misiones, Northeastern Argentina. CTY, Chacra Tuyuyu; PEP, Parque El Puma; SIR, Santa Inés Ranch; CSJ, Campo San Juan.
Field work in Capital Department was conducted at two sites: Santa Inés Ranch (SIR), a farm and cattle ranch including pine tree and “yerba mate” plantations, and small natural forest patches; and Chacra Tuyuyu (CTY), a small farm with citric plantations and a small natural forest patch surrounded by a gated community and grasslands. Field work in Candelaria Department was conducted in Parque El Puma (PEP), a 25 ha. multiple-use reserve situated in a rural area, and in Campo San Juan Reserve (CSJ), a well-preserved area of 5800 ha, including gallery forests, grasslands and fragments of “Urunday” (Astronium balansae) native forests. CSJ was transformed into a protected nature preserve in 2007, and had a few abandoned buildings with no permanent residents, few cattle and remnants of old plantations (Fig. 1).
Capture and handling of wild mammals
Captures were carried out by means of georeferenced traps and manual catches (Garmin Legend C; Garmin, Olathe, KS) in October 2012, June 2013 and April 2014. Tomahawk traps were deployed every 25 m along transect lines to capture medium-sized mammals (e.g. white-eared opossums). Traps were baited with beef or chicken scraps; checked every morning, and re-baited when appropriate. Small rodents and Woolly Mouse Opossums were caught using Sherman traps (H. B. Sherman Traps, Tallahassee, FL) deployed at ground level and on tree branches. Sherman traps were baited with seeds (oats, maize, seeds and local fruits), fresh (apple and banana) and dry fruits (walnuts, grape raisins) and pellets of a mixture of peanut butter, oatmeal and vanilla essence.
Bats were captured using mist nets (6·0 m wide, 2·6 m high, 38·0 mm black mesh; AFO Banding Supplies, Manomet, MA) opened at dusk and monitored every 30–40 min for 4 h. Four groups of two or three mist nets were placed in a zigzag formation at each capture site. The nets were set up in a cleared area free of vegetation where bat activity had previously been observed in CSJ and in PEP, and close to a D. rotundus refuge in SIR. In addition, D. rotundus bats from a colony found in a vacant house at CSJ (close to the site where the nets were deployed) were captured using a hand capture net. Black and Golden Howler Monkeys were caught with tranquilizing darts (Pneudart Type P 1 mL) shot with an air rifle (Pneudart Model 178B). Capture and transit permits were obtained from the government of Misiones Province.
The captured animals were transported to the field laboratory in PEP where each individual was subjected to a pre-anaesthetic examination and then given parenteral anaesthesia for induction and inhalatory anaesthesia for maintenance. The former was performed with tiletamine clorhydrate and zolazepan clorhydrate (Zelazol®; Fort Dodge, Buenos Aires, Argentina) or ketamine clorhydrate (Vetaset; Fort Dodge) combined with dexmedetomidine (Pfizer) at the minimum dose appropriate to species and weight (West et al. Reference West, Heard and Caulkett2007). Inhalatory anaesthesia with isoflurane was delivered with a vaporizer (IsoTec; Datex-Ohmeda GE Healthcare, Little Chalfont, UK) and medicinal O2 (0·25–3 L min−1). The anaesthetized animals were maintained on thermic cushioned surfaces in a quiet and comfortable environment, with their eyes protected with ophthalmic lubricant solutions and covered with home-made eyecups. The vital signs during the anaesthetic procedure were monitored with a multiparametric patient monitor (Contec CMS 6000).
Each individual was sexed, weighed (Pesola®), marked with a numeric metal tag (National Band and Tag Company, Newport, KY), its general condition determined, and then released at the capture site after full recovery from anaesthesia. Didelphis opossums were assigned to stage class based on tooth eruption (Schweigmann et al. Reference Schweigmann, Pietrokovsky, Bottazzi, Conti, Bujas and Wisnivesky-Colli1999).
Animals were bled by venepuncture and each blood sample was diluted 1:1 in 6 M guanidine hydrochloride, 0·2 M EDTA pH 8·0 buffer (GEB) for polymerase chain reaction (PCR) amplification. All specimens were tested by xenodiagnosis (XD) using 5–20 uninfected fourth-instar nymphs of T. infestans (depending on body size) contained in wooden boxes applied on the belly of the host for 25 min and checked for its degree of blood engorgement.
Road-killed mammals found in Candelaria routes during fieldwork; an adult male of A. caraya captured in a neighbouring locality, and mammals rescued at PEP were also examined for T. cruzi infection.
XD, parasite isolation and culture
The rectal contents from five XD bugs exposed to an identified host were pooled, diluted with physiological saline solution and examined microscopically (Zeiss, Oberkochen, Germany) at 400× magnification 30 and 60 days post-exposure (Gürtler et al. Reference Gürtler, Cecere, Lauricella, Cardinal, Kitron and Cohen2007). The numbers of exuviae and of dead bugs in each box were recorded as indices of XD quality. When a pool was positive for trypanosomes, all bugs were re-examined individually to assess the individual host's infectiousness to the vector (i.e. the proportion of infected bugs fed on a given individual relative to the total number of insects examined for infection at least once, excluding the insects that died prior to the first examination). Estimates of infectiousness included specimens positive by XD or by kDNA-PCR from GEB samples.
For parasite culture, the rectal contents from two microscope-positive bugs from each XD-positive animal were inoculated separately into culture tubes that contained a biphasic medium, and then incubated at 28 °C and a relative humidity of 50% (Cardinal et al. Reference Cardinal, Lauricella, Ceballos, Lanati, Marcet, Levin, Kitron, Gürtler and Schijman2008). Parasite growth was monitored weekly during 4 months until reaching 3 × 105 parasites mL−1, and the isolates were cryopreserved in liquid nitrogen.
Molecular diagnosis and genotyping
Total DNA was extracted from GEB blood samples boiled for 15 min using the DNeasy Blood & Tissue Kit (QIAGEN, Valencia, CA) according to manufacturer's instructions. Infections with T. cruzi were identified by PCR amplification of the 330-basepair (bp) fragment from the kDNA minicircles of T. cruzi (kDNA-PCR) using primers and cycling conditions described (Burgos et al. Reference Burgos, Begher, Freitas, Bisio, Duffy, Altcheh, Teijeiro, Lopez Alcoba, Deccarlini, Freilij, Levin, Levalle, Macedo and Schijman2005) with the incorporation of Taq Platinum® (Invitrogen, Carlsbad, CA, USA). Samples of 12 µL of PCR products were visualized under ultraviolet light after electrophoresis in 3% agarose gels containing GelRed® (Biotium, Inc., Hayward, CA). kDNA-PCR-positive hosts displayed the 330 bp band indicative of T. cruzi infection.
Samples from individual hosts that were XD-negative and kDNA-PCR-positive (in GEB samples) were subsequently tested by SAT-DNA-PCR, by sequence analyses of the 24S alpha ribosomal DNA (24Sα rDNA-PCR) or by kDNA-PCR of the rectal contents of XD bugs for confirmation of kDNA-PCR results. The rectal contents were diluted in 50 µL of deionized sterile water, boiled for 15 min., and DNA was extracted with DNAzol (Invitrogen) as described elsewhere (Cardinal et al. Reference Cardinal, Lauricella, Ceballos, Lanati, Marcet, Levin, Kitron, Gürtler and Schijman2008).
Trypanosoma cruzi DTUs were identified in culture samples of each infected host by PCR-based strategies targeting the intergenic region of spliced-leader genes (SL-IRac, SLIRII, SL-IRI) and 24S alpha ribosomal DNA, and A-10 genomic markers as described by Burgos et al. (Reference Burgos, Altcheh, Bisio, Duffy, Valadares, Seidenstein, Piccinali, Freitas, Levin, Macchi, Macedo, Freilij and Schijman2007). For confirmation of DTU and phylogenetic analysis, PCR amplification and sequencing of the partial sequences of CytB and H2B genes from two isolates obtained from two XD-positive D. rotundus was performed as described (Marcili et al. Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009a ). The obtained amplifications were sequenced in an external service (Macrogen Inc, Korea) and compared with available sequences stored at GenBank using BLAST (Basic Local Alignment Search Tool). For phylogenetic analyses, the sequences obtained in the present study were aligned with available sequences of T. cruzi reference strains of all DTUs, from Colombian bats (Ramírez et al. Reference Ramírez, Tapia-Calle, Muñoz-Cruz, Poveda, Rendón, Hincapié and Guhl2014) and Brazilian samples (Marcili et al. Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009a ; Cavazzana et al. Reference Cavazzana, Marcili, Lima, da Silva, Junqueira, Veludo, Viola, Campaner, Nunes, Paiva, Coura, Camargo and Teixeira2010; Lima et al. Reference Lima, Espinosa-Álvarez, Ortiz, Trejo-Varón, Carranza, Pinto, Serrano, Buck, Camargo and Teixeira2015) (Table S1 – Supplementary Material). Alignments were performed using Clustal W and then were manually refined. The phylogenies were inferred using the Kimura two-parameter model and maximum-likelihood methods using Mega 6·06. The bootstrap was acquired from 1000 replicate trees.
Experimental infection and histopathology
To assess the pathogenicity and parasite amplification capability of bat trypanosomes, four Balb C strain mice were inoculated intraperitoneally with 105 parasites from the XD-positive D. rotundus bat (Murci 159). Each mouse was subsequently tested by XD as described above. Mice were anaesthetized with Zelazol® (20 mg kg−1) 30 days post-inoculation and killed by cervical dislocation. Complete necropsies and histopathological analyses were performed. Blood samples were obtained by intracardiac puncture for haemoculture, and heart, spleen, liver and intestine were removed for histopathology. Tissues were fixed in 10% buffered formalin, dehydrated in absolute ethanol, cleared in xylene, and embedded in paraffin. Sections (5 µm) were stained with haematoxylin and eosin and examined by light microscopy. Tissue parasite burden was classified in four levels: with no parasite nests (−); one to three nests (+); 3–6 (++), and more than six nests (+++). Inflammation of the myocardium (myocarditis) was scored according to Sun and Tarleton (Reference Sun and Tarleton1993), and skeletal muscle inflammation was based on a modification of a previously described method (Ben Younés-Chennoufi et al. Reference Ben Younés-Chennoufi, Hontebeyrie-Joskowicz, Tricottet, Eisen, Reynes and Said1988). Liver damage was evaluated by the presence of hepatocellular degeneration, necrosis, inflammation and hyperplasia of Kupffer cells. Spleen abnormalities included reactive changes and/or follicular hyperplasia.
Search of sylvatic triatomines
Searches for sylvatic foci of triatomine bugs were conducted by using mouse-baited sticky traps (Noireau traps), dissection of potential bug ecotopes, and tracking of opossums during 2 weeks in April 2014 at the sites where T. cruzi-infected opossums had previously been collected in 2012 and 2013. Baited sticky traps were deployed in hollow tree trunks (live or dead), fallen logs, and in the entrance of burrows on late evenings. All sites were georeferenced and identified with a numbered piece of cloth as described elsewhere (Ceballos et al. Reference Ceballos, Piccinali, Berkunsky, Kitron and Gürtler2009). High tree holes were accessed by means of a telescopic ladder. Traps were left overnight and inspected early on the following morning.
Triatomine bug searches performed in association with opossum burrows and tree holes, seven Didelphis opossums were fitted with a spool-and-line tracking device at the site of capture (Miles et al. Reference Miles, Souza and Póvoa1981), released in the evening, and tracked during the next day. Animals could not be tracked more than one day because the spool-and-line device was frequently dropped off when the animals entered some refuges or the line was used up. The burrows or tree holes in which the tracked animals sought shelter were marked with a coloured tape and georeferenced for subsequent searches of triatomine bugs by direct inspection or by deploying Noireau traps inside them.
Data analysis
Estimates of prevalence of infection excluded the 55 opossum cubs because they were not yet exposed to vector-borne infection. Proportions and 95% confidence intervals (95% CI) were estimated using Wilson's formula implemented in Epitools (Sergeant, Reference Sergeant2015).
RESULTS
A total of 200 mammals from at least 18 species were captured, including 55 opossum cubs within the marsupial bag and 17 unidentified cricetid rodents (Table 1). The total catch effort was 1182 trap-nights with Tomahawk traps; 1479 trap-nights with Sherman traps and 92 mist net-hours. A total of 56 medium-sized mammals (all were D. albiventris) were captured with Tomahawk traps (4·7 per 100 trap-nights) (Table 2), eight of which were females carrying the 55 cubs. Additional catches included 19 micro-mammals (1·3 per 100 Sherman trap-nights), 39 bats (42 per 100 mist net-hours), 11 bats caught manually, ten mammals rescued at PEP, six road kills and four howler monkeys.
Table 1. Number of T. cruzi-infected wild mammals from Capital and Candelaria Departments, Misiones, Argentina, as determined by xenodiagnosis (XD) and kDNA-PCR
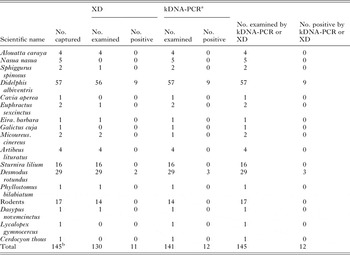
a Only includes kDNA-PCR-positive samples confirmed by other more specific methods.
b Total does not include 55 opossum cubs within the mother's marsupium.
Table 2. Catch per unit effort (CPUE) and prevalence of T. cruzi infection in D. albiventris over time in the rural and preserved areas

The composite prevalence of T. cruzi infection (as determined by XD or kDNA-PCR) over the 145 mammals examined was 8% (95% CI, 5–14%) (Table 1). The infected animals included nine (16%) D. albiventris opossums both kDNA-PCR- and XD-positive, and three (10%) D. rotundus, two of which were both kDNA-PCR and XD-positive and one was both kDNA-PCR- and SAT-DNA-PCR-positive. One (6%) Sturnira lilium bat (collected in the same site as the positive D. rotundus) that was positive by kDNA-PCR of blood sample and of the rectal contents of XD bugs, failed to be confirmed by SAT-DNA-PCR or by sequence analyses of 24Sα-rDNA-PCR from GEB and XD bug rectal contents; thus this specimen was considered doubtful (i.e. negative for T. cruzi). All 55 opossum cubs and their mothers tested XD negative.
Parasite isolation through culture was successful in all XD-positive individuals. Among those positive by XD or kDNA-PCR, on average opossums infected 62% (95% CI, 54–69%) of nymphs whereas D. rotundus infected 50% (95% CI, 25–75%). Of 1605 bugs used in XD, 90% were alive on first examination and 10% of them moulted within the 60-day observation period. The S. lilium bat that was negative both by XD and SAT-DNA-PCR had two of five xenodiagnostic bugs positive by kDNA-PCR of rectal contents.
Didelphis opossums were found infected in CSJ (7), SIR (1) and CTY (1). The observed prevalence rates of infection in Didelphis from the rural area (12%; 95% CI, 3–35%) and the preserved area (18%; 95% CI, 9–32%) were not significantly different (χ 2 test, d.f. 1, P = 0·58) (Table 2). The catch per unit effort (CPUE, per 100 trap-nights) of opossums was significantly greater in the preserved area (7·1; 95% CI, 5·2–9·5) than in the rural area (2·6; 95% CI, 1·6–4·2) (χ 2 test, d.f. 1, P = 0·0003) (Table 2). Similarly, rodents were slightly more abundant in the preserved area (1·6; 95% CI, 0·08–3·1) than in the rural area (1·1; 95% CI, 0·06–2·0).
All of the infected opossums were captured in 2012 and 2013 in the rural and in the preserved area, respectively, whereas the 12 opossums examined in 2014 (all from the preserved area, CSJ) were negative by XD and kDNA-PCR (Table 2). The infection prevalence in CSJ dropped from 25% (95% CI, 13–43%) in 2013 to 0% in 2014 despite catches were performed in same season and CPUE remained at similar levels (7·2 vs 6·6 per 100 trap-nights, respectively) (Table 2). Trypanosoma cruzi infection in D. albiventris steadily increased with developmental stage (Fig. 2).
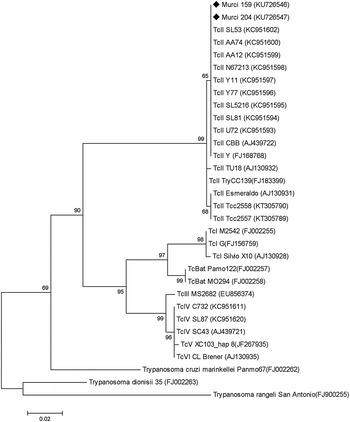
Fig. 2. Phylogenetic tree based on partial CytB gene sequences isolated from bats in this study and those obtained from Ramírez et al. (Reference Ramírez, Tapia-Calle, Muñoz-Cruz, Poveda, Rendón, Hincapié and Guhl2014) and Marcili et al. (Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009a , Reference Marcili, Valente, Valente, Junqueira, Da Silva, Pinto, Naiff, Campaner, Coura, Camargo, Miles and Teixeira b ) with their corresponding accession numbers at GenBank (in parenthesis). The phylogenies were inferred using the Kimura two-parameter model and maximum-likelihood methods. Numbers at nodes indicates bootstrap values derived from 1000 replicates. ■ Sequences obtained from this study.
A total of 50 bats from four species were captured (Table 1), 21 in the rural area and 29 in the preserved area. Vampire bats (D. rotundus) were most frequent (58%), followed by the frugivorous little yellow-shouldered bats (S. lilium) (32%), great fruit-eating bats (Artibeus lituratus) (8%), and Ipanema bats (Pygoderma bilabiatum) (2%). Four bats were kDNA-PCR-positive for T. cruzi, only three confirmed by SAT-DNA-PCR (Table 1). The three confirmed T. cruzi-infected vampire bats and the doubtful S. lilium were from the preserved area: three were captured by mist netting and one from the colony in the vacant dwelling. The prevalence of infection of vampire bats in CSJ was 21% (3/14; 95% CI, 8–48%) by kDNA-PCR and 14% (2/14; 95% CI, 4–40%) by XD.
TcI was identified in all infected D. albiventris, and TcII in both XD-positive specimens of D. rotundus (Table 2). For both CytB and H2B genes, the identity with TcII reference strains Esmeraldo and CBB was >98% (GenBank accession numbers are Murci159 CytB KU726546 and H2B KU726548, Murci204 CytB KU726547and H2B KU726549). We were not able to distinguish between TcII, TcV or TcVI in the XD-negative D. rotundus bat that was positive both by kDNA-and SAT-DNA-PCR. The phylogenetic analysis of CytB performed confirmed the two isolates were TCII and clustered together with Brazilian and Colombian bat strains (Fig. 3).

Fig. 3. Frequency of D. albiventris opossums captured and prevalence of T. cruzi infection according to stage of opossums and date of survey.
Heart and skeletal muscle of mice inoculated with the vampire parasite strain displayed mild to intense myocarditis and/or myositis with scattered amastigote nests (Fig. 4C and D). Calcification foci were observed in skeletal muscle. Liver and spleen exhibited non-specific reactive changes (Fig. 4A and B). Spleen microarchitecture showed reactive changes with enlargement of the lymphoid follicles and the liver suffered mild hepatitis with hyperplasia of Kupffer cells. Intestine did not present relevant findings.

Fig. 4. Histopathology of the liver (A), spleen (B), heart (C) and skeletal muscle (D) of balb-C mice inoculated with T. cruzi parasites isolated from a XD-positive D. rotundus; (A) Liver showing hyperplasia of Kupffer cell lining sinusoids discreetly dilated (black arrow). A binucleated liver cell can be observed in the bottom left corner (scale bar 400 µm); (B) Spleen with folicular hyperplasia. Microarchitecture of the spleen with lymphoid follicles separated by rows of mature lymphocytes. Note on the left middle and at the top right side of the picture, in the centrofolicular area, numerous dendritic cells giving a starry sky image (scale bar 100 µm); (C) Heart section displaying an intense mononuclear inflammation. Black arrows point foci of inflammation with nuclear debris and amastigotes, oedema and rupture of cardiac muscle cells (scale bar 200 µm). Insert shows parasite nest scale bar 400 µm); (D) Neurovascular bundle of skeletal muscle with development of young collagen (arrow) around a vascular structure with isolated mononuclear cells and oedema (scale bar 200 µm). Insert shows foci of mononuclear inflammation (scale bar 200 µm).
Searches for triatomines in SIR and CSJ included a total of 270 Noireau trap-nights, 45 ecotopes dissected, and seven opossums tracked. No triatomine was captured during these surveys.
DISCUSSION
Our results document the first finding of TcII in two D. rotundus vampire bats from Northeastern Argentina. In addition, we initially recorded a possible T. cruzi infection in one S. lilium bat captured in (peri)domestic habitats of the preserved area (as determined by kDNA-PCR of blood samples and from XD bug feces), which failed to be confirmed via the less sensitive SAT-DNA-PCR or 24Sα-rDNA-PCR.
Bats have been found infected with all T. cruzi DTUs (Marcili et al. Reference Marcili, Lima, Cavazzana, Junqueira, Veludo, Maia Da Silva, Campaner, Paiva, Nunes and Teixeira2009a ; Cavazzana et al. Reference Cavazzana, Marcili, Lima, da Silva, Junqueira, Veludo, Viola, Campaner, Nunes, Paiva, Coura, Camargo and Teixeira2010; García et al. Reference García, Ortiz, Osorio, Torrico, Torrico and Solari2012; Ramírez et al. Reference Ramírez, Tapia-Calle, Muñoz-Cruz, Poveda, Rendón, Hincapié and Guhl2014; Lima et al. Reference Lima, Espinosa-Álvarez, Ortiz, Trejo-Varón, Carranza, Pinto, Serrano, Buck, Camargo and Teixeira2015). TcII has been detected in various bat species from Brazil including the Atlantic Forest and Cerrado biomes (Lisboa et al. Reference Lisboa, Pinho, Herrera, Gerhardt, Cupolillo and Jansen2008; Lima et al. Reference Lima, Espinosa-Álvarez, Ortiz, Trejo-Varón, Carranza, Pinto, Serrano, Buck, Camargo and Teixeira2015), remnants of which reach our study area. In addition, TcII was identified in bats from Colombia, but not in D. rotundus vampire bats which were infected with TcI and TcIV (Ramírez et al. Reference Ramírez, Tapia-Calle, Muñoz-Cruz, Poveda, Rendón, Hincapié and Guhl2014).
In South America TcII occurs both in sylvatic habitats (Jansen et al. Reference Jansen, Xavier and Roque2015) and in domestic transmission cycles from central and Southeastern Brazil and Colombia, and in the Gran Chaco region (Brisse et al. Reference Brisse, Barnabé and Tibayrenc2000; Cura et al. Reference Cura, Lucero, Bisio, Oshiro, Formichelli, Burgos, Lejona, Brusés, Hernández, Severini, Velazquez, Duffy, Anchart, Lattes, Altcheh, Freilij, Diez, Nagel, Vigliano, Favaloro, Favaloro, Merino, Sosa-Estani and Schijman2012; Zingales et al. Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012). In Brazil, besides the bats mentioned above, TcII was isolated in all biomes, including dogs residing in an area with residual peridomestic infestations adjacent to a conservation unit where the local wild mammals were infected with TcI (Rocha et al. Reference Rocha, Roque, Arrais, Santos, Lima, Xavier, Cordeir-Estrela, D'Andrea and Jansen2012); coatis (Nasua nasua) from the Brazilian “cerrado” (Rocha et al. Reference Rocha, Roque, de Lima, Cheida, Lemos, de Azevedo, Arrais, Bilac, Herrera, Mourão and Jansen2013), Leontopithecus rosalia and Leontopithecus chrysomelas monkeys and opossums from the Atlantic forest (Jansen et al. Reference Jansen, Xavier and Roque2015; Lisboa et al. Reference Lisboa, Monteiro, Martins, Xavier, Lima and Jansen2015), in six-banded armadillos (Euphractus sexcinctus) (Yeo et al. Reference Yeo, Acosta, Llewellyn, Sánchez, Adamson, Miles, López, González, Patterson, Gaunt, Rojas De Arias and Miles2005), and in dogs and bats from the Amazon basin where no domestic transmission cycle occurs (Jansen et al. Reference Jansen, Xavier and Roque2015; Lima et al. Reference Lima, das C. Xavier, Maldonado, Roque, Vicente and Jansen2014, Reference Lima, Espinosa-Álvarez, Ortiz, Trejo-Varón, Carranza, Pinto, Serrano, Buck, Camargo and Teixeira2015). Our phylogenetic analyses of CytB sequences showed that the isolates clearly fitted into the TcII group. Further studies are required to elucidate whether the weakly supported phylogenetic relationships with Colombian bats strains inferred by this method, are congruent or not. The TcII-infected D. rotundus bats (including one with an undetermined DTU) and the dubious S. lilium were captured indoors and in the yard of a vacant dwelling from an old ranch which had been transformed into a protected area 5 years prior to our survey. The closest inhabited human dwelling at the time of our study was 5 km away. Given the observed association of TcII with domestic and sylvatic transmission cycles in other regions, combined with the extended lifespan (>10 years) and broad flight range (up to 10 km) of D. rotundus (Greenhall et al. Reference Greenhall, Joermann and Schmidt1983), the putative origins of TcII infections and transmission pathways in the study bats are several: the infected specimens could be part of a residual domestic transmission cycle, a domestic spillover in the past, or part of an undetected local sylvatic cycle of TcII involving bats only or other mammal species, as suggested by the increasing association of TcII with sylvatic transmission cycles in Amazonia. Bats may become infected by vertical transmission as in Molossus molossus (Añez et al. Reference Añez, Crisante and Soriano2009), or by horizontal transmission when grooming (Thomas et al. Reference Thomas, Rasweiler and D'Alessandro2007). The original source of parasites is also uncertain and may range from a domestic to a wild host.
Failure to confirm the dubious T. cruzi infection in S. lilium could be attributed to a lack of sensitivity (scarce T. cruzi DNA), or that the kDNA-PCR-positive outcome is detecting other bat trypanosomes (e.g. Trypanosoma vespertilionis and Trypanosoma dionisi). Our attempts to amplify the 24Sα rDNA fragment for further confirmation (Schijman et al. Reference Schijman, Lauricella, Marcet, Duffy, Cardinal, Bisio, Levin, Kitron and Gürtler2006) were unsuccessful. Given that both markers differ in the number of copies per genome, 24Sα rDNA-PCR is less sensitive than the kDNA-PCR, thus no conclusive identification of the trypanosome species involved could be achieved.
Although we did not detect triatomines or their signs in or around the vacant dwelling with the infected bats and in wild habitats, several species of Triatominae occur in Southern Misiones, including Triatoma sordida, Triatoma melanosoma (syn T. infestans), Triatoma rubrovaria and Panstrongylus megistus (Abalos et al. Reference Abalos, de Mischis and Kufner1980; Carcavallo et al. Reference Carcavallo, Girón, Jurberg and Lent1999). Of special interest is P. megistus, which usually has wild colonies and frequently invades domestic premises in Southern Brazil (Litvoc et al. Reference Litvoc, Goldbaum and da. Silva1990). Wild colonies of P. megistus were found in refuges of opossums, monkeys, rodents, birds and bats (Lisboa et al. Reference Lisboa, Mangia, Rubião, De Lima, Das Chagas Xavier, Picinatti, Ferreira, Fernandes and Jansen2004), and in bromeliads, palm trees and hollow trees in the neighbouring Rio Grande do Sul State (Santos et al. Reference Santos, Viola, Lorosa, Machado, Ruas Neto and Corseui2013). Panstrongylus megistus has been occasionally found in domestic premises in Northeastern Argentina (Damborsky et al. Reference Damborsky, Bar and Oscherov2001).
The haematophagous habits of D. rotundus suggest the possibility of an oral route of infection when the bat blood-feeds on a T. cruzi-infected mammal. Potential domestic mammalian hosts within the bats’ broad home range within the study area included dogs, cats, cattle, equines and sheep. Among these, dogs and cats are well-known reservoir hosts of T. cruzi throughout the Americas and are more infectious than domestic ungulates (Gürtler and Cardinal, Reference Gürtler and Cardinal2015). In addition to the wild mammals we captured, the diverse suite of species inhabiting the gallery forest and grasslands include cebus monkeys (Sapajus caí), crab-eating raccoons (Procyon cancrivorus), lesser grisons (Galictis cuja), tayra (Eira barbara), margay cats (Leopardus wiedii), maned wolves (Chrysocyon brachyurus), and southern tamandua (Tamandua tetradactyla) (H. Argibay, unpublished observations). Some of these species are competent reservoir hosts of T. cruzi (Noireau et al. Reference Noireau, Diosque and Jansen2009) and a potential source of infection for vampire bats.
The infected D. rotundus bats were highly infectious to the vector (50%), and the T. cruzi isolate was pathogenic to mice and was further passed on to the vector. The histopathological findings (miocarditis and myositis with scattered amastigote nests) are consistent with an acute infection by T. cruzi (Sun and Tarleton, Reference Sun and Tarleton1993). These results are relevant because the behaviour of T. cruzi strains in bats has rarely been investigated, and because some strains are known to be highly virulent, whereas others are weakly or non-pathogenic in experimentally inoculated mice (Martínez-Díaz et al. Reference Martínez-Díaz, Escario, Nogal-Ruiz and Gómez-Barrio2001; Roellig and Yabsley, Reference Roellig and Yabsley2010). Whether vampire bats are able to transmit T. cruzi when they blood-feed on susceptible mammals is unknown. Successful transmission occurred in Trypanosoma evansi, in which D. rotundus acted both as a reservoir host and a vector (Desquesnes et al. Reference Desquesnes, Holzmuller, Lai, Dargantes, Lun and Jittaplapong2013). Because T. cruzi has been detected in the saliva and salivary glands of experimentally infected dogs and mice during the acute phase (Marsden and Hagstrom, Reference Marsden and Hagstrom1966; Lopes et al. Reference Lopes, Ribeiro, Carvalho, de Albuquerque and Watanabe1991), the probability of transmission from a T. cruzi-infected vampire bat to a susceptible mammal may not be excluded.
The finding of TcI-infected white-eared opossums was expected given previous findings within the same region (Bar et al. Reference Bar, Mabel Alvarez, Oscherov, Damborsky and Jörg1999; Vaz et al. Reference Vaz, D'Andrea and Jansen2007), and because Didelphis opossums are the most widely distributed sylvatic reservoir host of TcI throughout the Americas (Noireau et al. Reference Noireau, Diosque and Jansen2009; Marcili et al. Reference Marcili, Valente, Valente, Junqueira, Da Silva, Pinto, Naiff, Campaner, Coura, Camargo, Miles and Teixeira2009b ; Zingales et al. Reference Zingales, Miles, Campbell, Tibayrenc, Macedo, Teixeira, Schijman, Llewellyn, Lages-Silva, Machado, Andrade and Sturm2012; Orozco et al. Reference Orozco, Enriquez, Alvarado-Otegui, Cardinal, Schijman, Kitron and Gürtler2013). The prevalence of T. cruzi infection in the opossums (16%) was lower than elsewhere in the dry Chaco and surrounding areas (Bar et al. Reference Bar, Mabel Alvarez, Oscherov, Damborsky and Jörg1999; Yeo et al. Reference Yeo, Acosta, Llewellyn, Sánchez, Adamson, Miles, López, González, Patterson, Gaunt, Rojas De Arias and Miles2005; Vaz et al. Reference Vaz, D'Andrea and Jansen2007; Alvarado-Otegui et al. Reference Alvarado-Otegui, Ceballos, Orozco, Enriquez, Cardinal, Cura, Schijman, Kitron and Gürtler2012; Orozco et al. Reference Orozco, Enriquez, Alvarado-Otegui, Cardinal, Schijman, Kitron and Gürtler2013), whereas their mean infectiousness to the vector (62%) was similarly high (Orozco et al. Reference Orozco, Enriquez, Alvarado-Otegui, Cardinal, Schijman, Kitron and Gürtler2013). The infected opossums occurred both in the rural and preserved areas, and eventually may act as a bridge between (peri)domestic and sylvatic cycles. The drastic drop of opossum infection prevalence in 2014 reveals the large fluctuations in the sylvatic transmission of T. cruzi even in a protected area not subject to anthropic disturbance. Such large variations may be overlooked by cross-sectional surveys with limited sample sizes.
Our results may have public health relevance. We found two different transmission cycles of T. cruzi involving mammalian host species that occupy preserved areas and (peri)domestic habitats. Didelphis opossums were infected in both areas, whereas vampire bats captured indoors or in the vicinities of a vacant house harboured a T. cruzi DTU found both in domestic and sylvatic habitats. The finding of vampire bats infected with a genotype of T. cruzi identified in other domestic and sylvatic reservoir hosts elsewhere, in a site with no permanent human residents considered to be free of domestic vector-borne transmission, deserves further research efforts.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0031182016000925.
ACKNOWLEDGEMENTS
We are grateful two anonymous reviewers and Alejandro Schijman for their comments that improved this manuscript, to Gustavo Enriquez, María del Pilar Fernández, Lucía Rodríguez Planes, Flavia Netto, Ana Carbajal de la Fuente, Tomás Franzese, Esteban Actis, Yanina Berra, Juan Pablo Zurano, José Ferreyra, Héctor Zeballos, Patricia Sandoval and Ernesto Centurión for valuable field and laboratory assistance; Romina Piccinalli for advice on sequencing and sequence interpretation; Natalia Macchiaverna for support on parasite isolation and phylogenetic analyses; Jacqueline Bua for performing mice inoculation, and Santa Ines Ranch and Tuyuyu owners, Entidad Binacional Yaciretá, and the Ministry of Ecology and Natural Resources of Misiones.
FINANCIAL SUPPORT
This study was supported by awards from Roemmers Foundation to H.A., Universidad de Buenos Aires (UBACYT 2011-2014), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2012-2015), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2011-2072, PICTO-Glaxo 2011-0062) to R.E.G. M.M.O., M.V.C. and R.E.G. are members of CONICET Researcher's Career.









