INTRODUCTION
Reproductive cycles of temperate echinoid species are typically annual or semi-annual (Lawrence, Reference Lawrence1987; Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991) and gametogenesis is considered to be influenced by factors such as seasonal changes in photoperiod (Pearse et al., Reference Pearse, Pearse and Davis1986; Bay-Schmith & Pearse, Reference Bay-Schmith and Pearse1987), water temperature (Yamamoto et al., Reference Yamamoto, Ishine and Yoshida1988; Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991) and nutrition (Lawrence, Reference Lawrence1987; Lamare et al., Reference Lamare, Brewin, Barker and Wing2002).
The green sea urchin Arbacia dufresnii (Blainville, 1825) is one of the most abundant echinoids along the Argentinean coast. It is distributed around South America at depths of 0–315 m from the Rio de la Plata (35°S) in the Atlantic Ocean to Puerto Montt (41°S) in the Pacific and is also found on islands of the South Atlantic Ocean (Bernasconi, Reference Bernasconi1953). In Golfo Nuevo, Patagonia, individuals of this species live on mixed gravel and sandy bottoms and amongst Macrocystis blades and holdfasts, where they live with another sea urchin, Pseudechinus magellanicus (Philippi, 1857) (Bigatti et al., Reference Bigatti, Marzinelli, Cledón, Penchaszadeh, Heinzeller and Nebelsick2004; Marzinelli et al., Reference Marzinelli, Bigatti, Gimenez and Penchaszadeh2006). In this location the sea surface temperature ranges from 9°C to 18°C (Esteves & De Vido, Reference Esteves and De Vido1980) and day length varies from 9 to 16 hours.
Arbacia dufresnii is primarily carnivorous and the prey varies depending on the habitat (Penchaszadeh & Lawrence, Reference Penchaszadeh, Lawrence, M.D. Candia and Bonasoro1999; Penchaszadeh et al., Reference Penchaszadeh, Bigatti and Miloslavich2004). Only a short report with preliminary data on the reproductive cycle of this sea urchin has been published so far (Brogger et al., Reference Brogger, Martinez, Penchaszadeh and Lawrence2004).
The purpose of the present study is to determine the reproductive pattern of A. dufresnii in Golfo Nuevo, Argentina. Histological examination of gonad development, gonad index, sex-ratio and the relationship between environmental factors and gametogenesis are examined.
MATERIALS AND METHODS
Samples of Arbacia dufresnii were collected monthly by SCUBA diving at depths between 5 and 10 m off Puerto Madryn, Golfo Nuevo (42°46′S 65°02′W) from September 2000 to March 2003. Specimens were fixed in Bouin's solution over two days and then preserved in 70% ethanol. The test diameter and test height of the sea urchins was measured with a Vernier caliper. Only full adult-sized sea urchins and thus mature individuals (N = 20 per sample; test diameter > 16 mm) were used for the reproductive cycle study. Total body wet weight and gonad wet weight were measured with a Mettler precision balance (0.0001 g) to calculate gonad index (GI = (gonad wet weight/total wet weight) × 100) (Grant & Tyler, Reference Grant and Tyler1983; Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). Sex discrimination was done by gonad colour and corroborated through gonad squash at the microscope.
To corroborate gonad index reliability, the gonads of sea urchins sampled from September 2001 to March 2003 (N = 95; 5 per month) were embedded in paraplast, sectioned at 8 µm and stained with haematoxylin and eosin. Sections were examined microscopically and stages of maturity for males and females were recognized. The diameter of 30 mature oocytes/ova with distinct nucleolus found in sectioned ovaries was measured. Additionally, from a supplementary sample of 20 individuals collected during summer 2003, fresh eggs' diameter (N = 30) of each sea urchin were measured under optical microscope. Spawning was induced by injecting each individual with 0.5 ml of KCl 0.5M. For size measurements, eggs were viewed in a suspension of Sumi ink. Images were taken with a Zeiss Axiostar microscope equipped with a Sound Vision 2.0 digital camera.
Water temperatures corresponded to the monthly mean value obtained from the daily measurement of the sea surface temperature at Golfo Nuevo by the Navy Coastal Ocean Model–Naval Research Laboratory (NCOM), of the US Navy. The photoperiod was obtained from the US Navy Observatory.
Statistical analysis
A Chi-squared test was carried out to study the male to female sex-ratio. Gonad index (GI) variations for each sex were examined using Kruskal–Wallis analysis followed by Dunn contrasts between months. A Pearson correlation analysis between GI for males and females was carried out to determine if there was a synchronism between their gametogenesis. Correlation between GI and photoperiod and surface water temperature was carried out in order to study if these environmental factors were influencing the gametogenesis. All statistical analysis was done using the STATISTICA 6 statistical software package.
RESULTS
A total of 658 sea urchins were examined and used for the reproduction study. Test diameter size-range was 16.2–44.3 mm, with a mean test diameter of 30.7 mm (SD = 4.9 mm).
Sex-ratio and gonad index
The male to female ratio in the population studied was 1.26:1, which differed significantly from 1:1 (χ2 = 8.549; P < 0.05). There were no months when gonad colour and examination under the microscope showed lack of gametes which would have made it difficult to sex individuals.
Both the male and female GI varied through the studied period (males: Kruskal–Wallis H = 247.1263; P < 0.05; and females: Kruskal–Wallis H = 168.7414; P < 0.05) and those variations were highly correlated with each other (r = 0.93; N = 31; P < 0.05). No statistical differences were found between the male and female GI (Kruskal–Wallis H = 0.0306; P = 0.8612), so gonadal relative size was studied for both sexes jointly. The combined GI showed a significant variation for each month (Kruskal–Wallis H = 407.2511; P < 0.05; Figure 1). The GI data indicate that in Golfo Nuevo Arbacia dufresnii runs through a pre-spawning peak during the austral spring months (November 2000, October 2001 and October 2002) with a post-spawning minimum in the summer months (December 2000, December 2001 and December 2002). A second and more notorious pre-spawning peak was observed during summer (February 2001, January 2002 and January 2003) with a post-spawning minimum in the autumn and winter months. Significant differences were observed between pre-spawning and post-spawning peak months (P < 0.05 Dunn).
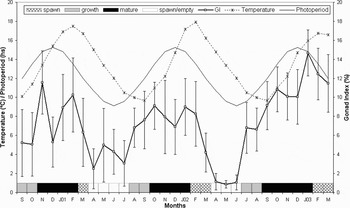
Fig. 1. Arbacia dufresnii. Changes (means±SE) in gonad index for pooled males and females (N = 20; test diameter > 16 mm) collected between September 2000 and March 2003 at Golfo Nuevo. Surface water temperature from Golfo Nuevo taken from Navy Coastal Ocean Model (Naval Research Laboratory, US Navy). Photoperiod obtained from the US Navy Observatory. The bars show the predominant stage at each month, resulting from the histological examination.
Statistical analyses showed a positive correlation between GI and photoperiod (r = 0.67; N = 31; P < 0.05; Figure 1). No correlation was found between GI and water temperature (r = 0.23; N = 31; P > 0.05; Figure 1).
Fresh mean egg diameter had a value of 87.1 µm (SD = 1.3 µm) with a jelly coat of 12.7 µm (SD = 0.9 µm). Total mean egg diameter was 112.5 µm (SD = 1.9 µm).
Gonad histology
Typical sea urchin gonad stages were observed and photographed during the studied time-period. Gonad histology showed two spawning events, one during spring and another in summer. The presence of mature gonads was synchronous among males and females during the spring and the summer pre-spawning stages. In autumn the gonads were almost completely on the resorption stage, with nutritive phagocytes (Figure 2A&E) and only few relict gametes were present. After that, during winter, gamete growth and proliferation began (Figure 2B,C&F) and continued until the first pre-spawning phase, when the presence of fully mature gonads was observed (Figure 2D&G). Spawning periods were evidenced by a decrease in the amount of mature gametes in the gonads, with free spaces appearing between the gametes (Figure 2H). Proliferation and differentiation of gametes, and the presence of mature gonads were synchronous among males and females. Through the studied time these different stages were repeated in a cycle, only observing a monthly displacement of the predominant stage for a few months (Figure 1). Observed at the histological sections, mature oocytes mean diameter was 67.7 µm (SD = 7.6 µm) with a mean jelly coat of 2.8 µm (SD = 0.4 µm).

Fig. 2. Arbacia dufresnii. (A–D) Histological sections of testes: (A) post-spawn resorption; (B) proliferation and growth; (C) partially mature; (D) mature. (E–H) Histological sections of ovaries: (E) post-spawn resorption; (F) proliferation and growth; (G) mature; (H) partially spawned. Spz, spermatozoa; Spc, spermatocytes; L, lumen; Ph, nutritive phagocytes; Ov, ovum; Oo, oocytes. Scale bars: A, B, E, F, 50 µm; C, D, G, H, 100 µm.
DISCUSSION
Arbacia dufresnii from Golfo Nuevo develops an annual reproductive cycle with two spawning events, one in spring and another one in summer, with the first being a partial spawning event. For each month the reproductive state observed from histological analysis was reflecting the gonad index analysis. Spawning events in summer were reported for Arbacia lixula (Linnaeus, 1758) at the coast of France in the Mediterranean Sea (Harvey, Reference Harvey1956). In the Pacific Ocean, Arbacia spatuligera (Valenciennes, 1846) spawn during a short period of time in spring (Bay-Schmith, Reference Bay-Schmith1981). A spring and summer spawning pattern was also observed for Lytechinus variegatus (Lamarck, 1816) in three habitats in Saint Joseph Bay, Florida (Beddingfield & McClintock, Reference Beddingfield and McClintock2000). In a recent study on Pseudechinus magellanicus (Marzinelli et al., Reference Marzinelli, Bigatti, Gimenez and Penchaszadeh2006) conducted at the same location and time as the present study, an annual reproductive cycle with two spawns was observed: one major spawn in winter and a partial, shorter spawn in summer. Such seasonal differences between A. dufresnii and P. magellanicus may be a result of an asynchronal reproductive nest, which implies a minor competition of the food resources at the larval stages.
The sex-ratio observed for the population of A. dufresnii studied was statistically different from 1:1. This difference was observed during four of the 31 months studied. A 1:1 proportion between males and females has been common in most sea urchins (Lamare et al., Reference Lamare, Brewin, Barker and Wing2002) including Arbacia punctulata (Lamarck, 1816) and A. lixula (Harvey, Reference Harvey1956). Some other authors have observed significant differences in the sex proportion, sometimes between different populations, and have proposed that the differences observed may be a reflection of the ambient conditions that influence sex determination (Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991).
Mature oocytes mean diameter measured from histological sections was smaller than that registered for fresh eggs. This difference is not only due to a shrink on the histological treatment, but also because of the difficulty of knowing whether one is measuring the largest diameter even when the nucleolus is evident. According to Tyler (Reference Tyler1986) egg size is indicative of the kind of larval development. Small eggs (mean diameter 100 µm) correspond to a planktotrophic development. Whether measured from fresh or from histological sections, eggs of A. dufresnii are around 100 µm, so corresponded to this type of development.
No significant difference was observed between gametogenic phases of males and females. This synchrony could be affected by ambient factors that regulate the reproductive cycle (Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). In the case of A. dufresnii in Golfo Nuevo, the principal factor that could be related to the reproductive cycle is the photoperiod. Pearse et al. (Reference Pearse, Pearse and Davis1986) found a positive control of the photoperiod for the gametogenesis in Strongylocentrotus purpuratus (Stimpson, 1857). Walker & Lesser (Reference Walker and Lesser1998) found the same for S. droebachiensis (Müller, 1776). At Golfo Nuevo Marzinelli et al. (Reference Marzinelli, Bigatti, Gimenez and Penchaszadeh2006) found a negative correlation between the reproduction and the photoperiod for P. magellanicus, which could explain the difference in the spawning time observed for this species and A. dufresnii.
The intake of food allows the production of gametes in adults and growth of juveniles and larvae, and is particularly important in determining and maintaining the time of the reproductive period (Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). Inter-annual variation of the observed values of the gonadal index of A. dufresnii could be caused by the seasonal difference of the availability of food for adults in the population.
Some authors propose that temperature, photoperiod, and phytoplankton blooms are not the only ambient factors that affect the reproduction of sea urchins: others are tides, salinity, turbulence, lunar phases and access to and abundance of food (Lawrence, Reference Lawrence1987; Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). In the Golfo Nuevo zone, salinity is constant throughout the year (Pastor & Bala, Reference Pastor and Bala1995) and could be not a significant influence on the reproduction of A. dufresnii.
In the zone of the Norpatagonic gulfs phytoplankton blooms occur during the spring and summer months (Esteves et al., Reference Esteves, De Vido, Cejas and Frontali1981, Reference Esteves, Santinelli, Sastre, Diaz and Rivas1992). This is coincident with reproductive events of A. dufresnii, so reproduction could be related to food resources and larvae development. Some authors have found a significant relation between the concentration of chlorophyll-a and spawning events for different sea urchins (Himmelman, Reference Himmelman1975; Pearse & Cameron, Reference Pearse, Cameron, Giese, Pearse and Pearse1991). In aquaria experiments Himmelman (Reference Himmelman1975, Reference Himmelman1978) found chemical substances associated with the phytoplankton blooms in spring that could stimulate spawning on S. droebachiensis.
In conclusion Arbacia dufresnii, from the Golfo Nuevo's population, displays an annual reproductive cycle with a spring and summer spawning pattern related to seasonal changes in day length. This information extends the data base about reproductive cycles of temperate echinoid species and especially from a poorly studied species in an understudied region.
ACKNOWLEDGEMENTS
Special thanks to Victoria Zavattieri, Eugenia Zavattieri and Oscar Wheeler for taking samples and to Jennifer Antonides for reading and correcting an earlier version of the manuscript. Two anonymous referees are acknowledged for their very helpful suggestions that improved this paper. This work was partially supported by PIP 2788, UBACyT X171 and PICT-2007 1869.




