Introduction
The evolution and stability of resistance to insecticides or parasites is believed to be strongly influenced by the development of concurrent fitness costs (McKenzie, Reference McKenzie1996; Cotter et al., Reference Cotter, Kruuk and Wilson2004). Trade-offs between costs and advantages of insecticide resistance may explain why so few insects develop resistance to the widely-used microbial insecticide Bacillus thuringiensis (Bt) in the field, and why most systems show a decrease in resistance when insecticide use is discontinued (Ferré & van Rie, Reference Ferré and van Rie2002). Bacillus thuringiensis, a soil bacterium that produces crystals that are highly toxic to some insects, is an effective biopesticide that is used worldwide. Bt has minimal impact on the environment and human health and does not disrupt natural enemies such as parasitoids (reviewed by Tabashnik, Reference Tabashnik1994). The frequency of use of Bt toxins has dramatically increased with the development of genetically modified crops expressing the genes responsible for toxin production (Gould, Reference Gould1998).
Bacillus thuringiensis has been extensively used for over 30 years, and for some time it was thought that resistance would not develop in targeted pests (Tabashnik, Reference Tabashnik1994). Despite their slow development of resistance, low levels of resistance were first found in the lepidopteran Plodia interpunctella in stored grain by McGaughey (Reference McGaughey1985). Since then, resistance to Bt has been selected for under laboratory conditions in several species of Lepidoptera, Diptera and Coleoptera (Tabashnik, Reference Tabashnik1994; Ferré & van Rie, Reference Ferré and van Rie2002). In the field, resistance has been rare and; so far, even with widespread use of Bt, only three insects have been demonstrated to have developed resistance under field conditions, Plutella xylostella (Tabashnik, Reference Tabashnik1994), Trichoplusia ni (Janmaat & Myers, Reference Janmaat and Myers2003) and Helicoverpa armigera (Gunning et al., Reference Gunning, Dang, Kemp, Nicholson and Moores2005). Resistance has various associated costs that vary among different species and have received considerable attention in recent years. These include lower survival (Groeters et al., Reference Groeters, Tabashnik, Finson and Johnson1994; Tabashnik et al., Reference Tabashnik, Finson, Groeters, Moar, Johnson, Luo and Adang1994; Oppert et al., Reference Oppert, Hammel, Thorne and Krammer2000; Carrière et al., Reference Carrière, Ellers-Kirk, Liu, Sims, Patin, Dennehy and Tabashnik2001a), lower pupal weight or fecundity (Groeters et al., Reference Groeters, Tabashnik, Finson and Johnson1994; Sayyed & Wright, Reference Sayyed and Wright2001; Janmaat & Myers, Reference Janmaat and Myers2003), lower mating success (Groeters et al., Reference Groeters, Tabashnik, Finson and Johnson1993) and slower development time (Liu et al., Reference Liu, Tabashnik, Denneby, Patin and Bartlett1999; Oppert et al., Reference Oppert, Hammel, Thorne and Krammer2000; Akhurst et al., Reference Akhurst, James, Bird and Beard2003; Higginson et al., Reference Higginson, Morin, Nyober, Biggs, Tabashnik and Carrière2005). In some species, however, no difference could be detected between susceptible and resistant individuals (Gould & Anderson, Reference Gould and Anderson1991; Ramachandran et al., Reference Ramachandran, Buntin, All, Tabashnik, Raymer, Adang, Pulliam and Steward1998).
Consequences of resistance to insecticides are not always apparent, and some are manifested only in environments where intra-specific competition is strong. For example, lower survival resulting from resistance to an organophosphate insecticide in Culex pipiens is especially apparent under conditions of crowding (Bourguet et al., Reference Bourguet, Guillemaud, Chevillon and Raymond2004). In some cases, reduced mating success of resistant males can only be detected in competitive environments (Higginson et al., Reference Higginson, Morin, Nyober, Biggs, Tabashnik and Carrière2005). Diet can also exacerbate resistance effect, resistant T. ni feeding on lower quality food plants had lower fitness (Janmaat & Myers, Reference Janmaat and Myers2005). Adverse abiotic conditions can also increase the impact of resistance. Winter represents a stressful period for many insects and the overwintering period can affect the frequency of resistant alleles in a population (McKenzie, Reference McKenzie1996). Despite the potential relevance of overwintering conditions to the evolution of resistance, few studies have considered the impact of winter conditions on resistant insects. McKenzie (Reference McKenzie1994) showed that mortality was higher for the diazinon resistant phenotype of the fly Lucilia cuprina during overwintering. Similarly, Foster et al. (Reference Foster, Denholm and Devonshire2000) showed that peach-potato aphids (Myzus persicae) resistant to ester-based insecticides have lower winter survival. Gazave et al. (Reference Gazave, Chevillon, Lenormand, Marquine and Raymond2001) studied the relative level of insecticide resistance controlled by two genetic loci in Culex pipiens. The frequency of resistant traits decreased over winter, indicating reduced survival for resistant individuals. In a study with Bt and Pectinophora gossypiella (pink bollworm), resistant individuals had reduced overwinter survival (Carrière et al., Reference Carrière, Ellers-Kirk, Patin, Sims, Meyer, Liu, Dennehy and Tabashnik2001b).
Trichoplusia ni is the only lepidopteran causing significant economic damage in vegetable greenhouses in British Columbia. Greenhouse populations at several geographically separate locations in the lower mainland of British Columbia have developed strong resistance to Bt subsp. kurstaki (Btk), making control of the pest problematic (Janmaat & Myers, Reference Janmaat and Myers2003). In British Columbia, greenhouses are in production for approximately ten months of the year, followed by an intense clean-up procedure to prevent the carryover of pests between production seasons. During this period, the greenhouse is unheated. It has been shown that cabbage looper pupae can survive the cold period during clean-up (Cervantes & Myers, unpublished) while they cannot overwinter in field conditions in British Columbia (Mitchell & Chalfant, Reference Mitchell, Chalfant, Lingren and Green1984). Unless there is a reduced survival or fecundity of resistant individuals, overwintering resistant individuals are likely to persist in greenhouses. This has consequences for resistance levels in the next growing season and the long-term efficacy of Btk as a biological control in the system. This system represents a unique situation to test the relationship between resistance and stress, relating to the evolution of resistance in-situ.
Materials and methods
Colony rearing
The cabbage loopers used in this study are from two colonies designated as RC and Gip. RC is a colony that has been reared under laboratory conditions for more than 15 years and has never been in contact with Btk. Gip originated from insects collected in 2001 from a commercial tomato greenhouse in British Columbia. This initial population of cabbage loopers showed high levels of resistance to Btk (labelled T2C in Janmaat & Myers, Reference Janmaat and Myers2003). The Gip colony was split in two; one half was selected for resistance to Btk every one to two generations (hereafter called Gip Bt); the other half (Gip C) was never exposed again to Btk and lost its resistance.
Cabbage loopers were reared in groups of 15 larvae in 175 ml Styrofoam cups containing 20 ml of wheat germ-based artificial diet (Ignoffo, Reference Ignoffo1963) at a temperature of 25°C and light:dark photoperiod of 16:8 h. Pupae were removed from their cocoons and soaked for 5 min in a 0.6% sodium hypochlorite solution to disinfect them against potential viral or bacterial contamination. Pupae were rinsed and dried before being placed in 30 cm high cylindrical wire mesh cages with a 20 cm diameter at 20–25°C until hatching. Upon emergence, adults were provided with dental wicks soaked in a 10% sucrose solution for food. Paper towels were put on the outside of the cage to provide oviposition sites. Egg sheets were changed every 2 to 3 days and sprayed with a 0.2% bleach solution, allowed to dry and then stored at 9°C until use (maximum of ten days). Egg sheets were placed in 4-litre buckets at 25°C until hatching.
Selection
Neonates were put in groups of 25 in 175 ml Styrofoam cups containing 20 ml of wheat germ-based artificial diet at a temperature of 25°C and photoperiod of 16:8. In order to generate resistance, the larvae were exposed to Btk at doses reflecting those encountered in greenhouses. At the third instar (five days old), larvae were transferred to new Styrofoam cups containing artificial diet mixed with a commercial (Dipel, Abbott Laboratories) wettable powder of Btk solution (ratio Bt solution:diet=1:10). Btk solution was added to cooling diet before solidification. Two days later, the surviving larvae were transferred to normal artificial diet in groups of 20 individuals. Surviving pupae were bleached to reduce viral infections and caged.
Gip Bt colony was divided into four and selected at different Btk concentrations. Four new colonies were formed and named after the concentration of Btk used for selection (Gip Bt20 was selected at 20,000 IU ml−1, Gip Bt40 at 40,000 IU ml−1, Gip Bt60 at 60,000 IU ml−1 and Gip Bt160 at 160,000 IU ml−1). In greenhouses, the recommended concentration for Dipel solution to be sprayed ranges between 9,600 and 19,200 IU ml−1, but producers often increase the dosage (Caron, personal observation). These colonies showed a range of resistance levels to Btk; each colony represents a treatment in the experiment. In order to counteract the potential direct effect of Btk ingestion, colonies were left for one generation without Btk exposure before the experiments were started.
Assessment of resistance
The lethal concentration required to kill half the population (LC50) of each colony was measured for the generation that was used to test overwintering capability (after Janmaat & Myers, Reference Janmaat and Myers2003). Neonates were put on artificial diet in groups of 25 in 175 ml Styrofoam cups for five days at 25°C. Three hundred individuals were taken from each colony (unless stated otherwise), and divided into six groups of 50, each of which was subjected to a different dose of Btk. Doses were made by serial dilution of the original solution (4 g of Dipel into 40 ml of water giving 160,000 IU ml−1) and incorporated into the diet. Colonies showing resistance were assigned a range of high Btk doses (0, 10,000, 20,000, 40,000, 80,000 and 160,000 IU ml−1), while susceptible colonies were given a range of lower doses (0, 625, 1250, 2500, 5000 and 10,000 IU ml−1). Larvae were transferred to the Btk diet in groups of five. Mortality was assessed after three days by touching the larvae gently with a toothpick. An individual was considered dead if it did not move on probing.
Adult survival, deformity and offspring resistance levels following cold treatment
Colonies RC, Gip C, Gip Bt20, Gip Bt40, Gip Bt60 and Gip Bt160 were assessed. Neonates from respective colonies were reared in groups of 15 on artificial diet until pupation as described above. Pupae were put singly in 30 ml plastic cups and randomly assigned to spend 0, 1, 2, 3, 4, 5 or 6 weeks at 10°C. The overwintering temperature of 10°C was chosen as it represents the average temperature in greenhouses in the winter when neither light nor heat is provided. Pupae in the control were kept at 25°C with a photoperiod of 18:6 until emergence. Pupae in the cold temperature treatments were first kept for 24 h at 17.5°C before being placed at 10°C (photoperiod 12:12) to reduce potential thermal shock. After the required time at 10°C, pupae were put at 17.5°C for 24 h and then at 25°C with a photoperiod of 18:6. Adult emergence was recorded every 2–3 days. Adult deformity was assessed visually; moths showing wing abnormalities were recorded as deformed. Adults were caged separately for each colony and treatment combination, and eggs were collected every 2–3 days. The resistance levels of the offspring were assessed as described above.
Statistical analysis
All LC50s were determined with Probit analysis using Genstat 5 (Genstat, 1998). When mortality in the control was less than 5%, the control was removed from the analysis. A 95% fiducial limit was calculated for all colonies. Resistance ratios were calculated by dividing the LC50 of resistant colonies by the LC50 of their respective susceptible colony.
All further statistical analyses were done in JMPIN 4.0.3 (2000). The relationship between Btk resistance and mortality over time at 10°C was assessed using a logistic regression with the log transformed LC50 values for each week spent at 10°C. To test the importance of time spent at 10°C on the proportion of mortality and deformed moths, Chi-square analysis followed by Pearson tests were carried out. Nominal logistic analysis and Wald tests were used to test for an interaction between the effects of overwintering treatment and resistance on deformity.
Results
Resistance levels
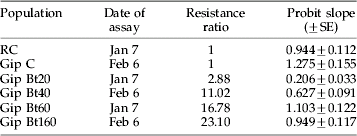
Selected colonies had a range of resistance levels which would be meaningful under field situations. The LC50 values of the different populations were significantly different (df=7, deviance ratio=69.30, p<0.001) (fig. 1). RC and Gip C were highly susceptible to Btk and were significantly different from all the resistant colonies, but not from each other. Gip Bt20 showed low levels of resistance while Gip Bt40, 60 and 160 had higher resistance ratios (table 1). Gip Bt40 and Gip Bt160 were different from each other, but not from Gip Bt60.

Fig. 1. LC50 data and 95% fiducial limits of T. ni colonies used to assess overwintering capabilities. LC50s were considered significantly different when error bars were not overlapping.
Table 1. Resistance ratio (resistant: susceptible) of colonies used to assess overwintering capabilities. Results for Probit analyses of the effect of dose on mortality are shown (all slopes are significant at p<0.001). Resistant ratios were calculated relative to LC50 of the reciprocal susceptible colony.
Adult survival, deformity and offspring resistance levels following cold treatment
Cold kills pupae and the time spent at 10°C as pupae was negatively correlated with the proportion of moths that emerged (Pearson χ2=376, p<0.001). Mortality after exposure to 10°C at the pupal stage was related to the resistance level of the colony for most of the colonies and was significantly higher for susceptible pupae than resistant pupae after 2, 4, 5 and 6 weeks exposure (control: χ21, 269=0.05, p=0.818; 1 week: χ21, 272=0.60, p=0.437; 2 weeks: χ21, 271=6.18, p=0.013; 3 weeks: χ21, 270=0.00, p=0.974; 4 weeks: χ21, 269=5.95, p=0.015; 5 weeks: χ21, 270=5.30, p=0.021; 6 weeks: χ21, 271=20.64, p<0.001) (fig. 2).

Fig. 2. Relationship between the proportion of T. ni pupae that died after being exposed to 10°C for two, four and six weeks and the LC50s of the colonies (![]() , 2 weeks; ●, 4 weeks; □, 6 weeks).
, 2 weeks; ●, 4 weeks; □, 6 weeks).
Moth deformity was strongly influenced by the time pupae spent at 10°C (Pearson χ2=882, p<0.001). The proportion of perfectly formed moths decreased with the number of weeks spent at 10°C, with no moths being normal after three weeks. Some mating was observed between mildly deformed moths. The resistance level had no effect on deformity; perfectly formed and deformed moths were distributed equally among colonies having different LC50s (Wald=3.23×10−9, p>0.999).
Offspring resistance level
No moths laid eggs after having spent three weeks at 10°C as pupae. After two weeks the most resistant colonies, Gip Bt40, Gip Bt60 and Gip Bt160, produced few live larvae (approximately 15 surviving after five days) compared to Gip C (101 larvae) and RC (133 larvae) and Gip Bt 20 (168 larvae), indicating a significant reproductive fitness cost of resistance. The LC50 at two weeks cold exposure could only be assayed for the last three colonies.
Only the most resistant colony, Gip Bt160, had reduced levels of resistance in subsequent generations, i.e. LC50s of the offspring were lower than the LC50 of their respective parents (fig. 3). The overwintering treatment had no significant effect on resistance of offspring.

Fig. 3. LC50s and 95% fiducial limits of T. ni parents and their offspring related to the time parents spent at 10°C as pupae. Probit analysis between dose and mortality are not shown. All slopes were significant at p<0.001. LC50s were considered significantly different when error bars were not overlapping (![]() , parents;
, parents;![]() , 0 week; □, 1 week; ■, 2 weeks).
, 0 week; □, 1 week; ■, 2 weeks).
Discussion
The persistence of insecticide resistant genotypes in populations of insects are likely to be strongly influenced by trade-offs with any related costs. Adverse conditions may be more challenging for insects resistant to insecticide. Winter can be a stressful period in an insect's life (McKenzie, Reference McKenzie1996). For this reason, we predicted that Btk resistant T. ni could have reduced ability to survive the overwintering period. The length of the overwintering period reduced survival and fecundity and increased rates of deformity seen in T. ni. Contrary to the original prediction, when exposed to a stress mimicking the overwintering period in greenhouses, the survival of resistant individuals was reduced less than that of susceptible moths. This strongly contrasts with other studies which have found that overwintering success of resistant phenotypes is lower (McKenzie, Reference McKenzie1994; Foster et al., Reference Foster, Denholm and Devonshire2000; Carrière et al., Reference Carrière, Ellers-Kirk, Patin, Sims, Meyer, Liu, Dennehy and Tabashnik2001b; Gazave et al., Reference Gazave, Chevillon, Lenormand, Marquine and Raymond2001) or that overwintering mortality is not related to resistance (Daly & Fitt, Reference Daly and Fitt.1990). A single case from the literature has shown any positive effect of resistance; Hollingsworth et al. (Reference Hollingsworth, Tabashnik, Johnson, Messing and Ullman1997) found an increase in progeny production of methomyl resistant cotton aphids. This study, therefore, is the first to describe an increase in survival associated with resistance, and is only the second account of a potential advantage due to resistance.
Increased survival of the resistant phenotype in this study could be due to pleiotropic effects (positive or negative effects of one trait resulting from selection for another trait) (Hedrick, Reference Hedrick1999). In this case, it is possible that an allele responsible for resistance is positively influencing one of the components required for longer survival at cold temperatures. More than one gene is involved in resistance of T. ni to Btk (Janmaat et al., Reference Janmaat, Wang, Kain, Zhao and Myers2004). It is, therefore, possible that these different genes have pleiotropic effects on life-history traits of T. ni, although we know of no possible mechanisms. Investigation of the genetic basis of insecticide resistance and the potential influence of these on other traits has been little explored to date, and certainly warrants attention.
Although there were clear positive effects of resistance on survival in this study, survival of resistant individuals is only significant in evolutionary terms if these individuals transmit the resistant alleles to the next generation. Our results suggest that in the greenhouse study system there is potential for resistant genes to be transferred to subsequent generations. The frequency of the resistant phenotype was not reduced by the overwintering period, and offspring had the same resistance level as offspring from parents kept at constant temperature. Therefore, surviving resistant moths capable of reproduction will transmit resistant alleles to the next generation, and these could be at a competitive advantage under future selection associated with Btk applications.
Reduced pupal weight and, therefore, fecundity of T. ni has been associated with Btk resistance (Janmaat & Myers, Reference Janmaat and Myers2003; Caron, unpublished data). In this study, after a two week overwintering period, resistant colonies produced fewer offspring than susceptible colonies. This reduced fecundity indicates a major reproductive fitness cost that seems exacerbated by the stress of the overwintering period. This has the potential to decrease the levels of resistance in the population, even if overwintering survival is higher for resistant pupae. If resistant and susceptible phenotypes survive the overwintering period, the susceptible moths, with their higher fecundity, will have more progeny and, therefore, the frequency of heterozygous, susceptible individuals will increase in subsequent generations. While it is likely that the levels of deformity influence reproductive fitness as well, this did not differ between resistant and non-resistant populations.
These results could have important consequences for the management of T. ni in greenhouses because populations persisting from one generation to the next will continue to be resistant to Btk even though they have reduced fecundity. Trichoplusia ni is a subtropical species and does not overwinter in field conditions in British Columbia, where populations are re-established by annual migration from southern California (Mitchell & Chalfant, Reference Mitchell, Chalfant, Lingren and Green1984), but was shown to survive in unheated greenhouses (Cervantes and Myers, unpublished). Field populations have low levels of resistance (Janmaat & Myers, Reference Janmaat and Myers2003). If resistant populations surviving in greenhouses and non-resistant immigrants mate, the level of Bt resistance would decline. Outbreeding with susceptible moths reduces resistance development over time, even if overwintering is not impeded by resistance (Daly and Fitt, Reference Daly and Fitt.1990). The beginning of crop production in the greenhouse, however, precedes the arrival of migrant moths in the outside environment. Therefore, if the frequency of the resistant allele is already high at the beginning of the season and selection continues, resistance will increase. We know that early season cabbage looper populations can be highly resistant to Btk in vegetable greenhouses in British Columbia (Janmaat and Franklin, personal communication), and the results described here are consistent with that observation. These results help to explain the rapid evolution of resistance to Btk of cabbage loopers in greenhouses observed by Janmaat and Myers (Reference Janmaat and Myers2003) and emphasize the necessity of eliminating moth populations for resistance management in greenhouses.
In conclusion, resistance to Btk in T. ni increases potential overwintering capability. Survival rates of resistant pupae were higher than Btk susceptible pupae when kept longer at cold temperatures. It is extremely unusual for insecticide resistance to be associated with positive effects on fitness when exposed to another stressor. This may have important implications for the development of resistance, although in this particular case, all moths surviving after spending a few weeks at cold temperatures were highly deformed and incapable of reproducing. There was also a fecundity cost associated with Btk resistance that reduced the number of viable offspring, potentially counteracting the advantage given by an increased survival in the face of cold stress. Therefore, this increase in cold-stressed survival by resistant individuals may not infer an important overall increase in fitness, unless only the resistant phenotype is present after the overwintering period. This study emphasizes the need to study fitness costs associated with the development to insecticide resistance carefully. More importantly, this study warns against the assumption that there will necessarily be negative trade-offs associated with insecticide resistance.
Acknowledgements
We thank Dave Gillespie, Alida F. Janmaat, Veronica Cervantes, Michelle Franklin, Jenny Cory, Arianne Albert and Ross Thompson for useful discussions, and Patricia Duffels, Tamara Richardson and Tahmineh Ziae for technical assistance. This research was funded by the Biocontrol Network and Canadian NSERC.






