INTRODUCTION
Differential parasite abundance in hosts belonging to different age cohorts has been reported for various host and parasite taxa (Goater and Ward, Reference Goater and Ward1992; Fichet-Calvet et al. Reference Fichet-Calvet, Wang, Jomaa, Ben Ismail and Ashford2003; Krasnov et al. Reference Krasnov, Stanko and Morand2006; Alarcos and Timi, Reference Alarcos and Timi2012). However, the effect of host age on the distribution pattern of parasite abundance differs among different host-parasite associations (Hudson and Dobson, Reference Hudson, Dobson, Grenfell and Dobson1995). In some host-parasite associations, parasite abundance increases (linearly or asymptotically) with host age (e.g., Johansen et al. Reference Johansen, Lydersen, Aspholm, Haug and Kovacs2010; Body et al. Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011), while in other associations parasite abundance either increases or decreases in the youngest and oldest hosts compared with median age hosts (Gregory et al. Reference Gregory, Montgomery and Montgomery1992; Krasnov et al. Reference Krasnov, Stanko and Morand2006).
Age-dependent development of defence tools coupled with parasite-dependent host mortality are thought to be the main mechanisms generating these patterns (Pacala and Dobson, Reference Pacala and Dobson1988; Woolhouse, Reference Woolhouse1998), although host behaviour and ecology may also be responsible (Krasnov et al. Reference Krasnov, Stanko and Morand2006). Indeed, the acquired resistance against parasites can be lower in young and/or old hosts than in median age hosts. Young hosts may merely not have enough time to acquire resistance against parasites (Gallie, Reference Gallie1973), while old hosts may lose the capacity to withstand parasites due to immunosenescence (Møller and de Lope, Reference Møller and de Lope1999; Gruver et al. Reference Gruver, Hudson and Sempowski2007; Praet et al. Reference Praet, Speybroeck, Rodriguez-Hidalgo, Benitez-Ortiz, Berkvens, Brandt, Saegerman and Dorny2010). As a result, hosts belonging to the youngest and oldest cohorts would represent better habitats for parasites (that is, habitats that allow higher fitness reward), so that the shape of the relationship between parasite abundance and host age would be convex. However, if high parasite burdens cause host mortality, then heavily infested young and old hosts will be lost from the population, transforming the relationship between parasite abundance and host age into a hump-shaped curve (Rousset et al. Reference Rousset, Thomas, de Meeûs and Renaud1996). In addition, if the negative effect of heavy parasite burdens causes mortality mainly in young rather than old hosts, then parasite abundance will increase with an increase in host age. In any of these cases, parasites are expected to perform better on young and old hosts (due to their lower defences) than on median age hosts (further referred to as ‘adult hosts’).
From the ecological and evolutionary perspectives of host-parasite interactions, both responses of hosts to parasites and responses of parasites to hosts are equally important (Combes, Reference Combes2001). However, the majority of studies of anti-parasitic defences rarely focused on parasites but rather assessed anti-parasitic responses of hosts (but see Jørgensen et al. Reference Jørgensen, Leathwick, Charleston, Godfrey, Vlassoff and Sutherland1998; Sargison et al. Reference Sargison, Jackson and Gilleard2011).
Recently, we tested the effect of age of a rodent host (Meriones crassus) on feeding performance of a flea (Xenopsylla ramesis) and predicted that fleas would perform better on juvenile and senescent hosts than on subadult and adult hosts (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011). Fleas are obligatory haematophagous insects that alternate periods when they occur on host body and when they occur in its burrow or nest. In contrast to the imago, flea larvae are not parasitic and usually develop off-host. Upon emergence from a pupa, a new imago flea finds a host because flea reproduction is not possible without blood feeding (Krasnov, Reference Krasnov2008). Unequivocal support for our prediction was not found but rather the effect of host age was mediated strongly by the effect of host gender. In particular, from the perspective of resource acquisition (that is, bloodmeal size), a better quality of young and old age cohorts was manifested in female but not in male hosts, while, from the perspective of resource processing (that is, digestion of blood), some trends of age-dependent host quality were found in male but not female hosts. In other words, the results of the study by Liberman et al. (Reference Liberman, Khokhlova, Degen and Krasnov2011) suggested that host age could not unequivocally predict whether it is more or less beneficial for a flea. Another reason for inconsistencies in these results could be that feeding performance in hosts belonging to different age cohorts might not necessarily be a good proxy for fitness achieved in these hosts (see also Khokhlova et al. Reference Khokhlova, Fielden, Degen and Krasnov2012). Consequently, investigating mechanisms of the effect of host age on parasite abundance requires experimental measurement of direct fitness-related variables.
Here, the direct effect of host age on parasite fitness was studied using the same model host-parasite association as in our earlier study (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011), namely a rodent M. crassus and a flea X. ramesis. Flea fitness was measured in terms of both number and quality of offspring. The number of flea offspring was measured via egg and new imago production, while quality was assessed via duration of development, resistance to starvation and body size. A higher emergence success was assumed to indicate a higher quality of offspring because it mirrors the mortality of pre-imaginal fleas. A shorter duration of pre-imaginal development of a flea may be an indicator of higher quality because (a) earlier-hatched flea larvae often cannibalize later-hatched larvae (Lawrence and Foil, Reference Lawrence and Foil2002), (b) earlier-hatched flea larvae may have an advantage over later-hatched larvae in competition for food (Krasnov et al. Reference Krasnov, Burdelova, Khokhlova, Shenbrot and Degen2005), and (c) earlier-emerged fleas likely have a higher probability to find a host than later-emerged fleas. The resistance to starvation in newly emerged imagoes is a measure of their quality because when a flea emerges from a cocoon, it possesses energy storage in the fat tissue. This energy allows the flea to survive until it finds a host. The ability to survive unpredictable and sometimes lengthy periods without a bloodmeal is thus extremely important. Finally, body size may be considered as an additional indicator of a flea's quality because larger body size is intraspecifically associated with higher fecundity in insects (Honek, Reference Honek1993), although this has never been studied in fleas.
We predicted that fleas would produce more eggs and the emergence success of their offspring would be higher when they exploit juvenile (20–21 days old) and senescent (>4 years old) rodent hosts than subadult (45–60 days old) and adult (6–12 months old) rodent hosts. We also predicted that the offspring of fleas fed on juvenile and old rodents would (a) develop faster, (b) be larger and (c) survive without a bloodmeal longer than those fed on subadult and adult rodents.
MATERIALS AND METHODS
Fleas and rodents
We used fleas and rodents from our laboratory colonies. A detailed description of maintenance of these colonies can be found elsewhere (Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009, Reference Khokhlova, Serobyan, Degen and Krasnov2010; Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011). Fleas were maintained on M. crassus kept individually in plastic cages with a wire mesh floor over a pan containing a mixture of sand and dried bovine blood. Air temperature was kept at 25 °C and photoperiod at 12:12 (L: D) h. Every 2 weeks, all substrate and bedding material from the rodent's nest box and the pan were collected and transferred to an incubator (FOC225E, Velp Scientifica srl, Milano, Italy; 25 °C air temperature and 75% relative humidity [RH]) where the fleas developed. Newly-emerged fleas were collected from these boxes every 2 weeks. Fleas used in experiments were newly-emerged and were selected randomly from a colony.
Rodents were maintained in plastic cages (60 × 50 × 40 cm), and with sawdust and dried grass as bedding material. They were offered millet seed and fresh alfalfa (Medicago sp.) ad libitum daily. In this study, we used sexually-naïve juvenile, subadult, adult, and senescent M. crassus (107 males and 70 females). Adult rodents (mean body mass 140·2 g and 107·7 g for males and females, respectively) were 6–12 months old and were randomly selected from a laboratory colony. Old rodents (mean body mass 119·2 g and 86·1 g for males and females, respectively) were maintained individually from 2 months of age for 4 years. Juvenile rodents (mean body mass 25·8 g for both males and females) were 20–21 days old and pre-weaned. Subadult rodents (mean body mass 65·6 g for both males and females) were 45–60 days old and weaned at 30 days of age. In total, we used 44 juvenile, 57 subadult, 36 adult and 40 old rodents. No sibling rodents were used in the same treatment. For biological reasons, juvenile and subadult rodents were never parasitized by fleas, while adult and old rodents were exposed to fleas on at least 5 occasions previously.
Experimental procedures and design
We used 2 methods for feeding fleas. In the first method, we placed rodents individually in wire mesh (5 × 5 mm) tubes (15 cm length and 4 cm diameter for juvenile rodents and 18 cm length and 6 cm diameter for subadult, adult and old rodents) which prevented movement and self-grooming. Then, the tubes were placed in individual white plastic pans and 10, 20 or 30 fleas (equal number of males and females) were placed on each rodent (juvenile, subadult and adult/old, respectively) for 1 h. We brushed the hair of the rodent several times with soft custom-made forceps until all fleas were recovered. This procedure was repeated for 8 consecutive days. Each group of fleas was fed on the same rodent individual. In the second method, an individual rodent or a female with pre-weaned juveniles were placed in a plastic cage (60 cm by 50 cm by 40 cm) with a floor of 3–5 mm of clean sand covered by a wire mesh (5 mm by 5 mm). Ten (times number of juveniles in the litter), 20 or 30 fleas (equal number of males and females) were released into the cage (see above) and allowed to stay with a rodent for 3 days. Our preliminary observations demonstrated that fleas start to oviposit no sooner than the second day with a host under these conditions. After 3 days of an uninterrupted stay in a rodent's cage, fleas were collected as described above. Then, these fleas were fed daily on the same rodent individual using the first method during 5 days. Between feeding events (1–8 days for the first method and 4–8 days for the second method), fleas of each group (i.e., recovered from the same rodent individual) were placed in 50 ml glass vials and were then transferred to an incubator (see above) at 25 °C air temperature and 90% RH for 24 h. Each day, we collected all fleas from each vial and released them onto a rodent for 1 h. Eggs produced by each group of fleas in each day of oviposition were counted. Only eggs produced during days 6 to 8 from the onset of experiments were taken for subsequent analyses.
Eggs produced in the same day were transferred into new vials. These vials were filled with a 3 mm layer of sand and larval food medium (94% dry bovine blood, 5% millet flour, and 1% grinded excrements of M. crassus) and were covered by perforated lids. To ensure excess food for each larva, the amount of larval medium added to each vial was calculated as the necessary daily amount, times the maximum duration of larval stage (see details in Khokhlova et al. Reference Khokhlova, Serobyan, Degen and Krasnov2010) times the number of eggs in a vial and then tripled. Vials were than maintained at 25 °C air temperature and 90% RH. Temperature was regulated in refrigerated incubators (see above) and humidity was regulated in 38 × 23 × 13 cm acrylic humidity chambers using saturated salt solutions. Temperature and humidity were monitored using a Fisherbrand Traceable Humidity/Temperature Pen with Memory (Fisher Scientific International, NJ, USA).
Minimal duration of metamorphosis (i.e., from egg to adult) of X. ramesis at 25 °C air temperature and 92% RH is 25 days (Krasnov et al. Reference Krasnov, Khokhlova, Fielden and Burdelova2001). Consequently, starting from the 18th day after an egg was produced, we checked each vial daily until all adults emerged (i.e., the number of emerged adults was equal to the number of eggs) or for 60 consecutive days. We counted new imagos produced by each group of parent fleas. After emergence, each adult was transferred to an Eppendorf vial with a perforated lid and the bottom covered by a thin layer of clean sand and left in the incubator at the same air temperature and RH. Vials with newly-emerged adults were checked daily until all adults died. After the death of each imago, we identified its sex by examination of its genitalia under light microscopy.
After the death of an adult, we measured its body size via maximal length of its right hind femur. The use of direct measure of body size (e.g., body length) of a dead adult was not possible because the body shape of a flea could be distorted after starvation and desiccation. The reason behind this distortion is the high flexibility of the thorax and abdomen because thoracic and abdominal segments do not possess posterior walls (Medvedev and Krasnov, Reference Medvedev, Krasnov, Morand, Krasnov and Poulin2006). In addition, body length of fleas may vary with pressure applied to the specimens when preparing them between slides and cover-slides making body length an inaccurate indicator of body size (Tripet et al. Reference Tripet, Christe and Møller2002). In contrast, the length of a femur was shown to be a reliable indicator of body size in fleas because these traits were strongly correlated (Krasnov et al. Reference Krasnov, Burdelov, Khokhlova and Burdelova2003; Khokhlova et al. Reference Khokhlova, Serobyan, Degen and Krasnov2010). The length of the femur was measured on a screen using a digital microscope camera Moticam 2000 with the Motic Images Plus 2.0ML program (Motic, Speed Fair Cp., Ltd, Causeway Bay, Honkong) to the nearest 0·01 mm under a magnification of 40X and with calibration using an object-micrometer.
Data analyses
In the analyses of egg and new imago production, a replicate represented a group of fleas feeding simultaneously on a rodent. In total, there were 177 groups of female fleas that produced eggs. In 24 of these groups, all larvae died prior to pupation due to unknown reasons. These groups were excluded from the analysis. We calculated the number of eggs produced per female flea during 6–8 days of feeding and the number of new imago emerged from these eggs. Initially, these dependent variables were analysed using 3-way ANOVAs with host age, host gender and method of flea feeding as independent variables. No effect of the method of feeding on any of the dependent variables was found (F 1,145=0·39 and F 1,145=0·22 ; P > 0·50 for both). Consequently, in the final analyses of egg and new imago production, data obtained using the two methods of feeding were pooled, and dependent variables were analysed by 2-way ANOVAs with host age and gender as independent variables. We used univariate tests of significance for planned comparisons to compare dependent variables within host age and gender.
In the remaining analyses, a replicate represented an individual flea. In total, there were 2369 newly emerged fleas (1170 males and 1199 females). Of these, we measured the length of the right femur in 1888 fleas (932 males and 956 females). For each newly emerged flea, we calculated (a) time of development from oviposition till emergence and (b) time from emergence until death under starvation. These data were analysed using 3-way ANOVAs with host age, host gender and flea gender as independent variables. We used univariate tests of significance for planned comparisons to compare dependent variables within host age, host gender and flea gender.
All dependent variables, except for emergence success, were log-transformed prior to analysis. We applied angular transformation to emergence success. Figures represent non-transformed data.
RESULTS
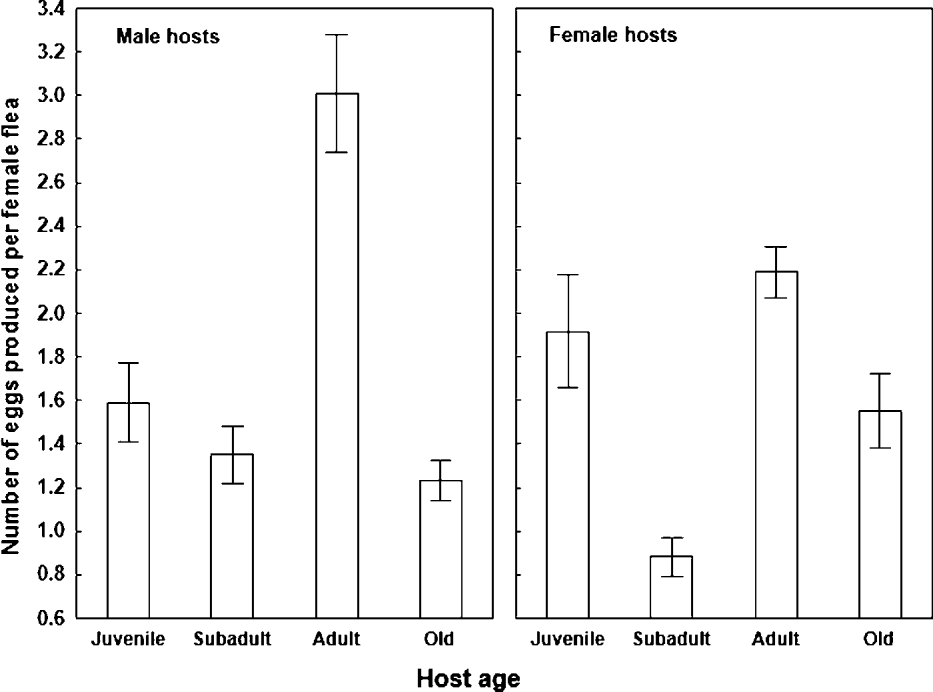
Summary of ANOVAs for the effect of host age and gender and/or gender of a newly emerged flea on number and quality of flea offspring are presented in Table 1. A significant independent effect of host age, but not host gender on flea egg production was found. However, a significant interaction between host age and gender demonstrated that the effect of host age on egg production differed in the response of a parent flea exploiting either a male or female host. In fleas fed on subadult and adult rodents, egg production was significantly higher for those fed on male than on female hosts (F = 5·1 and F = 3·9, respectively; P < 0·05 for both; Fig. 1). However, this was not the case for fleas fed on either juvenile or old hosts (F = 1·9 and F = 1·5, respectively; P > 0·16 for both; Fig. 1). In general, fleas fed on male hosts produced significantly more eggs on adult hosts than the other age groups (F = 49·2, P < 0·001; Fig. 1), which did not differ among themselves (F = 0·01, P = 0·9; Fig. 1). In experiments with female hosts, fleas produced more eggs (a) on juvenile and adult than on subadult and old rodents (F = 20·2, P < 0·001; Fig. 1) and (b) on adult than on subadult rodents (F = 9·3, P < 0·01; Fig. 1).
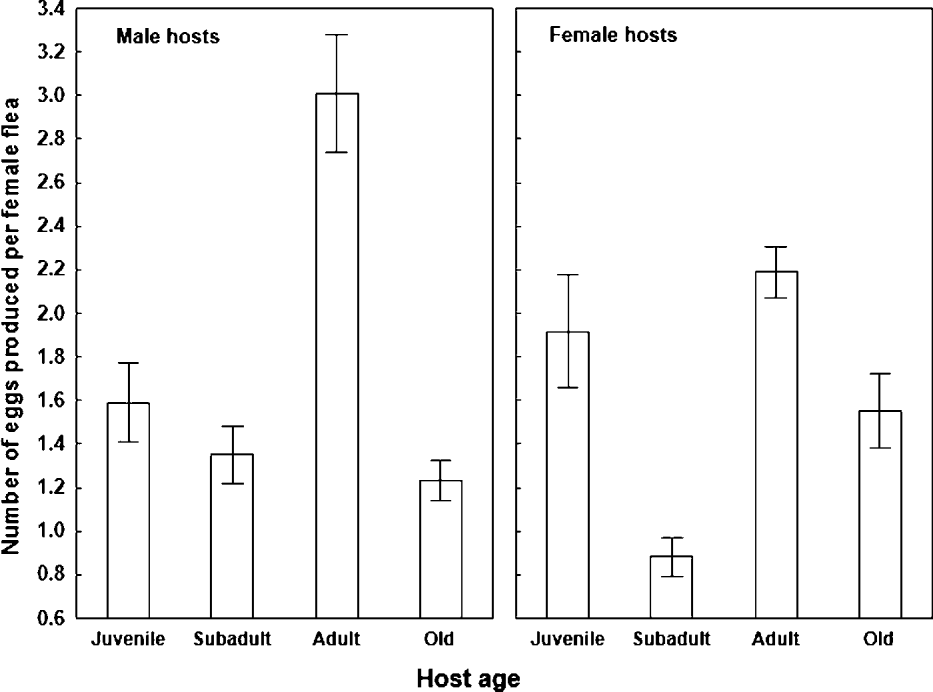
Fig. 1. Mean (±s.e.) number of eggs produced per female Xenopsylla ramesis during 6–8 days of feeding on juvenile, subadult, adult and old male and female Meriones crassus.
Table 1. Summary of significant (P < 0·05 for all) effects in 2- and 3-way ANOVAs of flea (X. ramesis) reproductive variables as affected by age and gender of a rodent (Meriones crassus) host and/or gender of a flea

The number of new imago was affected significantly by host age and gender, while interaction between these factors was not significant (Table 1). Significantly more new imago emerged if a parent flea fed on juvenile and old than on subadult or adult rodents (F = 19·0 for male hosts and F = 14·4 for female hosts; P < 0·01 for both; Fig. 2). Significantly more new imago emerged from eggs laid by females fed on female than on male adult rodents (F = 4·0, P < 0·05; Fig. 2), while female fleas fed on hosts belonging to the 3 remaining cohorts produced similar number of new imago, independent of host gender (F = 0·01–1·7, P > 0·19 for all; Fig. 2).

Fig. 2. Mean (±s.e.) number of new imago produced per female Xenopsylla ramesis during 6–8 days of feeding on juvenile, subadult, adult and old male and female Meriones crassus.
Duration of development of flea offspring was affected significantly by host age and gender and differed significantly between male and female fleas, while all between-factor interactions were significant (Table 1). It took fleas a significantly longer time to develop if their mothers fed on either adult or both subadult and adult hosts (F = 150·8 for male rodents and F = 128·3 for female rodents; P < 0·001 for both; Fig. 3) than if they fed on hosts belonging to other age cohorts (Fig. 3). Female fleas generally developed faster than male fleas (F = 1003·2, P < 0·01; Fig. 3). Regarding the effect of host gender, female offspring of fleas on subadult and adult male hosts developed faster than those on subadult and adult female hosts (F = 22·4 and F = 38·8, respectively; P < 0·01 for both). The same was true for male offspring of fleas fed on subadult hosts (F = 15·4, P < 0·01; Fig. 3), whereas male offspring developed longer if their parents fed on adult male than female rodents (F = 19·5, P < 0·01; Fig. 3). No effect of host gender on duration of development was found for flea offspring from parents fed on juvenile and old hosts (F = 0·16–2·21, P > 0·13 for all; Fig. 3).

Fig. 3. Mean (±s.e.) duration of development from egg to imago in Xenopsylla ramesis from females fed on juvenile, subadult, adult and old male and female Meriones crassus.
Among the 3 independent factors, only host age and interactions between host age and either host or flea gender significantly affected time of survival under starvation of flea offspring (Table 1). Fleas from mothers fed on old hosts died under starvation faster than those on juvenile, subadult and adult hosts (F = 150·6, P < 0·01; Fig. 4), except for male offspring of fleas fed on female hosts. The latter survived starvation longer if their hosts belonged to younger (juvenile and subadult) than older (adult and old) age cohorts (F = 25·0, P < 0·01; Fig. 4). The effect of interaction between host age and gender showed that male (but not female) offspring from fleas fed on adult male hosts survived longer than those on adult female hosts (F = 16·2, P < 0·01; Fig. 4), while no difference was found between male and female hosts of other age categories (F = 0·3–2·3; P > 0·31 for all; Fig. 4). Interaction between host age and flea gender showed significant difference in survival under starvation between male and female flea offspring only for adult female hosts (female fleas survived longer than males; F = 6·3, P = 0·01; Fig. 4).

Fig. 4. Mean (±s.e.) time of survival from emergence under starvation in Xenopsylla ramesis from females fed on juvenile, subadult, adult and old male and female Meriones crassus.
Body size of flea offspring was affected by both host age and gender and differed significantly between males and females (larger females; Table 1, Fig. 5). In addition, two 2-way and the 3-way interactions were significant (Table 1). Fleas fed on old hosts produced the largest offspring (F = 36·4 for males and F = 45·7 for females; P < 0·001 for both; Fig. 5), while the size of newly emerged females did not differ among the remaining age cohorts (F = 1·14 for male hosts and F = 0·45 for female hosts; P < 0·28 for both; Fig. 5). The same was true for newly emerged males from parents fed on male hosts (F = 0·2, P = 0·65). In contrast, fleas fed on adult female hosts produced the smallest male fleas (F = 18·6, P <0·001; Fig. 5). The interaction among factors showed a significantly different size of (a) new male imago from fleas fed on male than female adult hosts (F = 13·6, P < 0·001 versus F = 0·03–2·2, P > 0·05; larger offspring produced from male hosts; Fig. 5) and (b) new female imago from fleas fed on male than female juvenile hosts (F = 9·5, P < 0·01 versus F = 0·22–0·99, P < 0·30; larger offspring produced from female hosts; Fig. 5).

Fig. 5. Mean (±s.e.) length of right hind femur in male and female offspring Xenopsylla ramesis produced by females fed on juvenile, subadult, adult and old Meriones crassus.
DISCUSSION
Results of this study supported our predictions. Although fleas produced more eggs when they exploited adult hosts than when they exploited juvenile, subadult and old hosts, the net results of their reproduction (that is, number of individuals of new generation) undoubtedly pointed to a better reproductive performance on juvenile and old hosts. The difference between the pattern of egg and new imago production indicated that the quality of eggs produced from juvenile and old hosts was higher than those from subadult and adult hosts because a higher proportion of them attained emergence. Furthermore, fleas performed better when they fed on juvenile and/or old hosts in 2 of 3 measures of offspring quality (duration of development and body size). Nevertheless, when offspring quality was estimated via resistance to starvation of a new imago, fleas demonstrated good performance when exploiting young (juvenile and subadult) hosts, while they performed poorly when exploiting old hosts. In general, thus, reproductive performance of fleas was better when they exploited the youngest and the oldest hosts when compared to hosts of median age. However, the effect of host age on flea reproductive performance was manifested somewhat differently (a) when fed on male and female hosts and (b) between male and female flea offspring. In other words, the effect of host age on flea reproduction was mediated by host gender. The effect of host gender on flea reproduction has been discussed in our earlier studies (Khokhlova et al. Reference Khokhlova, Serobyan, Krasnov and Degen2009, Reference Khokhlova, Serobyan, Degen and Krasnov2010). Consequently, we focus here mainly on the effect of host age.
Reproductive success of an individual is a net result of quantity of a resource and its quality. Furthermore, the amount of the resource available for a consumer depends not only on the amount of the resource in the surrounding environment (a host, in case of parasites) but can be affected by the pattern of its acquisition. Indeed, it is highly unlikely that the amount of resource varies among hosts belonging to different age cohorts, especially given that fleas usually consume only a small portion of host blood (Khokhlova et al. Reference Khokhlova, Krasnov, Kam, Burdelova and Degen2002). However, these hosts possess different defence abilities which can affect the amount of blood a flea can obtain. An individual host attacked repeatedly by an ectoparasite develops acquired resistance against this ectoparasite (Willadsen, Reference Willadsen1980; Rechav, Reference Rechav1992) which is manifested by decreased feeding and reproduction of the ectoparasite (e.g., Rechav et al. Reference Rechav, Heller-Haupt and Varma1989; Fielden et al. Reference Fielden, Rechav and Bryson1992; Khokhlova et al. Reference Khokhlova, Ghazaryan, Krasnov and Degen2008). For example, a study of acquired resistance in guinea pigs showed that repeated infestation of guinea pigs by tick larvae resulted in a sharp reduction in body mass of the larvae (Fielden et al. Reference Fielden, Rechav and Bryson1992). Thus, higher reproductive outcome and higher quality of offspring in juvenile and, partly, old hosts when compared with subadult and adult hosts can be explained by lower defence abilities of the former, which affects the amount of blood a flea is able to take. Indeed, in our earlier study, we found that fleas took more blood from juvenile and old than from subadult and adult animals (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011).
The most likely reason behind lower defence abilities of juveniles is their under-developed immune system. Although they can have some protective, albeit not especially effective, immunity transferred from their mothers during pregnancy and lactation (Knopf and Coghlan, Reference Knopf and Coghlan1989; Carlier and Truyens, Reference Carlier and Truyens1995; Hasselquist and Nilsson, Reference Hasselquist and Nilsson2009), their skin immunity is functionally immature (Dewar et al. Reference Dewar, Doherty, Woods, Lyons and Muller2001). Nevertheless, additional nutrition may provide additional resources to be invested in immune defence which may increase anti-ectoparasite resistance (McCoy et al. Reference McCoy, Boulinier, Schjorring and Michalakis2002; Tschirren and Richner Reference Tschirren and Richner2006). An increase in immunity of nestlings due to additional provisioning by parents has been reported for birds (Saino et al. Reference Saino, Calza and Møller1997), but an increase in immunity due to increased parent provisional effort (via foraging or lactation) is unlikely to occur in pre-weaned small mammals. This is supported by the fact that milk production and milk fat concentration in female rodents decreases with the age of pre-weaned pups (Kam and Degen Reference Kam and Degen1993; Hinde, Reference Hinde2007) likely because the latter start to feed independently from about the 17th day of age.
Lower defence abilities of old hosts could result from immunosenescence. It is well known that immune defences often deteriorate with age (Tarazona et al. Reference Tarazona, Solana, Ouyang and Pawalec2002; Gruver et al. Reference Gruver, Hudson and Sempowski2007) and, as a result, anti-parasitic defence in old animals is generally weak (Klein, Reference Klein2004). The decline of anti-parasitic defence with age has been reported for both birds (e.g., Saino et al. Reference Saino, Ferrari, Romano, Rubolini and Møller2003) and mammals (Pelletier et al. Reference Pelletier, Page, Ostiguy and Festa-Bianchet2005; Body et al. Reference Body, Ferté, Gaillard, Delorme, Klein and Gilot-Fromont2011). The degree of deterioration of the immune function with age can be, however, affected by environmental conditions (e.g., environmental stress; Hayward et al. Reference Hayward, Wilson, Pilkington, Pemberton and Kruuk2009) and may differ between males and females (due to faster aging of males; Clutton-Brock and Isvaran, Reference Clutton-Brock and Isvaran2007). However, we did not find any indication of the latter effect in our study.
The pattern of the effect of host age on survival of new imago under starvation differed from that of the 2 other measures of flea offspring quality in this study. Survival of new imago was the shortest if their parents fed on old hosts. This suggested that quality of parasite offspring might be affected not only by the amount of resource (which is relatively high in the case of old hosts; see Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011), but also by the quality of this resource and that nutritional value of blood taken from an old host is likely to be low. The nutritional value of host's blood consumed by a flea can affect its offspring via transfer from a parent as well as in a direct way. As noted above, a newly emerged flea possesses stored energy in fat tissue. It is commonly accepted that the larval stages in holometabolous insects serve to accumulate substrates that allow the imago to emerge with significant fat body stores (Gilbert and Chino, Reference Gilbert and Chino1974; Anand and Lorenz, Reference Anand and Lorenz2008). Flea larvae are not parasitic and feed on all kinds of organic debris found in the host's burrow or nest. This debris often includes flea faeces (Silverman and Appel, Reference Silverman and Appel1994). Moreover, in some species, females have been shown to expel faecal pellets near the clutch which can later serve as a food source for larvae (see review in Krasnov, Reference Krasnov2008). The protein content of flea feces was actually higher than the blood upon which they fed (Hinkle et al. Reference Hinkle, Koehler and Kern1991). Consequently, flea larvae consume host blood via fecal pellets of parent fleas and may be, thus, be directly affected by the quality of this blood. Shortest survival of new imago from parents fed on old hosts hinted on some deterioration of nutritional value of their blood, although resistance to starvation in parent fleas after a direct bloodmeal from a senescent host was not compromised (Liberman et al. Reference Liberman, Khokhlova, Degen and Krasnov2011).
In conclusion, our results suggest that the reproductive performance of a flea is affected by the age of its host and is a trade-off between quantity (determined by defence abilities of a host) and quality (nutritional value) of the resource taken from a host. Furthermore, different manifestation of the host age effect on reproductive performance of parasites (a) due to host gender and (b) in male and female offspring may be the reasons behind variation in age-related patterns of parasite infestation (Krasnov et al. Reference Krasnov, Stanko and Morand2006).
ACKNOWLEDGEMENTS
This study was supported by the Israel Science Foundation (Grant no. 27/08 to B.R.K., I.S.K. and A.A.D.). The experimental procedures complied with the laws of the State of Israel. This is publication no. 783 of the Mitrani Department of Desert Ecology.