Background
Truncus arteriosus is characterised by a single great artery giving rise to the ascending aorta, pulmonary arteries, and coronary arteries. Truncus arteriosus accounts for approximately 0.7% of all congenital heart lesions with approximately 300 cases occurring in the United States annually.Reference Parker, Mai and Canfield1 Truncus arteriosus is further classified using the Van Praagh and Collette and Edwards classification systems.Reference Van Praagh and Van Praagh2, Reference Collett and Edwards3 Various rare aortic anomalies associated with truncus arteriosus have been described; however, no cases of retrograde-dependent aortic flow were documented in our literature review.
Case presentation
A fetal echo was performed at 23 weeks gestation due to suspected fetal heart disease on screening obstetric ultrasound. The fetal echocardiogram showed hypoplastic ascending and transverse aortic arch, membranous ventricular septal defect, and the dysplastic valve thought to be the pulmonary valve with main pulmonary artery dilation. There was no obvious flow seen through the ascending aorta.
The infant was delivered at 39 weeks via vaginal delivery with a birth weight of 3205 grams. Vitals signs included a blood pressure of 68/35 and oxygen saturation ranging from 75 to 90%. Prostaglandin E1 was initiated based on fetal findings. A postnatal echocardiogram revealed situs solitus, D-loop, atrioventricular concordance, and right ventricular hypertrophy. Additionally, there was a single arterial trunk arising from both ventricles with a non-restrictive sub-aortic ventricular septal defect and a large patent ductus arteriosus with bidirectional shunting. The truncal valve appeared significantly dysplastic with severe stenosis, leading to preferential streaming into the main pulmonary artery. The peak instantaneous pressure gradient across the truncal valve was 84 mmHg with a mean gradient of 43 mmHg. There was trivial truncal valve regurgitation. There was a left aortic arch with mild hypoplasia of the ascending aorta and a transverse arch supplied exclusively via retrograde filling through the patent ductus arteriosus (Fig 1).
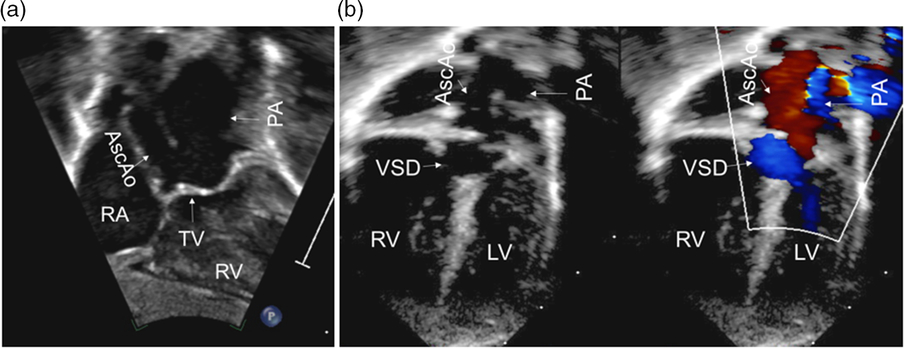
Figure 1. Subcostal coronal view (a) showing the anatomy of the TA. Apical color compare view (b) showing the antegrade flow through the PA (blue color) and retrograde flow in the AscAo (red color). AscAo=ascending aorta; LV=left ventricle; PA=pulmonary artery; RA=right atrium; RV=right ventricle; TV=truncal valve; VSD=ventricular septal defect.
Due to the abnormal constellation of findings on echocardiography, a gated cardiac CT angiogram was performed which confirmed the findings of the echocardiography including the potential development of aortic coarctation once the duct closed (Fig 2). The effective orifice of the dysplastic truncal valve measured 1.1 cm2. The findings were consistent with type A1 truncus arteriosus with hypoplastic ascending and transverse aortic arch. On day of life four, the patient underwent complete repair of the truncus arteriosus including ventricular septal defect closure with bovine pericardial patch, truncal valvotomy, aortic arch reconstruction with a large homograft patch, ligation, and resection of the patent ductus arteriosus and placement of an 11-mm right ventricle to pulmonary artery homograft. Intraoperatively, the truncal valve was bicuspid and dysplastic with an incomplete commissure. The post-procedural transesophageal echocardiogram revealed an 8-mmHg gradient across the truncal valve without significant valve regurgitation. Post-operatively, the patient has been recovering well.

Figure 2. Anterior (a) and posterior (b) view of the cardiac CT angiogram showing the hypoplastic aortic arch, MPA and large PDA. Ao=Aorta; DAo=Descending aorta; LA=left atrium; LPA=left pulmonary artery; MPA=main pulmonary artery; PDA=patent ductus arteriosus; RPA=right pulmonary artery.
Discussion
The process of pulmonary and aortic septation in embryogenesis is known to be mediated via neural crest cell migration to the pharyngeal arches, developing aortic arches and outflow tract of the developing heart.Reference Collins-Nakai and McLaughlin4 Lack of neural crest cell migration to these areas is known to lead to defects such as truncus arteriosus and aortic arch abnormalities.Reference Collins-Nakai and McLaughlin4 The relationship of the aortic arch (fourth pharyngeal arch) and ductus arteriosus (sixth pharyngeal arch) development in hearts with truncus arteriosus is inversely proportional.Reference Calder, Van Praagh and Van Praagh5 This relationship is present in our case. It is likely that the abnormal development of the primitive arches due to improper neural crest cell migration during cardiogenesis may have led to the pharyngeal arch inequality seen in this case.
Various aortic arch anomalies and haemodynamic flow patterns have been associated with truncus arteriosus. These anatomical variants range from benign right-sided arches (~30%) to severe interrupted arches (10–15%).Reference Konstantinov, Karamlou and Blackstone6, Reference Butto, Lucas and Edwards7 Interrupted arches require a patent ductus arteriosus in order to provide blood supply to the descending aortic arch. Although the arch was not interrupted in our case, a patent ductus arteriosus was still required in order to provide flow to the hypoplastic ascending and transverse arch. More rarely, truncus arteriosus may also be associated with a double aortic arch where flow is bidirectionally split into two aortic arches, although it may favour one side over the other depending on resistance.Reference Alboliras, Lombardo and Antillon8 These aortic anomalies all have variation in haemodynamic flow patterns; however, they are all described as having antegrade flow supplying the aorta.
In the absence of aortic and pulmonary anomalies, the presence of a patent ductus arteriosus associated with truncus arteriosus is rare and only reported in approximately 2% of cases.Reference Mello, McElhinney, Parry, Silverman and Hanley9
Interestingly, there has been a report of truncus arteriosus with pulmonary atresia forming retrograde flow through the ductus arteriosus which then supplied the downstream pulmonary circulation.Reference Tongsong, Sirichotiyakul, Sukpan and Sittiwangkul10 This is a similar compensatory flow pattern as the one in our case. It is certainly possible that the absence of in utero antegrade aortic flow through the dysplastic truncal valve resulted in a large patent ductus arteriosus which supplied the ascending aorta in a retrograde manner.
The truncal valve is described as being bicuspid (7%), tricuspid (68%), or quadricuspid (25%), although occasionally more than four leaflets have also been reported.Reference Van Praagh and Van Praagh2 Severe valvular dysplasia has been described as being more common in cases with two or four leaflets than those with three.Reference Butto, Lucas and Edwards7 Our patient had a bicuspid dysplastic valve with incomplete commissure. We believe that the incomplete valve commissure in our case led to occlusion of flow through the truncal valve and subsequent preferential flow through the main pulmonary artery.
Conclusion
This fascinating case of retrograde aortic flow in truncus arteriosus has never been previously reported. Identifying variations in truncus arteriosus such as this is important in guiding both medical management and surgical planning. Valve dysplasia as described in this case should also entertain those of other potentially affected diagnoses including aortic atresia and ventricular septal defects with dysplastic pulmonary valves. Complimentary imaging modalities such as cardiac CT and MRI can aid in characterising these rare cases for optimal diagnosis and management.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.




