Introduction
The introduction of the fetal programming hypothesis in epidemiology and developmental biology has generated new interest in the role of phenotypic plasticity, the mechanisms that govern it, and its place in evolutionary biology. Several studies link low birth weight, assumed to be a consequence of constrained prenatal energy availability, with adverse effects on the risk of chronic diseases later in life.Reference Lesage, Sebaai and Leonhardt 1 – Reference Seckl 6 Many adverse events associated with pregnancy may be involved with pathological fetal programming, as shown in several experimental models.Reference Godfrey, Robinson, Barker, Osmond and Cox 7 – Reference Woodall, Johnston, Breier and Gluckman 12 Although the precise underlying mechanisms are still unclear, maternal undernutrition and hormonal deregulation have been proposed to induce intrauterine fetal programming.
The brain is responsive to gestational programmings and many humoral factors, such as angiotensin II, steroids and their receptors, transcriptional factors, and fluctuations in nutrient availability that can permanently affect neural development.Reference Matsumoto 13 , Reference Welberg and Seckl 14 In gestational protein restriction [low protein (LP)] models, a decreased activity and expression of the placental type-2 11-β-steroid-dehydrogenase has been demonstrated, resulting in excessive exposure of fetuses to maternal steroids.Reference Langley-Evans, Phillips and Benediktsson 8 , Reference Langley-Evans 15 – Reference Benediktsson, Lindsay, Noble, Seckl and Edwards 17 Among the neural structures, the hippocampus–hypothalamic–pituitary–adrenal (HHPA) axis has been demonstrated to be particularly susceptible to the programming effects of gestational stressors.Reference Darnaudéry and Maccari 18 Animal studies have long suggested that maternal emotional and nutritional stresses during pregnancy are associated with behavioral outcomes in offspring.Reference Kapoor, Dunn, Kostaki, Andrews and Matthews 19 Previously, Oliveira et al.Reference Oliveira, Rodrigues and Leão 20 demonstrated that subcutaneous injection of dexamethasone in pregnant dams modulated anxiety-like and fear behavior, in parallel to bed nucleus of the stria terminalis (BNST) and amygdala morphological, neurochemical and molecular changes in rats. Fear and anxiety represent specific aspects of emotional response that show behavioral similarity but are triggered by events that activate distinct neuronal pathways. Although the amygdala plays a crucial role in fear-conditioned to cue stimuli, the BNST is implicated in anxiety behavior and responses to contextual stimuli. Anatomically, the BNST is heterogeneous, containing many sub-nuclei that are modulated by an abundant diversity of neurotransmitters, such as serotonin and their receptors 5HT1A and 5HT2A. The BNST mediates anxiety-like behavior in rats and humans.Reference Duvarci, Bauer and Paré 21 – Reference Walker and Davis 25 Besides, by modulating corticotrophin-releasing factor (CRH)-expressing neurons in the paraventricular hypothalamic nucleus (PVN), the BNST plays a role in the regulation of HHPA axis.Reference Dunn 26 , Reference Herman, Cullinan and Watson 27
In the current study, to search whether BNST structural changes and neurochemical alterations are associated with anxiety-related behavior in adult gestational protein-restricted offspring, we conduct behavioral tests and BNST dendritic tree analysis, associated to immunoblotting–protein quantification (11β-HSD2, GR, MR, AT1R, 5HT1A and 5HT2A, CRH and CRH1).
Methods
Animals and diets
The Institutional Ethics Committee (CEUA/UNICAMP #29/08) approved the experimental protocol, and the general research guidelines established by the Brazilian College of Animal Experimentation were followed throughout the investigation. The experiments were conducted as described in detail previouslyReference Lopes, Torres and Rodrigues 28 on age-matched rats of 12-week-old sibling-mated Wistar HanUnib rats (250–300 g). Briefly, after weaning at 3 weeks of age, animals were maintained under controlled temperature (25°C) and lighting conditions (07:00–19:00 h, with free access to tap water and standard rodent chow (Nuvital, Curitiba, PR, Brazil). The 12-week-old dams were kept throughout the entire pregnancy on an isocaloric rodent laboratory chow ad libitum, with either normal protein content NP (n=20) (17% protein) or LP content LP (n=20) (6% protein). We designated day 1 of pregnancy as the day in which the vaginal smear showed sperm. The NP and LP maternal food consumption were determined daily (food intake data by 100 g of body weight), and body weight of dams was recorded weekly in both groups. All maternal groups returned to the NP diet intake after delivery. The male pups were weaned at 3 weeks, and only one offspring from each litter was used for each behavioral, hormonal and immunoblotting experiment. The male offspring also were followed and maintained with standard rodent chow under a controlled temperature (25°C), a 12-h light–dark cycle (07:00–19:00), and housed in cages with four rats until 16 weeks of age. The NP and LP offspring were handled weekly by a familiar person and followed until 16 weeks of age when behavioral tests were performed. The male pups were weighed at birth and weekly thereafter. Moreover, the anogenital distance was measured. Offspring food consumption was monitored daily and normalized to body weight.
Behavioral analysis
The experiments were conducted as described in http://www.bio-protocol.org/e1211. One male offspring from each litter and different mothers, NP (n=13) and LP (n=12) were used for behavioral analysis. The behavioral tests always were performed during the light cycle. The illumination in the test room was kept constant and controlled under low-intensity white lighting (5–30 Lux).
Open field test
Animals were tested individually for 5 min each in an open field arena (96 cm diameter and 57 cm across the center; model ENV-515; Med Associates Inc., St Albans, VT, USA). Each subject was placed initially in the center of the arena. Horizontal activity, and the instantaneous position was registered using a system of two 16 beam infrared arrays connected to a computer. Times and distances used as indicators of locomotor activity, in the predefined central and peripheral areas were recorded and used to calculate the ratio of time spent in the central area over the total time of the trial, and distance traveled in the central area as a function of the total area. Number and duration of rearing were recorded.
Elevated plus maze (EPM)
Animals were tested over 5 min in the EPM, a black polypropylene ‘plus’-shaped maze (ENV-560; Med Associates Inc.) at the height of 72 cm above the floor. The maze consisted of two facing open arms (50.8×10.2 cm) and two closed arms (50.8×10.2×40.6 cm). The time spent in the open arms, junction area, and closed arms, as well as the number of entrances and explorations in each section, were recorded using a system of infrared photo beams, the crossings of which were monitored by computer.
Histological procedures
In all, 16-week-old NP and LP offspring were anesthetized with a mixture of ketamine (75 mg/kg body weight, i.p.) and xylazine (10 mg/kg body weight, i.p.) and the level of anesthesia was controlled by monitoring the corneal reflex. The offspring were perfused transcardially with 0.9% saline, and the brains were weighed and immediately processed for Golgi–Cox staining according to a published protocol.Reference Gibbs and Kolb 29 Briefly, removed brains were immersed in Golgi–Cox solution; then transferred to 30% sucrose solution in 0.5% sodium azide (3 days) before being cut on a vibratome. Coronal vibratome sections (200 μm thick) were collected in 6% sucrose and blotted dry onto gelatin-coated microscope slides. These slides were subsequently alkalinized in 18.7% ammonia, developed in Dektol (Kodak, Linda-Velha, Portugal), fixed in Kodak Rapid Fix (prepared to manufacturer’s instructions), dehydrated through a graded series of ethanol, and cleared in xylene before being mounted and cover-slipped. A histological study was carried out as a blind analysis using coded slides before morphometric analysis in both sets.
Dendritic tree analysis
Dendritic tree analysis by three-dimensional reconstructions of representative Golgi-impregnated neurons from the BNST was performed. The criteria used to select neurons for reconstruction were as follows: (a) full impregnation of the neurons along the entire length of the dendritic tree, (b) dendrites without significant truncation of branches, (c) relative isolation from neighboring impregnated neurons to avoid interference with the analysis and (d) no morphological changes attributable to incomplete dendritic impregnation of Golgi–Cox stain.Reference Pêgo, Morgado and Pinto 22 Accordingly, we chose neurons with bipolar conformation confined to the anteromedial area for dendritic analysis using the following criteria: (i) presence of transverse anterior commissure, (ii) location rostral to the stria terminalis main bundle, and (iii) selection of neurons adjacent to the anterior commissure. These landmarks corresponded to the rostral portion of the medial division described by McDonaldReference McDonald 30 as being populated by cells with individual ovoid soma and polarized dendritic trees that branch sparingly. These cells are characteristically sparsely to moderately spiny, contrasting with the spine- and dendrite-rich cells of the lateral division. For each selected neuron, all branches of the dendritic tree were reconstructed at 600× magnification using a motorized microscope [Carl Zeiss (Jena, Germany) Axioplan 2, with oil immersion objectives], attached to a camera (DXC-390; Sony Co., Konan Minato-ku, Tokyo, Japan) and Neurolucida software (MicroBrightField, Williston, VT, USA). Three-dimensional analysis of the reconstructed neurons was performed using NeuroExplorer software (MicroBrightField). In each brain, neurons of the anteromedial division of the BNST were studied. As a result, in this study, we analyzed 92 BNST bipolar neurons of NP (n=4) and LP (n=4) groups. Two aspects of dendritic morphology were examined and compared between NP and LP groups: the dendritic length and the number of dendritic intersections.
Corticosterone (CORT) serum levels
To avoid any influence of fluctuating baseline CORT levels due to circadian variations, blood samples from a separate group of 16-week-old NP (n=6) and age-matched LP (n=6) male offspring from each litter were collected by tail venipuncture at 08:00 h to assess basal morning time CORT levels. All blood sampling and restraint were performed in a separate room adjacent to the animal housing room, and within a maximum time of 2 min from disturbing the animal’s cage. The blood was quickly centrifuged at high speed (14,800 g) for 5 min to separate the plasma and was kept frozen (−20°C) until the CORT assay was conducted. A double antibody radioimmunoassay method was used to assay CORT serum levels for the rats using a commercial kit (R&D corticosterone SystemsTM Biotechne, Minneapolis, MN, USA). The coefficient of variation (CV) of the test is 7.5%, the analytical sensitivity is 5 ng/ml, and the functional sensitivity is 25 ng/ml with a CV of intermediate precision of 20%.
Tissue extracts
In all, 16-week-old male rats from the NP (n=5) and LP (n=5) groups were used. After euthanasia by cervical dislocation, the BNST dissection was performed on ice with the help of a stereomicroscope, minced coarsely, and homogenized immediately in 10 volumes of solubilization buffer [10 ml/l Triton-X 100, 100 mmol/l Tris[hydroxymethyl]amino-methane (Tris) pH 7.4, 10 mmol/l sodium pyrophosphate, 100 mmol/l sodium fluoride, 10 mmol/l ethylendiaminetetracetic acid, 10 mmol/l sodium vanadate, 2 mmol phenylmethylsulfonyl fluoride and 0.1 mg/ml aprotinin at 4°C, using a polytron PTA 20S generator (model PT 10/35; Brinkmann Instruments, Westbury, NY, USA)] operated at maximum speed for 20 s. The tissue extracts were centrifuged at 11,000 rpm at 4°C for 40 min, and the supernatants were used as the samples.
Immunoblotting
Protein quantification was performed using the Bradford method. Both tissue and total extract samples (250 μg protein) were treated with Laemmli buffer containing 100 mmol/l dithiothreitol, heated in a boiling water bath for 4 min and subjected to 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a Bio-Rad minigel apparatus (Mini-Protean; Bio-Rad, Hercules, CA, USA). Electrotransfer of proteins from the gel to nitrocellulose membranes was performed for 90 min at 120 V (constant) in a Bio-Rad miniature transfer apparatus (Mini-Protean). Non-specific protein binding to the nitrocellulose was reduced by preincubating the filter for 2 h at 22°C in blocking buffer (5% non-fat dry milk, 10 mmol/l Tris, 150 mmol/l NaCl and 0.02% Tween 20). The nitrocellulose blots were incubated at 4°C overnight with primary antibodies against 11β-HSD2 (Santa Cruz anti-rabbit, 1:10,000), GR (H-300-Santa Cruz 8992, anti-rabbit, 1:300), MR (H-300-Santa Cruz, anti-rabbit, 1:300), AT1 goat, 5HT1A (ab101914, anti-goat,1:1.000), 5HT2A (ab160228 anti-rabbit, 1:1.000), CRH (S-19-Santa Cruz 1761, anti-goat 1:500) and CRH1 (ab59023 anti-goat 1:500) diluted in blocking buffer (3% non-fat dry milk, 10 mmol/l Tris, 150 mmol/l NaCl and 0.02% Tween 20) and anti-α tubulin antibody (ab7291) as loading control. Immunoreactivity bands were detected using the enhanced chemiluminescence method (RPN 2108 ECL Western blotting analysis system; Amersham Biosciences, Little Chalfont, Amersham, UK) and band intensities were quantified by optical densitometry (Scion Image Corporation, Frederick, MD, USA).
Data presentation and statistical analysis
All data were normally distributed (Kolmogorov–Smirnov test) and hence was analyzed using parametric tests and reported as means±SD. Data obtained over time were analyzed using repeated measures analysis of variance (ANOVA). When statistically significant differences were indicated between selected means by ANOVA, post-hoc comparisons were performed with Bonferroni’s contrast test. Comparisons involving only two means within or between groups were carried out using a Student’s t-test. Statistical significance for segmental dendritic plots (Sholl analysis) was obtained from mixed factors (within and between subject factors) repeated measures ANOVA with adequate Greenhouse–Geisser corrections on the significant values. Data analysis was performed with GraphPad Prism 5.00 for Windows (1992–2007 GraphPad Software Inc., La Jolla, CA, USA). The level of significance was set at P⩽0.05.
Results
Gestational LP diet affects birth weight, but the not adult body and brain weights
Daily maternal food intake by 100 g of body weight (NP: 10±4 g.100−1 g of body weight and LP 9±2 g.100−1 g of body weight) were similar in both groups of dams. Birth weight of the LP male pups (n=70) was significantly reduced when compared to NP male pups (n=83) (NP: 6±0.05 g, n=83 v. LP: 5.8±0.05 g, n=70, P=0.006, Fig. 1a). However, no difference in body weight of offspring was observed beyond the sixth week of life (NP: 185±12.6 g, n=26 v. LP: 185±14 g, n=29, P=0.1786, Fig. 1b). In 16-week-old offspring, the LP brain weight (0.5658±0.02613 g, n=16) was unchanged when compared to age-matched NP brains (0.5278±0.03704 g, n=25, P=0.3934).

Fig. 1 Offspring body weight (in g) at birth (a) and from 6 weeks to 16 weeks old in normal protein diet (NP) compared with low protein content (LP) offspring (b). The results are expressed as means±SD. Data obtained over time were analyzed using repeated measures analysis of variance (ANOVA). When statistically significant differences were indicated between selected means by ANOVA, post-hoc comparisons were performed with Bonferroni’s contrast test. Comparisons involving two means within or between groups were carried out using a Student’s t-test. The level of significance was set at *P<0.05.
Gestational LP diet triggers an anxiety-related phenotype in adulthood
Open field results of 16-week-old NP (n=13) v. age-matched LP (n=13) offspring. The results, reported as mean frequency of rearing, dislocation and entries per square, mean time freeze, or self-grooming and frequency of crossing throughout the open field, were not significantly different between 16-week-old NP and age-matched LP for all parameters analyzed (Table 1). However, the progeny of mothers with LP diets displayed a significant reduction of time spent in open arms as compared with NP animals (LP: 31.62±8.45 v. NP: 57.68±6.85 s, P=0.02, Fig. 2a). Moreover, the analysis of the number entries in open (LP: 2.7±0.7 v. NP: 3.8±0.4, P=0.0001) and in closed (LP: 9±0.5 v. NP: 8±0.6, P=0.0001) arms revealed significant differences between experimental groups (Fig. 2c). No difference was observed between the times spent in closed arms to both offspring groups (Fig. 2b).

Fig. 2 Elevated plus maze performance. Analysis of the progeny of mothers with an low protein content (LP) diet displayed a significant reduction of time spend in the open arms as compared with normal protein diet (NP) animals. Analysis of the number of open and closed arm entries revealed a significant differences between NP and LP groups. One offspring from each litter was used for each behavioral, hormonal, and immunoblotting experiments. A comparison involving two samples of independent observations tends made using a Student’s t-test. The level of significance was set at *P<0.05.
Table 1 Open field results from 16-week-old normal protein diet (NP) (n=13) v. age-matched low protein content (LP) (n=13) offspring

The results are exhibited as the mean frequency of rearing, dislocation, mean time freeze, or self-grooming, and frequency of crossing throughout the open field. No significant differences were found between 16-week-old NP and age-matched LP for all parameters analyzed (Student t-test).
Gestational LP diet triggers dendritic atrophy in BNST neurons
Dendritic tree analysis showed a significant reduction in dendritic length in BNST neurons of rats from the LP group when compared with NP (LP 637.8±37.3 µm, n=45 v. NP 763.0±50.02 µm, n=47) (P=0.04, Fig. 3a). Panel b shows a representative BNST neuron micrograph. Panels c, e, and f have exhibited the intersections and length of the dendritic tree between two progressive circles positioned at intervals of 10 μm radial and 30 μm from perikaryon. Moreover, Panel d depicts the graphic representation from Sholl analysis that revealed a reduction of dendritic intersections (between 180 and 330 μm from the perikarya) in LP when compared with that observed in age-matched NP offspring (Fig. 3d).
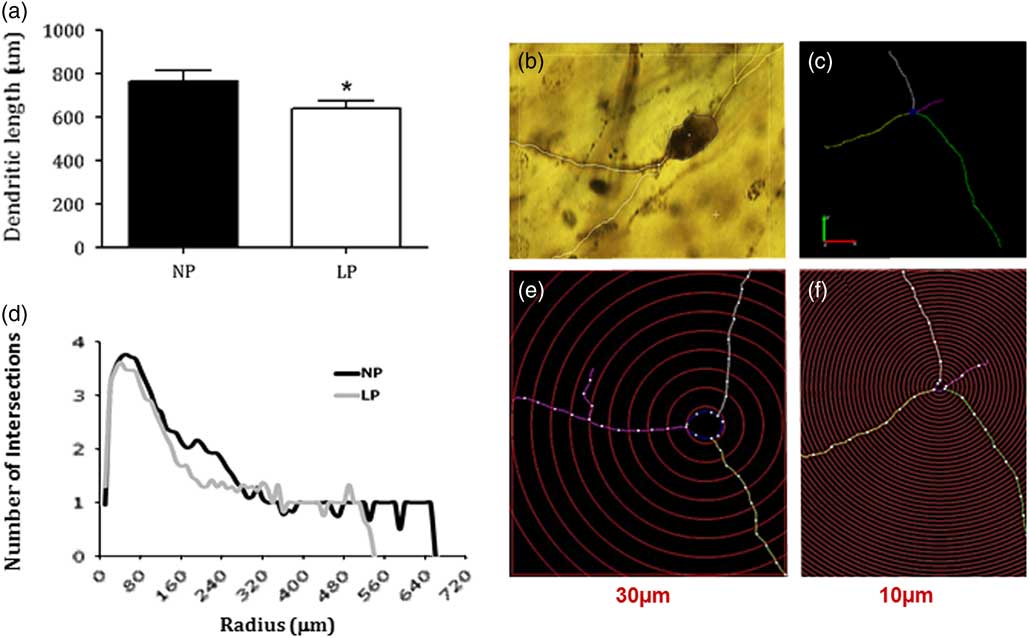
Fig. 3 Graphic depicts of the dendritic length of bed nucleus of the stria terminalis (BNST) neurons (a). Data expressed as mean±SD. A representative BNST neuron micrograph (b). The intersections and length of the dendritic tree between two progressive circles positioned at intervals of 10 μm radial and 30 μm from perikaryon (c, e, and f). The graphic showed from Sholl analysis (d). One offspring from each litter was used for histological analysis. Statistical analysis of segmental dendritic plots was conducted using repeated measures analysis of variance with adequate Greenhouse–Geisser corrections on the significant values. The level of significance was set at *P<0.05. NP, normal protein diet; LP, low protein content.
Gestational LP diet effect on CORT serum levels in adulthood
Basal diurnal CORT measurements revealed significant differences between the studied groups (Fig. 4), with LP animals displaying higher CORT serum levels in adult offspring when compared to NP rats (P=0.013).

Fig. 4 Basal plasma corticosterone levels in low protein content (LP) offspring (n=5) compared with the age-matched normal protein diet (NP) (n=6) group. One offspring from each litter was used for hormonal analysis. Data showed as mean±SD. Comparisons involving two means were carried out using a Student’s t-test. The level of significance was set at *P<0.05.
Western blot analysis
Western blot analysis in isolated BNST from 16-week-old LP offspring revealed 31% higher GR expression (n=6; P=0.044) and, respectively, 70, 84 and 33% reduced MR (P=0.0460), CRH1 receptor (P=0.042) and 11β-HSD2 (P=0.05) BNST expressions when compared with NP age-matched group (Fig. 5). The mean expression of BNTS 5HT1A and AT1R receptors was lower in LP relative to NP rats, but these differences are not statistically significant (Fig. 5). Moreover, the expression of 5HT2A and CRH were the same in both experimental groups (Fig. 5).
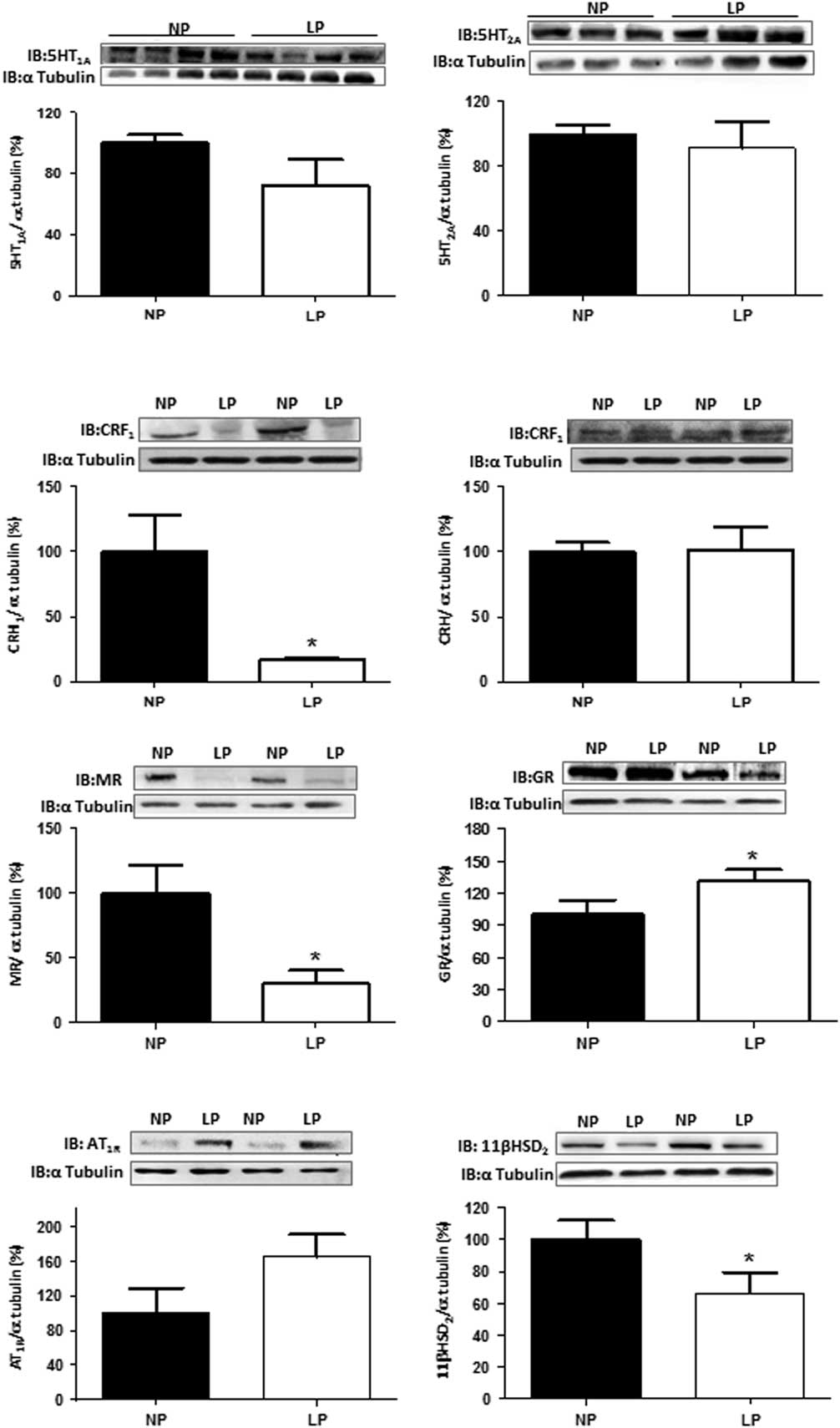
Fig. 5 Protein expression patterns in isolated bed nucleus of the stria terminalis from 16-week-old low protein content (LP) compared with age-matched normal protein diet (NP) rats. The results are expressed as means±SD. One offspring from each litter was used for immunoblotting experiments. Comparisons involving two means were carried out using a Student’s t-test. The level of significance was set at *P<0.05.
Discussion
The occurrence of maternal nutritional deficiency, especially reduced protein intake during the critical period of brain development, may result in morphological and neurochemical changes and, behavioral disorders.Reference Almeida, Tonkiss and Galler 31 – Reference Strupp and Levitsky 34 Studies have demonstrated that gestational protein restriction is followed by low birth weight in rats that lead to gender-related changes in blood pressure, glucose metabolism, and anxiety-like behaviors in male compared to female offspring.Reference Gillette, Reilly and Topper 35 – Reference Ozaki, Nishina, Hanson and Poston 37 Sex hormones contribute to a sexual phenotype dimorphism in the fetal programming model of adult disease by modulating regulatory pathways critical in the long-term control of neural, cardiovascular, and metabolic functions. Thus, this study was conducted only in male rats to ward-off interference from gender differences.
The current study confirms a reduced birth weight of rats whose mothers were fed a gestational restricted-protein diet compared to an NP intake.Reference Mesquita, Gontijo and Boer 9 , Reference Mesquita, Gontijo and Boer 10 , Reference Lopes, Torres and Rodrigues 28 However, beyond the sixth week of age, body mass in both groups was the same; a phenomenon known as catch-up growth. Moreover, we did not find differences in brain weight of 16-week-old LP rats when compared with age-matched NP offspring.
BNST is a rostral forebrain structure recognized as a relay station that links amygdala and hippocampus with PVN and brain stem regions.Reference Herman, Cullinan and Watson 27 , Reference Davis, Walker and Lee 38 , Reference Pacak, Palkovits, Kopin and Goldstein 39 Stimulation of BNST mimics stress-induced neuroendocrine and autonomic responses in rats.Reference Allen and Cechetto 40 , Reference Stoddard, Bergdall, Townsend and Levin 41 In the present study, the observation of adult progeny of mothers exposed to LP diet during gestation revealed an anxiety-like phenotype when compared with age-matched NP offspring. Thus, in the elevated plus-maze test, a significantly reduced time spent in open arms associated with an enhanced number of entries in protect-arms confirm the exacerbated anxiety-related behavior of LP offspring when compared with age-matched NP rats (Fig. 2).
Given the role of the BNST neurons in determining anxiety-like behavior,Reference Pêgo, Morgado and Pinto 22 , Reference Davis, Walker and Lee 38 we underwent a three-dimensional morphometric analysis of dendritic arborization in this brain nucleus. As demonstrated by the Golgi–Cox technique, the current study showed a significant reduction in dendritic length in BNST neurons from the LP offspring. The Sholl analysis also revealed a decrease in dendritic arborization, with a 16% reduction of the intersections of the dendritic tree in LP compared with age-matched NP offspring. To the best of our knowledge, there is no prior ontogenetic description of BNST cell plasticity in the adult offspring of protein-restricted mothers.
The current data are in line with previous studies demonstrating that protein restriction in pregnant rats resulted in elevated plasma corticosterone levels in adult offspring.Reference Langley-Evans, Phillips and Benediktsson 8 , Reference Lopes, Torres and Rodrigues 28 This study also confirms previous data from chronic gestational exposure to pharmacological doses of glucocorticoid (GC), in which changes in BNST plasticity were associated with increased plasma CORT levels in offspring.Reference Fameli, Kitraki and Stylianopoulou 42 , Reference Hammack, Roman and Lezak 43 It has been demonstrated that neurons in specific brain regions are vulnerable to GC excess. This effect has been implicated in behavioral disorders and neuron loss or atrophy,Reference Issa, Rowe, Gauthier and Meaney 44 – Reference Sapolsky 47 which can be prevented by adrenalectomy.Reference Landfield, Baskin and Pitler 48 The present study reaffirms the hypothesis that increased corticosterone levels, in maternal underfeeding offspring, are associated with reduced dendritic complexity and length in the BNST neuronsReference Vyas, Mitra, Shankaranarayana Rao and Chattarji 49 , Reference Vyas, Bernal and Chattarji 50 and enhanced anxiety-like responses in LP offspring.Reference Shepard, Chambers, Busch, Mount and Schulkin 51
Nutritional stress in adult rats caused a persistent and parallel increase in corticosterone levels and in the number of neurons expressing CRH, a peptide known to promote spine and dendrite loss.Reference Chen, Dubé, Rice and Baram 52 Surprisingly, in the present study, we found a striking reduction of BNST MR and type 1 CRH receptor expression associated with enhanced GR and unchanged CRH expression in 16-week-old LP offspring compared with controls. Our results suggest that BNST neurons of undernourished maternal offspring could be exposed to high levels of CORT, and these levels might suffice to stunt dendritic arborization associated with observed anxiety behavior.
11β-HSD2 acts as a major barrier to GC reaching the fetus and the neonate central nervous system expression.Reference Diaz, Brown and Seckl 53 – Reference Brown, Kotolevtsev and Leckie 55 Previous studies in the adult rodent brain hypothesized that there was an even more limited 11β-HSD2 expression confined to the cerebral areas, consistent with the central regulation of blood pressure and salt balance mediated by aldosterone and not reproduced by CORT, indicating aldosterone-selective MR in these specific actions.Reference Rowland and Fregly 56 However, the current study demonstrated the presence of 11β-HSD2 expression in the BNST of NP and LP offspring. Noticeably, the increased levels of systemic and local CORT levels (by decreased 11β-HSD2), along with an enhanced GR/MR ratio, likely may be of relevance for the increase in anxiety-like behavior in the EPM test, observed here in LP relative to NP offspring. We hypothesize that in gestational protein-restricted offspring, the reduced cerebral 11β-HSD2 expression compared with the NP group may maintain an elevated CORT brain concentration associated with stressful environments ex-uteri, and thus program the hypothalamic–pituitary–adrenal axis to alter the release of GC throughout life.
In conclusion, this work represents the first demonstration that BNST developmental plasticity is associated with gestational protein restriction, and it indicates fine structural changes and neurochemical alterations as potential underlying causes of the modified behavioral states. More thorough knowledge of these events is of paramount importance to determine therapeutic interventions that might mitigate such behavioral impairments.
Acknowledgements
None.
Authors’ contributions
D.B.T.: data curation, investigation, methodology, visualization, writing–original draft; A.L.: data curation, investigation, methodology; A.J.R.: investigation; J.J.C.: investigation; N.S.: investigation; J.A.R.G.: formal analysis, methodology, visualization, writing–original draft, writing–review and editing; P.A.B.: conceptualization, formal analysis, funding acquisition, methodology, resources, supervision, visualization, writing–original draft, writing–review and editing.
Financial Support
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (2005/54362-4 and 2013/12486-5) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of Interest
None.
Ethical Standards
The Institutional Ethics Committee (CEUA/UNICAMP #29/08) approved the experimental protocol; the general guidelines established by the Brazilian College of Animal Experimentation were followed throughout the investigation.








