Introduction
Temperature fluctuation is an absolute requirement for germination of many species (Probert, Reference Probert and Fenner1992). The ecological significance of this feature is associated with the strategies of seeds to detect canopy gaps, depth of burial of seeds in the soil or their positions under water (Roberts and Totterdell, Reference Roberts and Totterdell1981; Thompson and Grime, Reference Thompson and Grime1983; Pons and Schroder, Reference Pons and Schroder1985). Near to the soil surface, diurnal temperatures fluctuate less below a vegetation cover, or under water, than on bare soils (Balisky and Burton, Reference Balisky and Burton1993). While seed responses to fluctuating temperatures are widespread among many species, physiological and biochemical mechanisms underlying such responses are still largely unknown. Benech-Arnold et al. (Reference Benech-Arnold, Steinbach, Kristof and Sánchez1995) reported that fluctuating temperatures stimulate germination of immature dormant sorghum caryopses by reducing embryo sensitivity to abscisic acid (ABA). More recently, Huarte and Benech-Arnold (Reference Huarte and Benech-Arnold2005) and Huarte (Reference Huarte2006) performed a water relation analysis of seed germination at fluctuating and constant temperatures using Gummerson's hydrotime model (Gummerson, Reference Gummerson1986). In such an assessment, the authors found that incubation at fluctuating temperatures reduced seed mean base water potential ψb(50). This finding implies that fluctuating temperatures promote germination through an enhancement of embryo potential to overcome a physical restraint for germination.
Germination is promoted by gibberellins (GAs) and inhibited by ABA. GA de novo synthesis appears to be an important requirement for dormancy release (Jacobsen and Olszewski, 1993). Inhibitory effects of GA biosynthesis inhibitors such as paclobutrazol (PCB) and uniconazole on seed germination have been observed (Nambara et al., Reference Nambara, Akazawa and McCourt1991). Kucera et al. (Reference Kucera, Cohn and Leubner-Metzger2005) defined ABA as a positive regulator of dormancy induction. ABA plays a crucial role both in the acquisition and maintenance of seed dormancy (Le Page-Degivry et al., Reference Le Page-Degivry, Bianco, Barthe, Garello and Lang1996; Grappin et al., Reference Grappin, Bouinot, Sotta, Miginiac and Jullien2000). The role of ABA in dormancy induction was clearly demonstrated by the reduced dormancy in ABA-deficient or -insensitive mutants (Li and Foley, Reference Li and Foley1997). Dormancy maintenance in dormant ecotypes of Arabidopsis (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004) and other species requires de novo ABA synthesis upon imbibition (Le Page-Degivry et al., Reference Le Page-Degivry, Bianco and Barthe1997; Grappin et al., Reference Grappin, Bouinot, Sotta, Miginiac and Jullien2000). ABA is synthesized from a C40 carotenoid precursor; hence, chemical inhibitors of carotenoid biosynthesis, such as fluridone, inhibit ABA accumulation (Zeevaart, Reference Zeevaart1988). ABA effects are related to its endogenous level and seed sensitivity to its action (Corbineau et al., Reference Corbineau, Bianco, Garello and Come2002). From these and many other results, it was concluded that the maintenance of a dormancy state depends on high ABA–GA ratios and/or high ABA sensitivity. Dormancy release is associated with increased GA biosynthesis and ABA degradation, both of which contribute to low ABA–GA ratios, and a high GA sensitivity (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). This balance is also modulated by environmental factors. Toyomasu et al. (Reference Toyomasu, Tsuji, Yamane, Nakayama, Yamaguchi, Murofushi, Takahashi and Inoue1993, Reference Toyomasu, Kawaide, Mitsuhashi, Inoue and Kamiya1998) and Yamaguchi et al. (Reference Yamaguchi, Smith, Brown, Kamiya and Sun1998) have found that the promotion of germination by Pfr, the active form of phytochrome, after seed exposure to red light, is mediated by an increase in GA biosynthesis, due to the up-regulation of genes encoding GA3 β-hydroxylase, a key enzyme in the production of active GAs. This increases embryo growth potential and decreases a physical restraint for germination by the covering tissues of the seed. Pfr enhancement of embryo capacity to overcome a physical restraint for germination is also through a reduction in embryo sensitivity to ABA (Sánchez and Mella, Reference Sánchez, Mella, Benech-Arnold and Sánchez2004) and an increase in embryo sensitivity to GA (Yang et al., Reference Yang, Nagatani, Zhao, Kendrick and Kamiya1995; Arana et al., Reference Arana, de Miguel and Sánchez2006). Since fluctuating temperatures promote germination through an enhancement of embryo capacity to overcome a physical restraint, it is possible that this effect is also mediated by hormonal regulation. We hypothesized that seed responses to fluctuating temperatures in terms of dormancy termination are through the elicitation of a set of physiological processes that are similar to those underlying seed responses to red light.
In this study, we aimed to determine: (1) ABA content in and sensitivity of Cynara cardunculus seeds during incubation at fluctuating or constant temperatures; (2) seed responses to exogenous GA at constant or fluctuating temperatures; and (3) whether a fluctuating temperature requirement can be cancelled by red light or GA.
Materials and methods
Plant materials
C. cardunculus (L.) mature achenes (hereafter termed as seeds) were hand collected during January 2006 from an infested roadside at Alejandro Petion, Buenos Aires Province, Argentina (34°59′S, 58°40′W). After the initial cleaning, seeds were kept for 3 months in paper bags at room temperature (20 ± 2°C) before use. Three independent experiments were conducted during 2006.
Germination tests
Germination was expressed as cumulative percentage of total seeds. Each value was mean ± SE of three replicates of 25 seeds each. Germination was scored during 14 consecutive days. Seeds with visible radicle protrusion were considered to have germinated and were removed. Germination tests were performed in germination chambers at 20°C (constant temperature) or at fluctuating temperatures (25°C, 12 h/15°C, 12 h). Seeds were incubated in the dark in 9-cm Petri dishes covered with plastic film to prevent evaporation.
Hydrotime analysis
To determine the effects of fluctuating temperatures (25/15°C) on hydrotime parameters, seeds were placed on dishes containing water or different concentrations of polyethylene glycol solutions (PEG8000, Anedra, Buenos Aires, Argentina), which were prepared according to the Michel (Reference Michel1983) equation. The resulting osmotic potential was measured with a vapour-pressure osmometer (VPO, Model 5100 C, Wescor Inc., Logan, Utah, USA) calibrated with sodium chloride solutions. PEG solutions were replaced after 24 h and every 6 d thereafter. Hydrotime parameters were also determined in seeds incubated at a constant temperature (20°C). Under these conditions, the parameters were also calculated for red-light-treated, GA3-treated (throughout incubation) and dark-treated seeds. Irradiated seeds were pre-incubated in distilled water in darkness at 20°C for 24 h. After pre-incubation, seeds were irradiated for 2 h with a red light provided by Philips 40/15 40 W fluorescent lamps (Philips, Eindhoven, The Netherlands) to obtain a calculated proportion of Pfr of 0.87 (Casal et al., Reference Casal, Sánchez, Di Benedetto and Miguel1991). After light exposure, seeds were transferred to Petri dishes containing distilled water or the different concentrations of PEG solutions. All practices including germination counting were carried out under a green safety light. To determine the effects of GA3 on hydrotime parameters, a mixed solution of GA3 plus PEG was prepared by dissolving the PEG required according to the Michel equation in 100 μM GA3. GA3 plus PEG solution was replaced after 24 h and every 6 d thereafter.
Germination time courses were analysed according to the hydrotime model using Solver Tool of 2003 Microsoft Excel®. This module allows maximizing the fit between simulated values and experimentally recorded values. The optimization criterion used to obtain the best fit was minimum root-mean-square error (RMSE) between simulated and experimentally obtained data.
ABA, GA3, fluridone and paclobutrazol treatments
Sensitivity of seeds incubated at either fluctuating or constant temperatures to (+)-cis, trans-ABA (Sigma Chemical Company, St. Louis, Missouri, USA) was evaluated through incubation in 6 ml of ABA solutions at different concentrations (0, 1, 50 and 100 μM). Sensitivity of seeds to GA3 at fluctuating or constant temperature was determined through incubation in 6 ml of GA3 solutions at different concentrations (0, 1, 25, 50 and 100 μM). Seeds were also incubated in the presence of 6 ml of 50 μM fluridone {1-methyl-3-phenyl-5-[3-triuoromethyl-(phenyl)(-4-(1H)-pyridinone} (Phytotechnology Laboratories, Shawnee Mission, Kansas, USA) and 68 μM paclobutrazol (PCB) [2RS, 3RS-1-(4-clorofenil)-4,4-dimetil-2-(1H1,2,4-Triazol-1-il)pentan-3ol] (CRESTAR, Syngenta Crop Protection AG, Birsfelden, Switzerland). Fluridone solutions were prepared by dissolving the compound in 0.1% acetone until complete dissolution and then diluting it with water. Control experiments showed no acetone effect on germination. Seed germination in a mixed solution of PCB (68 μM) plus GA3 (50 μM) was also tested for seeds incubated at 25/15°C.
In ABA experiments, the treatments were factorial combinations of four ABA doses and two thermal conditions (20°C vs. 25/15°C). In GA experiments the combinations were five GA doses and two thermal conditions (20°C vs. 25/15°C). The germination data were subjected to analysis of variance (Statistix 8.0, Analytical Software, Tallahassee, Florida, USA). Tukey's test at 5% level of probability was used for comparison between means.
ABA quantification
To quantify ABA content in seeds incubated at fluctuating (25/15°C) or constant (20°C) temperatures, three replicates of approximately 100 mg seeds were collected with 12-h intervals for the first 4 d of incubation. Ungerminated seeds incubated at 20°C were also sampled at 24-h intervals from the fourth day of incubation onwards. The samples were immediately frozen in liquid nitrogen. Seeds were then lyophilized, powdered, weighed and stored at − 18°C until assayed for ABA content with a radio-immunoassay using the monoclonal antibody MAC 252, as described by Steinbach et al. (Reference Steinbach, Benech-Arnold, Kristof, Sánchez and Marcucci Pultri1995). The results presented are the means of three measurements ± SE.
Results
Hydrotime analysis of germination of seeds incubated at fluctuating temperatures and seeds incubated at constant temperatures with red light or GA treatment
Fluctuating temperatures (25/15°C) promoted the final germination percentage of seeds compared to that of seeds incubated at a constant temperature (20°C) (Fig. 1a and d). Seeds incubated at reduced osmotic potentials displayed a decrease in total germination, except for GA3-treated seeds incubated at − 0.3 MPa, (Fig. 1c). Germination data observed for each treatment was analysed using the hydrotime model in order to quantify changes in water relation parameters. The resulting parameters were used to predict seed germination time courses according to: probit (g) = [ψ − (θH/t g) − ψb(50)]/σψb(50), converting probit values back to germination percentages. Incubation at fluctuating temperatures or at constant temperature (20°C) in the presence of GA3 reduced the ψb(50) of the population to a similar extent, compared to that observed for seeds incubated at 20°C without the hormone (Fig. 1h and g). Irradiation with red light prior to incubation at 20°C reduced ψb(50) to a lesser extent than incubation at 25/15°C or in the presence of GA3 (Fig. 1e and f). The reduction in ψb(50) by fluctuating temperatures and GA3 was accompanied by a reduction of their θH compared to the 20°C treatment On the other hand, there were no major changes in σψb between the dark control and the rest of the treatments.

Figure 1 Cumulative germination time courses of Cynara cardunculus seeds incubated at (a) 20°C under water potentials of 0, − 0.3 and − 0.6 MPa; (b) at 20°C under water potentials of 0, − 0.3 and − 0.6 MPa after red light exposure; (c) at 20°C in the presence of 100 μM GA3; and (d) at 25/15°C under water potentials of 0, − 0.3 and 0.6 MPa. The symbols represent the experimental data, and the lines are the time courses predicted by the hydrotime model. (e) to (h) Normal distributions showing the relative frequencies of ψb(g) values corresponding to panels (a) to (d), respectively. The median, standard deviation (δψb), and hydrotime constant (θH) values are shown.
Role of ABA in seed responses to constant or fluctuating temperatures
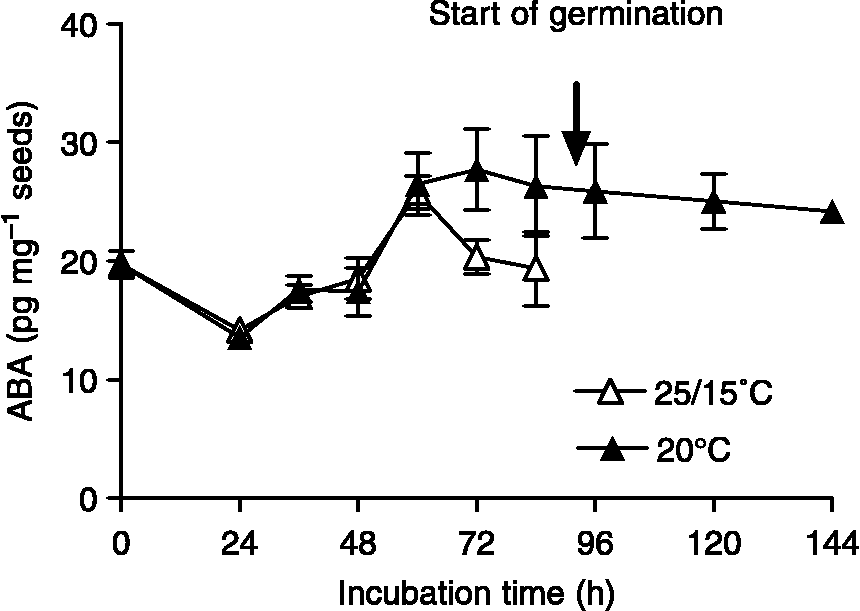
The two main effects, ABA doses and the thermal treatment on germination, were significant (P < 0.001). On the other hand, the interaction between ABA doses and thermal treatments was not significant (P = 0.96). Seeds incubated at fluctuating temperatures exhibited higher germination compared to that of seeds incubated at 20°C at the same ABA concentrations (Fig. 2). Total germination in water (ABA 0 μM) was 96.4 ± 1.7% (mean ± SE) at 25/15°C and 36.8 ± 8.3% at 20°C, respectively. The final germination percentage at 25/15°C dropped from 96.4 ± 1.7% in water to 56.6 ± 8.3% in 100 μM ABA. In contrast, when incubation was performed at 20°C, the final germination percentages in water and 100 μM ABA were 36.8 ± 8.3% and 1.6 ± 1.6%, respectively (Fig. 2). Fluridone (50 μM) promoted germination of seeds incubated at 20°C (85.6 ± 3.8%) (Fig. 2), suggesting that ABA biosynthesis upon seed imbibition might be attributable to the inhibition of germination at 20°C. To examine this possibility, we measured ABA content in seeds incubated at constant and fluctuating temperatures throughout imbibition. ABA content in dry seeds was 19.71 ± 1.16 pg mg− 1 seeds (0 h of imbibition) (Fig. 3). ABA contents in seeds incubated at 25/15°C and 20°C were similar until 60 h after imbibition (Fig. 3). ABA content decreased in seeds incubated at 25/15°C prior to radicle emergence (Fig. 3; timing of radicle emergence indicated by an arrow). While ABA contents in seeds incubated at 20°C were 27.7 ± 3.4 pg mg− 1 seeds at 72 h and 26.3 ± 4.19 pg mg− 1 seeds at 84 h, ABA contents in seeds incubated at 25/15°C were 20.3 ± 1.41 pg mg− 1 seeds at 72 h and 19.37 ± 3.12 pg mg− 1 seeds at 84 h. A steady ABA content was observed in seeds incubated at 20°C after 72 h.

Figure 2 Final germination percentages of Cynara cardunculus seeds incubated at different ABA concentrations. Open bars show germination under fluctuating temperatures (25/15°C) and filled bars show germination scored at a constant temperature (20°C). Error bars indicate SEs. The germination experiments were performed for 14 d.
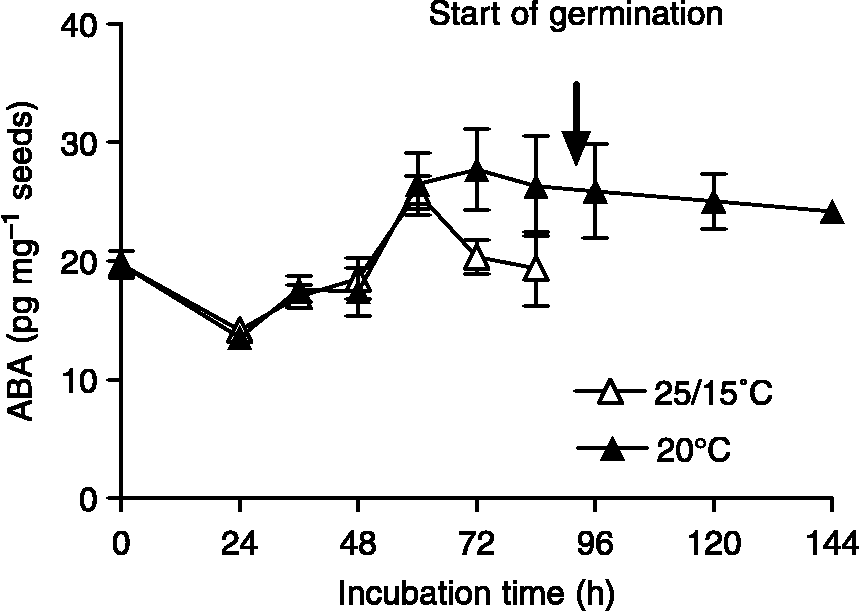
Figure 3 ABA content (pg mg− 1 dry seeds) in Cynara cardunculus seeds at different time points of incubation. Open symbols represent ABA content of seeds incubated at 25/15°C, closed symbols represent ABA content of seeds incubated at 20°C. Data are means of triplicates ± SEs.
Role of GA in seed responses to constant and fluctuating temperatures
The two main effects, thermal treatment (20°C and 25/15°C) and seed responses to GA doses, and their interaction, were statistically significant (P < 0.001). Germination did not differ between 25/15°C and 20°C when seeds were incubated in the presence of GA (1–100 μM). Germination of these seeds in the presence of GA was similar to that observed in seeds incubated in water at 25/15°C (Fig. 4). On the other hand, a reduced total germination was scored both in water at 20°C (31.6 ± 9.2%) (mean ± SE) and PCB (68 μM) (21.3 ± 3.3% and 16 ± 4% for 20°C and 25/15°C, respectively) (Fig. 4). Incubation in the mixture solution of PCB (68 μM) plus GA3 (50 μM) at 25/15°C restored germination (88.8 ± 8%) to a similar extent to that observed in water (90.8 ± 3.3%) (Fig. 4).

Figure 4 Final germination percentages of Cynara cardunculus seeds incubated at fluctuating temperatures (25/15°C) (open bars) or at a constant temperature (20°C) (filled bars) incubated in different solutions (see legend beneath horizontal axis). Vertical bars indicate the SEs. Germination experiments were performed for 14 d. Different letters at the top of each bar indicate significant differences according Tukey's test (α = 0.05).
Discussion
Fluctuating temperatures and light are environmental factors that terminate seed dormancy in many species (Batlla et al., Reference Batlla, Kruk, Benech-Arnold, Benech-Arnold and Sánchez2005). A large body of information is available for light signalling, including its perception and signal transduction. In contrast, little is known about physiological and biochemical mechanisms involved in the responses of seeds to fluctuating temperatures. Our previous work on the enhancement of germination of C. cardunculus by fluctuating temperatures clearly demonstrated that none of the single (constant) temperatures included in the fluctuating temperatures but fluctuation per se released C. cardunculus seeds from dormancy (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2005). The hydrotime model indicated that the promotion of germination by fluctuating temperatures was accompanied by a reduction in ψb, which provides an important physiological implication that fluctuating temperatures stimulate dormancy termination through an enhancement of embryo capacity to overcome either physical or osmotic restraint. Embryo growth potential is positively or negatively affected by GA or ABA, respectively (Karssen et al., Reference Karssen, Zagorski, Kepczynski and Groot1989; Sánchez and de Miguel, Reference Sánchez and de Miguel1997; da Silva et al., Reference da Silva, Toorop, van Aelst and Hilhorst2004). Hormone action could be explained by its content in seed tissues and cells and/or by tissue or cell sensitivity to hormones. In the present study, a clear reduction of ABA sensitivity at fluctuating temperatures was demonstrated (Fig. 2). This is in full agreement with the work of Benech-Arnold et al. (Reference Benech-Arnold, Steinbach, Kristof and Sánchez1995), Romagosa et al. (Reference Romagosa, Prada, Moralejo, Sopena, Muñoz, Casas, Swanston and Molina-Cano2001) and Corbineau et al. (Reference Corbineau, Bianco, Garello and Come2002). These authors also found a reduction in ABA sensitivity and its possible contribution to dormancy release. In contrast, only slight differences in the ABA content were found during seed incubation, which do not seem to be instrumental to explaining the different behaviour of C. cardunculus seeds at fluctuating and constant temperatures. So, ABA synthesis could be important to maintain seed dormancy at a constant temperature. Nevertheless, evidence to support the involvement of ABA in thermodormancy provided by previous reports showed more dramatic changes in ABA content than that reported in this paper (Yoshioka et al., Reference Yoshioka, Endo and Satoh1998; Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004; Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Come and Corbineau2006). Hence, our results suggest that ABA deactivation is not the major mechanism in termination of C. cardunculus dormancy. This is somewhat contradictory to the enhancement of germination observed with fluridone-treated seeds incubated at 20°C (Fig. 2), because fluridone is known to inhibit ABA biosynthesis by blocking carotenoid biosynthesis. However, at least one report points out that fluridone enhances germination of dormant seeds without modifying ABA content (Benech-Arnold et al., Reference Benech-Arnold, Enciso, Sánchez and Weipert1999). The application of exogenous GA3 to seeds incubated at fluctuating temperatures did not increase their germination percentage. On the other hand, GA3 treatment enhanced germination of seeds incubated at a constant temperature (Fig. 4). That is, GA3 replaced the requirement of fluctuating temperatures for seeds to germinate. This suggests that GA biosynthesis is involved in the promotion of germination at fluctuating temperatures. The importance of GA biosynthesis to promote germination under 25/15°C is also supported by the strong inhibition of germination by PCB, which was then completely reversed by co-incubation with GA3. Moreover, the results of the hydrotime analysis, where GA3-treated seeds at 20°C exhibited hydrotime parameters very similar to those obtained for seeds incubated at 25/15°C, strongly support the proposition that GAs are involved in the process. Taking these results together, we propose that fluctuating temperatures terminate dormancy of C. cardunculus seeds mainly by the promotion of GA biosynthesis and a reduction in ABA sensitivity.
Acknowledgements
We are grateful to Silvina Enciso for her guidance to H.R.H. in ABA determination, Sebastian Staltari and María Susana Pereyra for their help during the course of the experiments and Eduardo Fernandez for his help with the statistical analyses.