Introduction
Common vetch (Vicia sativa) is an economically important self-pollinating annual legume forage in the genus Vicia that has a short growth period, strong adaptability and the ability to fix nitrogen and improve soil structure. Common vetch can be used as feed, green manure, hay and farmland cover (Cakmakci et al., Reference Cakmakci, Aydinoglu, Karaca and Bilgen2006; Liu et al., Reference Liu, Ma, Nan and Wang2013, Reference Liu, Liu, Luo, Liu and Wang2014; Dong et al., Reference Dong, Jahufer, Dong, Wang and Liu2016). Due to its excellent traits and wide use, common vetch is widely planted in China, Turkey, New Zealand and other countries, and the area planted in Turkey amounts to 579 684 hectares (Firincioglu et al., Reference Firincioglu2014). This species is commonly planted in the middle and lower reaches of the Yangtze River, North China and Northwest China (Chen and Jia Reference Chen and Jia2002) and is an important pasture and green manure crop in China's high altitude regions.
There are approximately 190 species of Vicia in the world, with approximately 40 species in China (Abozeid et al., Reference Abozeid, Liu, Liu and Tang2017); the genus is mainly distributed in Europe, Asia and North America and extends to temperate South America and tropical Africa (Maxted, Reference Maxted1993; Jaaska, Reference Jaaska2005). The highest specific diversity is found in Turkey and Northwest Asia (Maxted and Hawkes, Reference Maxted and Hawkes1997). Ball (Reference Ball1968) divided the genus into four subgenera: Vicia, Cracca, Ervum and Faba. Afterwards, these species were classified by Kupicha (Reference Kupicha1976) into two subgenera (subgenus Vicilla and subgenus Vicia) and 22 sections. Maxted (Reference Maxted1993) and others further divided the subgenera into nine series, 38 species and 14 subspecies on the basis of phenotypic classification. Common vetch includes a large number of subspecies and accessions (Maxted, Reference Maxted1993) with high diversity, but the taxonomic information between subspecies is not yet complete. Taxonomic studies of four common vetch subspecies have been reported, but studies of other subspecies are still rare.
Conventional morphotypological taxonomy has been used as the basis for taxonomic treatments in the genus Vicia and diagnostic characters chosen on different bases have been used to delimit the subgenera and sections (Jaaska, Reference Jaaska2005). Later, new classification methods were also widely used. For example, certain methods such as DNA RAPD and restriction fragment characteristics by Potokina et al. (Reference Potokina, Tomooka, Vaughan, Alexandrova and Qiang1999) and isozymes by Jaaska (Reference Jaaska1997) have been utilized in studying the taxonomic relationships prevailing between species in the Vicia subgenus. Leht and Jaaska (Reference Leht and Jaaska2002) examined the subgenus based on cladistics and phenetics of both morphological and isozyme characteristics. Chung et al. (Reference Chung, Kim, Suresh, Lee and Cho2013) used next-generation sequencing to develop 65 novel polymorphic cDNA-SSR markers in Vicia sativa subsp. sativa to make further contributions in molecular and breeding genetics studies of this species.
The aim of this study was to carry out a comparative study of the pollen morphology of the 22 taxa belonging to Vicia sativa L. from 15 countries by using light microscopy (LM) and scanning electron microscopy (SEM) and to assess the systematic significance of the examined pollen characteristics in terms of understanding the variation among the sections and taxa. In addition to accumulating information for systematic taxonomy within common vetch species, this work also lays a foundation for related palynological research.
Materials and methods
Plant materials
A total of 20 wild accessions and two local cultivar checks, Lanjian No. 1 and Lanjian No. 3, of common vetch were used in this study (Table 1). The accessions were obtained from the National Plant Germplasm System of the USA and College of Pastoral Agriculture Science and Technology, Lanzhou University of China.
Table 1. Name and origin of 22 common vetch accessions

NPGS, National Plant Germplasm System of the USA.
Field trials
The experimental site was located at Yuzhong Experimental Station (104°09′ E, 35°89′ N, 1720 m a.s.l.), College of Pastoral Agricultural Science and Technology, Lanzhou University, Gansu Province. Yuzhong is located in the western Loess Plateau and belongs to a semi-arid climate zone. The soil type is loess and the area is characterized by an annual average temperature of 6.7 °C, average annual precipitation of 382 mm and average annual evaporation of 1343 mm (Dong et al., Reference Dong, Jahufer, Dong, Wang and Liu2016). The total monthly precipitation and the mean monthly minimum and maximum temperatures during the trial period are shown in Fig. 1.

Fig. 1. Mean monthly minimum temperature (°C), mean monthly maximum temperature (°C) and total monthly precipitation (mm) in 2016 in Yuzhong County.
Experimental method
The experimental layout of the field trial was a randomized complete block design containing three replicates. Each accession of each replicate was planted with 30 individual plants, and the spacing of each individual plant was 50 cm within rows and 50 cm between rows. The experimental materials were sown on 20 April 2016 and pollen samples were collected when the various accessions reached the flowering stage from June to August of the same year. Mature pollen grains were obtained from randomly selected mature flower buds of three individual plants from each accession of each replicate and used as materials for observation by LM and SEM.
Light microscopy
Pollen grains were first treated with 70% alcohol to remove oily substances and then embedded in glycerine jelly stained with basic fuchsin following the method of Wodehouse (Reference Wodehouse1935) (Kahraman et al., Reference Kahraman, Binzat and Doğan2013). The polar axis length (P), equatorial axis length (E), colpus length (Clg) and colpus width (Clt) were observed by a LEICA-DM3000 microscope and 30 pollen grains were repeatedly measured for each accession. Then, the P/E ratio was determined.
Scanning electron microscopy
A double-sided conductive adhesive was used to fix the dried pollen grains on a metal sample stand and low vacuum sputter was used to coat the samples with gold for 90 s (K650X, this is a Quorum Sputter Coating Systems). Samples were observed with a scanning electron microscope (JSM-5600 LV, JEOL SEM), the acceleration voltage was 20 kV and the working distance was 10 mm. We observed 30 pollen grains of each accession in each replicate to determine pollen ornamentation and pole characteristics.
The size of the pollen grains was represented by the polar axis length and equatorial axis length (P × E). The ratio of polar axis length and equatorial axis length (P/E) represented the shape of the pollen grain. A P/E of 0.5 ~ 0.8 is oblate, a P/E of 0.8 ~ 1.0 is oblate spherical and a P/E of 1.0 ~ 2.0 is prolate. The terminology of Punt et al. (Reference Punt, Hoen, Blackmore and Nilssont2007) was used in descriptions of the pollen.
Data processing
Data analysis was based on (1) variance component analysis to assess the significance and magnitude of the genotypic variation among accessions and (2) pattern analysis, which consisted of a combination of cluster analysis and principal component analysis (PCA), to provide a graphical summary of the accession-by-multi trait data matrices (Gabriel, Reference Gabriel1971; Kroonenberg, Reference Kroonenberg1994; Watson et al., Reference Watson, De Lacy, Podlich and Basford1995).
The residual maximum likelihood (REML) option in GenStat 7.1 (2003) was used for variance component analysis. A random linear model was used to analyse data within subspecies data. The analysis was based on best linear unbiased predictors (BLUP) (White and Hodge, Reference White and Hodge1989) and generated accession means for pollen traits. The mixed linear model analysis was also performed with accessions as fixed effects to study the differences among accessions for each of the traits measured. SPSS (22) was used to analyse the measures of dispersion indexes.
According to the Fehr (Reference Fehr1987) method, the variance components of genotypic (${\rm \sigma }_{\rm g}^ 2$![]() ) and experimental error (${\rm \sigma }_{\rm \varepsilon }^ 2$
) and experimental error (${\rm \sigma }_{\rm \varepsilon }^ 2$![]() ) and the number of replications (nr) in the REML analysis were used to estimate the accession mean repeatability (R) of the five traits of each accession:
) and the number of replications (nr) in the REML analysis were used to estimate the accession mean repeatability (R) of the five traits of each accession:

Results and analysis
The results of pollen morphological measurements are given in Table 2. SEM images of the representative pollen grains studied are shown in Figs 2–4.
Table 2. Main characteristics of the pollen morphology in 22 accessions of common vetch

(P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Ev) equatorial view, (Pv) polar view, (Clg) colpus length, (Clt) colpus width, (Orn) ornamentation.
Note: Different letters after the same data indicate significant differences at P < 0.050.

Fig. 2. Scanning electron microscopy (SEM) of pollen grains. Polar view: (a) V. sativa (No. 1), (b) V. sativa (No. 2), (c) V. sativa (No. 3), (d) V. sativa (No. 4), (e) V. sativa (No. 5), (f) V. sativa (No. 6), (g ) V. sativa (No. 7), (h ) V. sativa (No. 8), (i) V. sativa (No. 9), (j) V. sativa subsp. nigra (No. 10), (k) V. sativa subsp. nigra (No. 11), (l) V. sativa subsp. nigra (No. 12), (m) V. sativa subsp. nigra (No. 13), (n) V. sativa subsp. nigra (No. 14), (o) V. sativa subsp. nigra (No. 15), (p) V. sativa subsp. sativa, (No. 16), (q) V. sativa subsp. sativa (No. 17), (r) V. sativa subsp. sativa (No. 18), (s) V. sativa subsp. sativa (No. 19), (t) V. sativa subsp. cordata (No. 20), (u ) V. sativa (No. 21), (v) V. sativa (No. 22). Scale bars = 10 μm.
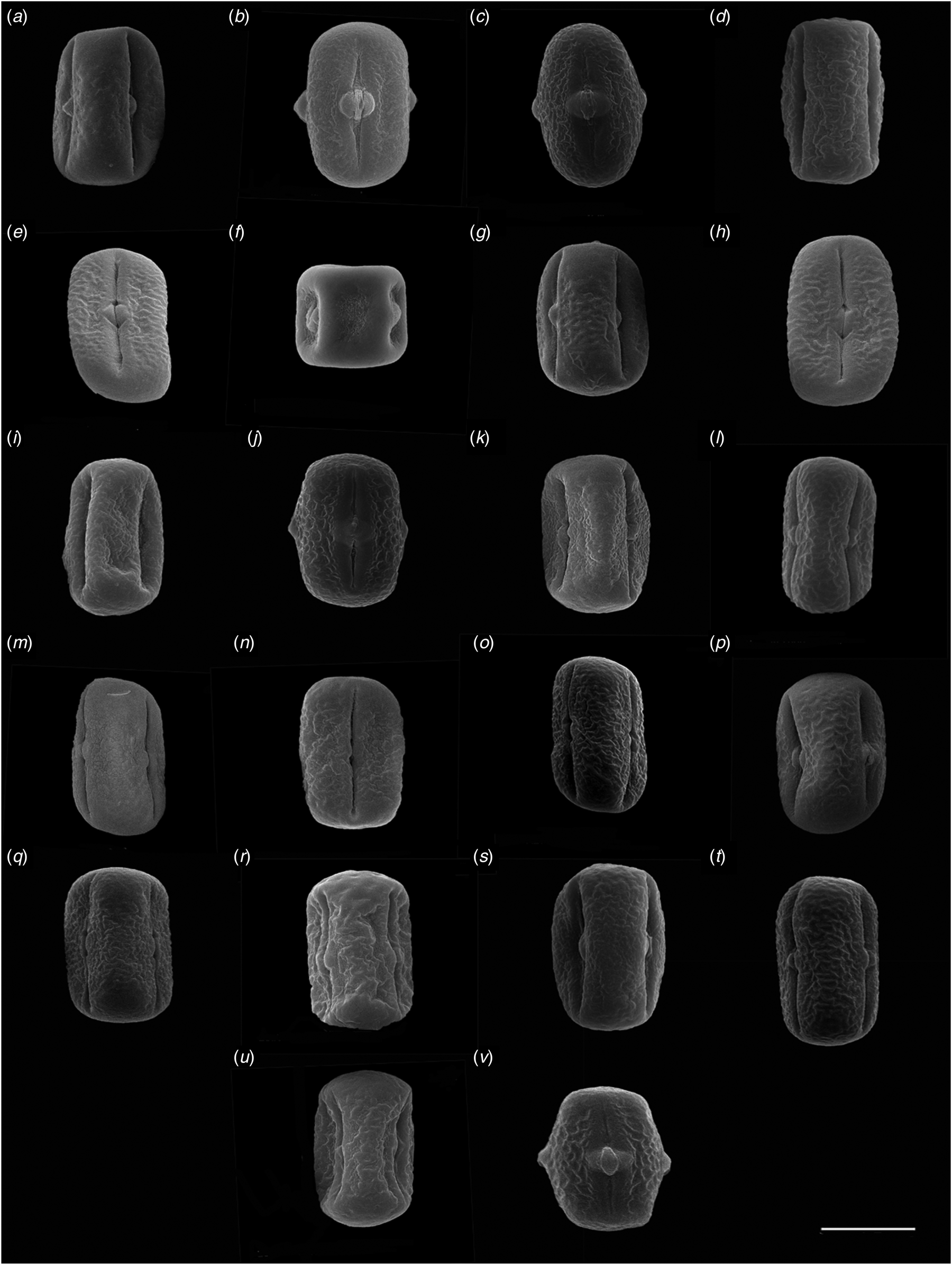
Fig. 3. Scanning electron microscopy (SEM) of pollen grains. Equatorial view: (a ) V. sativa (No. 1), (b ) V. sativa (No. 2), (c) V. sativa (No. 3), (d) V. sativa (No. 4), (e) V. sativa (No. 5), (f) V. sativa (No. 6), (g) V. sativa (No. 7), (h) V. sativa (No. 8), (i) V. sativa (No. 9), (j) V. sativa subsp. nigra (No. 10), (k) V. sativa subsp. nigra (No. 11), (l) V. sativa subsp. nigra (No. 12), (m) V. sativa subsp. nigra (No. 13), (n) V. sativa subsp. nigra (No. 14), (o ) V. sativa subsp. nigra (No. 15), (p ) V. sativa subsp. sativa, (No. 16), (q ) V. sativa subsp. sativa (No. 17), (r) V. sativa subsp. sativa (No. 18), (s) V. sativa subsp. sativa (No. 19), (t) V. sativa subsp. cordata (No. 20), (u) V. sativa (No. 21), (v) V. sativa (No. 22). Scale bars = 10 μm.

Fig. 4. Scanning electron microscopy (SEM) of pollen grains. Ornamentation: (a) V. sativa (No. 1), (b) V. sativa (No. 2), (c) V. sativa (No. 3), (d) V. sativa (No. 4), (e) V. sativa (No. 5), (f) V. sativa (No. 6), (g) V. sativa (No. 7), (h) V. sativa (No. 8), (i) V. sativa (No. 9), (j) V. sativa subsp. nigra (No. 10), (k) V. sativa subsp. nigra (No. 11), (l) V. sativa subsp. nigra (No. 12), (m) V. sativa subsp. nigra (No. 13), (n) V. sativa subsp. nigra (No. 14), (o) V. sativa subsp. nigra (No. 15), (p) V. sativa subsp. sativa (No. 16), (q) V. sativa subsp. sativa (No. 17), (r) V. sativa subsp. sativa (No. 18), (s) V. sativa subsp. sativa (No. 19), (t) V. sativa subsp. cordata (No. 20), (u) V. sativa (No. 21), (v) V. sativa (No. 22). Scale bars = 5 μm.
Morphological characteristics
The pollen of 22 common vetch accessions was observed and all accessions were monad pollen (Table 2). Among them, 21 accessions were oblate and one (No. 6) was oblate spherical. The polar views of 19 accessions revealed three-lobed circles and three accessions exhibited triangle shapes (Table 2). The comparison of pollen grain size showed that there were significant differences in pollen grain size among different accessions. Among them, the polar and equatorial axis lengths of accession No. 6 were significantly larger than those of the other 21 accessions (P > 0.05). In addition, seven types of pollen ornamentation were recognized: reticulate (seven accessions), rugulate (seven accessions), reticulate-rugulate (three accessions), quarse regulate (two accessions), psilate-reticulate-rugulate (one accession), psilate-perforate (one accession) and reticulate-retipilate (one accession). The pollen colpus size of the various accessions in common vetch was significantly different and the colpus length and width of accession No. 6 were significantly larger than those of the other 21 accessions. The apertures of 22 accessions were 3-zonocolporate and close to the poles, with different widths and the colpus was smooth or showed wart-like protrusions, but there were obvious depressions in the colpus of accession No. 11. The pollen polar axis length of the 22 accessions ranged from 19.39 ± 0.97 to 42.12 ± 0.76 μm and the equatorial axis length ranged from 35.97 ± 1.27 to 45.25 ± 0.81 μm. The colpus length was between 23.95 ± 1.87 and 32.23 ± 2.38 μm and the colpus width was between 2.22 ± 0.32 and 5.05 ± 1.13 μm for the 22 accessions.
In general, there were significant differences between accessions No. 6 and No. 11 and the other accessions in the qualitative pollen traits, such as equatorial view (with nearly all accessions being oblate), aperture (all 3-zonocolporate), ornamentation and polar view. The other 20 accessions were not significantly different and high conservation among the various accessions was observed.
Morphological analysis between subspecies
As shown in Table 3,
Table 3. Main characteristics of the pollen morphology of the three subspecies and two cultivars of 22 accessions of common vetch

(P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Ev) equatorial view, (Pv) polar view, (Clg) colpus length, (Clt) colpus width, (Orn) ornamentation.
Group one contains six accessions (No. 10–15). Group two contains four accessions (No. 16–19).
Group three contains one accession with No. 20. Group four contains two accessions with No. 21–22.
Note: Different letters after the same data indicate significant differences at P < 0.050.
Group 1: V. sativa subsp. nigra (Table 3; Figs 2(j—o), 3(j—o), (4j—o))
Pollen dimensions: P = 21.03–24.22 μm, E = 35.97–40.78 μm.
Pollen shape: oblate, P/E = 0.54–0.61 μm.
Apertures: 3-zonocolporate, apertures long, broader on pori in mesocolpium, Clg = 26.10–30.97 μm, Clt = 2.22–3.30 μm.
Outlines: equatorial view oblate; polar view three-lobed circular.
Ornamentation: reticulate-retipilate, reticulate or rugulate, obvious.
Group 2: V. sativa subsp. sativa (Table 3; Figs 2(p—s), 3(p—s), 4(p–s))).
Pollen dimensions: P = 20.72–24.71 μm, E = 35.70–40.41 μm.
Pollen shape: oblate, P/E = 0.51–0.65 μm.
Apertures: 3-zonocolporate, apertures long and slightly deep, broader on pori in mesocolpium, Clg = 24.99–31.33 μm, Clt = 2.41–3.49 μm.
Outlines: equatorial view oblate; polar view three-lobed circular or triangular.
Ornamentation: reticulate, regulate, reticulate-rugulate or quarse rugulate, obvious.
Group 3: V. sativa subsp. cordata (Table 3; Figs 2(t), 3(t), 4(t)).
Pollen dimensions: P = 23.37 μm, E = 43.67 μm.
Pollen shape: oblate, P/E = 0.54 μm.
Apertures: 3-zonocolporate, apertures long and slightly shallow, broader on pori in mesocolpium, Clg = 28.44 μm, Clt = 2.71 μm.
Outlines: equatorial view oblate; polar view three-lobed circular.
Ornamentation: reticulate, obvious.
Group 4: V. sativa (Table 3; Figs 2(u) and (v), 3(u) and (v), 4(u) and (v)).
Pollen dimensions: P = 23.21–23.50 μm, E = 37.95–40.40 μm.
Pollen shape: oblate, P/E = 0.57–0.62 μm.
Apertures: 3-zonocolporate, apertures long and slightly deep, broader on pori in mesocolpium, Clg = 27.25–30.74 μm, Clt = 2.56–2.88 μm.
Outlines: equatorial view oblate; polar view three-lobed circular.
Ornamentation: reticulate or quarse rugulate, obvious.
It was found that the values of P, E, Clg and Clt of V. sativa subsp. cordata subspecies were higher than those of the other three subspecies. This could be used to classify V. sativa subsp. cordata subspecies with other subspecies. Among all accessions, the traits P, E and P/E were significantly different, but the traits Clg and Clt were not significantly different.
Genotypic variation analysis of traits
The genotypic variance components estimated for five pollen traits showed significant differences (P < 0.05) among the 22 common vetch accessions (Table 4). The traits P, E, P/E, Clg and Clt all had higher accession mean repeatability (R). Among them, the R values of traits P, E and P/E were all above 0.98, and the traits Clg and Clt had relatively lower R values, which were 0.90 and 0.94, respectively. Higher R values indicate that pollen traits are more affected by genotypes and are more conservative.
Table 4. The estimated genotypic (${\rm \sigma }_{\rm g}^ 2$![]() ) and experimental error (${\rm \sigma }_{\rm \varepsilon }^ 2$
) and experimental error (${\rm \sigma }_{\rm \varepsilon }^ 2$![]() ) variance components and associated standard error (±s.e.) within 22 accessions of common vetch
) variance components and associated standard error (±s.e.) within 22 accessions of common vetch

(P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Clg) colpus length, (Clt) colpus width.
Pattern analysis and phenotypic correlation
Pattern analysis is a combination of cluster analysis and PCA, which provides a graphical summary of trial entry-by-multi trait data matrices. We analysed and compared the differences between the pollen traits of 22 common vetch accession pollens and visually displayed the correlation information (positive or negative) between the traits (Jahufer et al., Reference Jahufer, Da, Nichols, Crush, Li and Dunn2006). The pattern analysis results of 22 common vetch accessions based on seven traits, including P, E, P/E, Clg, Clt, Pv and Orn, are shown in Fig. 5. In the biplot, the first principal component explained 45% of the total trait variation, and the second principal component explained 24%. Among 22 common vetch accessions, the traits P, E, P/E, Clg and Clt showed a positive correlation (the angle between the direction vectors was <90°) (Fig. 5) and Pv also showed a positive correlation with P, E and Clg. Orn had a negative correlation with the traits P, E, P/E, Clg, Clt and Pv (the angle between the direction vectors is >90°).
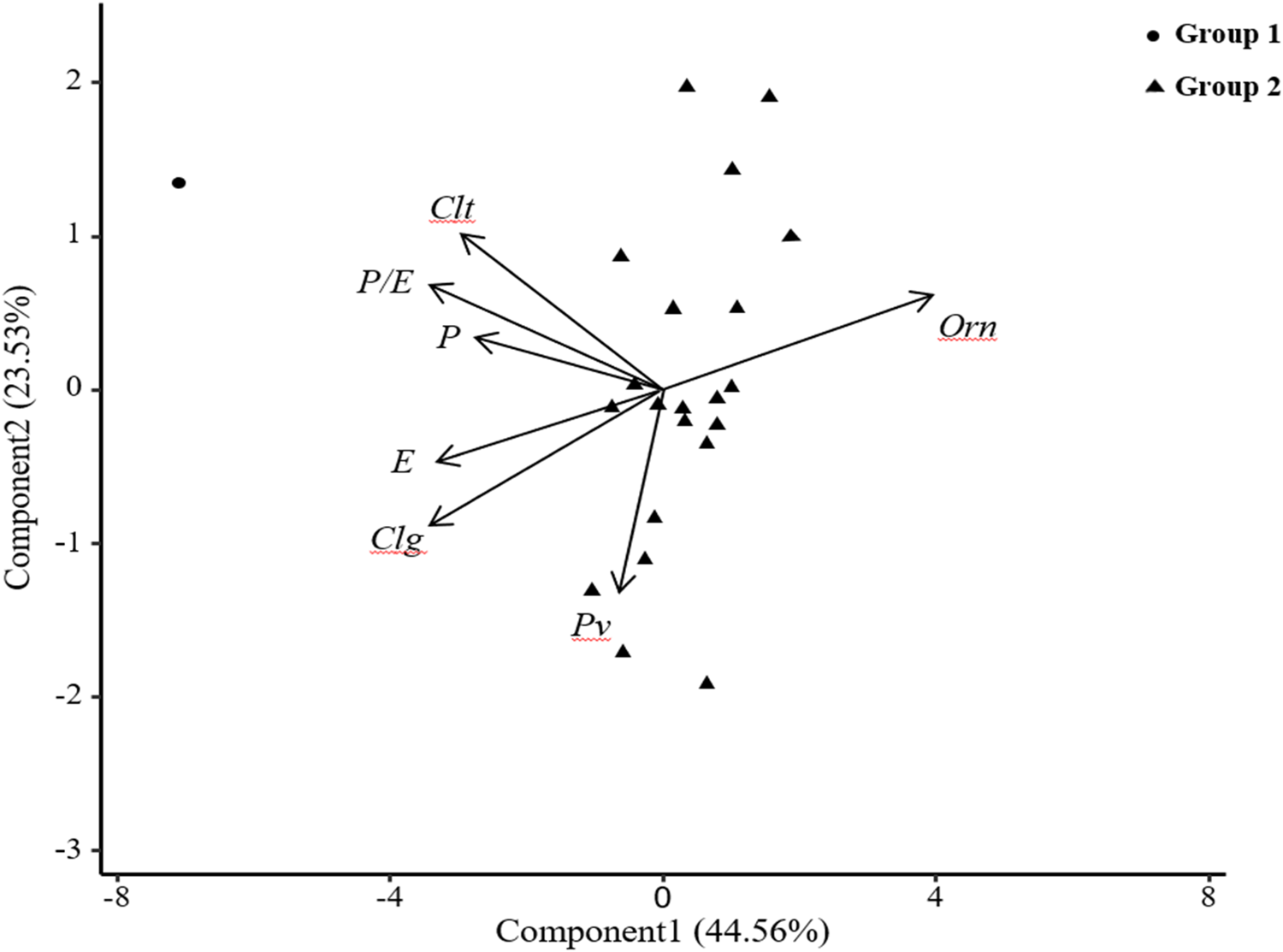
Fig. 5. Pattern analysis of pollen status for 22 common vetch accessions. (P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Pv) polar view, (Clg) colpus length, (Clt) colpus width, (Orn) ornamentation.
Using pattern analysis, 22 common vetch accessions were divided into two groups by K-means clustering (stats package). Among them, the first group included accession No. 6, and the second group included the other 21 accessions and the clustering results were consistent with the differences in pollen traits. The phenotypic correlation coefficient (rp) shown in Table 5 further verified the positive correlation between the traits P, E, P/E, Clg and Clt shown in Fig. 5; among them, P and E, P/E, Clt were extremely significantly correlated, and P and Clg were significantly correlated.
Table 5. Phenotypic correlation coefficients (rp) between traits based on 22 common vetch accessions

(P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Clg) colpus length, (Clt) colpus width, (Pv) polar view, (Orn) ornamentation.
**indicates that the difference is significant at P < 0.010.
*indicates that the difference is significant at P < 0.050.
Cluster analysis
To further study the common vetch accessions in this study, we selected seven traits of common vetch and used hierarchical clustering in the R Programming Language (NbClust package) for cluster analysis. The results are shown in Fig. 6. When the fusion level value was five, the clustering results were roughly grouped into two groups, which were determined to be the optimal clustering groups. The two groups were as follows:

Fig. 6. Cluster analysis of pollen status for 22 common vetch accessions.
Accession No. 6 was clustered into a single group, representing the first type. The pollen polar axis length of this accession was 42.12 μm, the equatorial axis length was 45.25 μm, the P/E value was 0.93, the colpus length was 32.23 μm and the width was 5.05 μm, as shown in Table 6. Regardless of the size of the pollen or the length and width of the colpus, the pollen was significantly larger than that of other accessions; additionally, these pollen grains alone, among all the common vetch accessions tested, exhibited an oblate spherical shape.
Table 6. Quantitative trait means for each of the two groups of the 22 common vetch accessions generated from cluster analysis

(P) polar axis, (E) equatorial axis, (P/E) ratio of equatorial and polar axes, (Clg) colpus length, (Clt) colpus width.
The other 21 common vetch accessions were grouped into the second group. The average length of the polar axis of this accession group was 22.53 μm, the average length of the equatorial axis was 38.59 μm, the average length of the colpus was 28.31 μm and the average width was 2.80 μm. These pollen shapes were oblate, the P/E values were less than 0.8, the pollen and colpus were similar in size and there was no significant difference among the various traits.
The above results are completely consistent with the results of the pattern analysis.
In this cluster analysis performed by selecting the relevant traits of common vetch, although the 22 accessions were roughly grouped, the classification results at the subspecies level were not ideal. For example, the four accessions No. 16, No. 17, No. 18 and No. 19 belonged to V. sativa subsp. sativa subspecies should have been clustered in the same group but were divided into four different groups. Through related research, we found that cluster analysis of common vetch accessions has been focused at levels above the subspecies level, but no related cluster analysis has been performed on accessions within a subspecies (e.g. LPWG, 2017; Banks and Lewis, Reference Banks and Lewis2018). The reasons for this finding need to be further explored.
Box-and-whisker plot analysis
As shown in Fig. 7, we conducted data measures of dispersion analysis on the five indexes of P, E, P/E, Clg and Clt of the 22 common vetch samples tested. Among the two indexes of P and P/E, the only accession No. 6 showed a relatively large degree of deviation from the other accessions, with significant differences. However, for the three indicators E, Clg and Clt, the degree of deviation among the 22 accessions was not significant.

Fig. 7. Box-and-whisker plots for the polar axis (P), equatorial diameter (E), P/E ratio, colpus length (Clg) and colpus width (Clt) of 22 common vetch accessions.
Discussion
Previous studies have studied the pollen morphology of Vicia. For example, Endo and Ohashi (Reference Endo and Ohashi1996) performed SEM observations of pollen from 32 species of the genus Vicia and found that the pollen interstitia of the examined species were regular columella, irregular columella or granular. Liu et al. (Reference Liu, Ma L, Wang and Liu2015) performed SEM observations of pollen from 16 Vicia plants from 11 countries and concluded that the pollen morphology of this genus is highly conserved. However, these studies mainly focused on the observation of pollen of different species of Vicia under SEM, and a comparative study of different subspecies and accessions within a single species of common vetch was not performed. This study collected 22 pollen materials from common vetch accessions from 15 countries to accumulate data for systematic taxonomy and palynology-related research within common vetch species.
Kahraman et al. (Reference Kahraman, Binzat and Doğan2013) carried out SEM observations of Vicia from 11 taxa in Turkey, in which the pollen polar axis length (P) of the accessions of V. sativa subsp. sativa was 30.88–36.67 μm, and the equatorial axis length (E) was 23.63–28.55 μm. In the present study, V. sativa subsp. sativa taxa showed P ranging from 20.72 to 24.71 μm, and E ranging between 35.70 and 40.41 μm, which is quite different from the experimental results of Kahraman et al. (Reference Kahraman, Binzat and Doğan2013). In the study of Liu et al. (Reference Liu, Ma L, Wang and Liu2015), the pollen equatorial view of accession No. 21 revealed an oblate spherical shape, and the equatorial axis length and polar axis length were relatively short. However, in this study the equatorial view of this accession revealed an oblate shape, and the equatorial axis length and polar axis length were both relatively long, which is inconsistent with the research results of Liu et al. (Reference Liu, Ma L, Wang and Liu2015) (Fig. 3(u)).
In this study, the similarity between Clg and Clt in the one subspecies accession of common vetch was higher than those of the other traits and the differences were not significant. At the same time, in the cluster analysis, we found that the accessions of the same subspecies could not be clustered in the same unit. Through other related studies, it was found that cluster analysis of common vetch accessions has only been reported above the subspecies level (e.g., LPWG, 2017; Banks and Lewis, Reference Banks and Lewis2018). In the boxplot analysis, we also found that apart from the special accession No. 6, the remaining 21 accessions had no significant degree of deviation in the five indicators of P, E, P/E, Clg and Clt. Common vetch is a strictly self-pollinated plant (Dong et al., Reference Dong, Jahufer, Dong, Wang and Liu2016) and its pollen morphology has strong conservation and genetic stability (Lee et al., Reference Lee, Heo, Cho, Lee, Chen and Kim2011). In this study, P, E, P/E, Clg and Clt all had high R values, which also indicates that the heredity of the pollen traits of common vetch is mainly affected by genotype and has high genetic stability. These reasons have caused the subspecies of common vetch to be poorly characterized in cluster analysis. However, the pollen morphology analysis among the subspecies of common vetch (Table 3) found that E and P/E have significant differences among the subspecies. This could provide extremely useful information for future work related to the classification of common vetch subspecies.
Pollen morphology plays an important role in the taxonomy and phylogenetic history of plants. Research in the field of palynology provides valuable information for the identification of closely related and complicated classifications as well as taxa whose taxonomy is contested (Jafar and Karm, Reference Jafar and Karm2007; Quamar et al., Reference Quamar, Ali, Pandita and Singh2017). In the process of generational transmission, pollen ornamentation, shape, surface, symmetry, colpus length, width and wall structure basically maintained their original morphological characteristics. These morphological and structural characteristics are important bases for identifying plant species (Bahadur et al., Reference Bahadur, Ahmad, Mir, Zafar, Sultana, Ashfaq and Arfan2018). After studying the pollen traits of 22 common vetch accessions, the pollen morphologies of various accessions not only share common characteristics but also have obvious specificities among some accessions. For example, the 22 common vetch accessions all showed pollen apertures that were 3-zonocolporate, with colpi close the poles and the colpi were smooth or had wart-like protrusions; however, there were obvious depressions in the middle of the colpus of accession No. 11, which provides important information for the identification of this accession. As another example, among the 22 common vetch accessions, 21 accessions had P/E values between 0.5 and 0.8 and an oblate pollen morphology, while only the pollen P/E value of accession No. 6 was greater than 0.8 and its pollen morphology was oblate spherical. These conclusions indicate that some accessions have their own unique characteristics at present and the identification of plant species can still mainly be based on morphological characteristics. The data from this study provide a basis for the identification of related species (Kahraman et al., Reference Kahraman, Binzat and Doğan2013).
Wodehouse (Reference Wodehouse1935) believed that the more evolved the pollen, the stronger its regulatory function and the longer the pollen. The equatorial axis lengths of 22 common vetch pollens in this study in order from large to small were No. 6, No. 20, No. 8, No. 5, No. 15, No. 7, No. 19, No. 22, No. 4, No. 10, No. 18, No. 2, No. 1, No. 21, No. 16, No. 12, No. 13, No. 9, No. 14, No. 11, No. 17 and No. 3. According to the evolution theory of Wodehouse (Reference Wodehouse1935), the pollens of accessions such as No. 6 and No. 20 were longer and more evolved, while the pollens of accessions No. 3 and No. 17 were shorter and more primitive. At the same time, Beck (Reference Beck1981) reported that pollen ornamentation can also reflect the evolution of pollen and pointed out that the evolutionary trend of ornamentation is surface psilate → surface foveolate, fossulate → surface scabrate, verrucate → surface granular → surface reticulate. The differences in the pollen ornamentation of the 22 common vetch accessions indicated that they have different degrees of evolution. According to the evolution theory of Beck (Reference Beck1981), the pollen ornamentations of accessions No. 3, No. 17 and No. 11 are rugulate and are thus more vague and primitive. The pollen ornamentations of No. 6, No. 20 and other accessions were reticulate and thus more evolved. The pollen morphological characteristics of 22 common vetch accessions observed in this experiment were verified by the research conclusions of Wodehouse (Reference Wodehouse1935) and Beck (Reference Beck1981), and the same results were obtained. This shows that it is feasible to study the evolution of the accessions of common vetch from the perspective of pollen morphology.
Conclusion
In conclusion, there are no previous detailed studies on the pollen morphology of different common vetch subspecies and their various accessions. In this study, the microscopic features of pollen from 22 accessions of common vetch were analysed using LM and SEM. The results showed that there are significant differences in the pollen traits, including polar axis length, equatorial axis length and colpus length and width, among accessions. The traits of the equatorial axis and the ratio of equatorial and polar axes have significant differences among subspecies. This trait information could be used for classification research between common vetch subspecies. The palynological properties of the concerned taxa are described in detail. Therefore, we suggest that the microscopic morphological structures of pollen can be useful to assess whether there are significant differences among accessions of the subspecies of common vetch. The palynological characteristics described for these taxa combined with molecular markers and other methods will help further research on this species.
Financial support
This research was supported by the Department of Science and Technology of Guizhou Province (Qian Ke Talent Introduction Project of Guizhou University (Gui Da Ren Ji He Zi [2017] 17)) and (Qian Ke He Ji Chu ([2019] 1074)).
Conflict of interest
None.
Ethical standards
Not applicable.


















