Introduction
Sorghum halepense is an allotetraploid species, obtained by hybridization between Sorghum propinquum and Sorghum bicolor (De Wet and Harlan, Reference De Wet and Harlan1971; Doggett, Reference Doggett1988; Paterson et al., Reference Paterson, Schertz, Lin, Liu and Chang1995; Dillon et al., Reference Dillon, Lawrence and Henry2001; Dillon et al., Reference Dillon, Lawrence, Henry, Ross, Price and Johnston2004). This species is considered to be the secondary gene pool of cultivated sorghum (Stenhouse et al., Reference Stenhouse, Prasada Rao, Gopal Reddy, Appa Rao, Fuccillo, Sears and Stapleton1997).
The greatest morphological diversity of S. halepense is in Southeast Asia (Celarier, Reference Celarier1958). Its natural geographical range is spanned from Southeast Asia to Mediterranean region, and it was introduced to other regions as an exotic plant. This species grows widely in different regions of Iran in a vast range of ecological conditions (Bor, Reference Bor and Rechinger1970).
S. bicolor ranked fifth among world's top cereal crops (FAOSTAT, 2013). Owing to successive selections performed on agricultural traits, the genetic diversity inherited by cultivated Sorghum has become narrower than that accumulated in the wild relative genetic resources such as S. halepense. These wild genetic resources are highly desirable and precious for improving crops and maintaining sustainable agroecosystems that are endangered by continuous climatic changes (Maxted et al., Reference Maxted, Kell, Ford-Lloyd, Maxted, Ford-Lloyd, Kell, Iriondo, Dulloo and Turok2008).
Investigations revealed that S. halepense harbours many useful traits such as resistance to many pests and diseases and broad adaptation to harsh environments (Holm et al., Reference Holm, Donald, Pancho and Herberger1977; Paterson et al., Reference Paterson, Schertz, Lin, Liu and Chang1995). These characteristics accentuated S. halepense as a potentially valuable gene source to probe useful alleles to be incorporated in the cultivated sorghum improvement (Ng'uni, Reference Ng'uni2011). On the other hand, many agronomically disadvantages such as ability to crowd out neighbouring seedlings and young plants (Newman, Reference Newman1993), inhibiting the growth of other plants and significantly reducing yields in crops (Holm et al., Reference Holm, Donald, Pancho and Herberger1977), are associated with this weed grass. Traits such as tolerance to some herbicides and reproduction ability through rhizome fragmentation have made these plants as pertinacious weeds.
Screening the genetic diversity encountered among natural populations of S. halepense is fundamental to understand the genetic bases of weediness, to increase knowledge of species biology, to perform a strategy for weed control, to find the germplasm with a higher priority for conservation and to understand evolution. However, the extent and spatial structure of genetic diversity present in this species, particularly those growing in Iran, have not been investigated well.
A major portion of plant and animal genomes is constituted of retrotransposons (Flavell et al., Reference Flavell, Smith and Kumar1992). Inter-retrotransposon amplified polymorphism (IRAP) illuminates polymorphisms that existed in DNA segments between the two adjacent retrotransposons in the genome among germplasms (Kalendar et al., Reference Kalendar, Grab, Regina, Suoniemi and Schulman1999). These polymorphisms can then be used as measures of genetic similarity/dissimilarity among plant materials. IRAP as a molecular marker with a high level of polymorphism and low running costs is largely used in studies concerning genetic diversity (Heslop-Harrison et al., Reference Heslop-Harrison, Brandes, Taketa, Schmidt, Vershinin, Alkhimova, Kamm, Doudrick, Schwarzacher, Katsiotis, Kubis, Kumar, Pearce, Flavell and Harrison1997; Barnaud et al., Reference Barnaud, Deu, Garine, Mckey and Joly2007; Branco et al., Reference Branco, Vieira, Malone, Kopp, Malone, Bernardes, Mistura, Carvalho and Oliveira2007; Saeidi et al., Reference Saeidi, Rahiminejad and Heslop-Harrison2008) and phylogeny (Shimamura et al., Reference Shimamura, Yasue, Ohshima, Abe, Kato, Kishiro, Goto, Munechika and Okada1997; Mansour, Reference Mansour2008).
The objectives of this study were to evaluate the genetic diversity of S. halepense in Iran and to superimpose the observed patterns of diversity on the geographical distribution of the species to elucidate the spatial structures of genetic diversity present within this species using IRAP markers.
Material and methods
Plant materials
A total of 38 accessions of S. halepense were collected from different regions of Iran (Fig. 1) during July–September 2013. In each site, five to eight individual culms were harvested from different rhizomes in an area measuring about 25 m2. Two accessions of S. bicolor were included in the study as outgroups. The accessions and herbarium voucher specimens are deposited in the herbarium of the University of Isfahan. Accessions were morphologically identified according to Bor (Reference Bor and Rechinger1970) and House (Reference House1985). Accession code, locality and other details regarding the plant materials used in this study are provided as supplementary online material in Table S1 (available online).

Fig. 1 Geographical distribution of 38 accessions of S. halepense and two accessions of S. bicolor studied in Iran. Circles illustrate six ecogeographical-related groups that were revealed based on IRAP markers. (SW = south-west, W = west, NW = north-west, C = centre, N.EC= north, eastern coast of Caspian Sea, N.WC = north, western coast of Caspian Sea, ● = S. bicolor, ▲ = S. halepense).
DNA extraction and molecular analyses
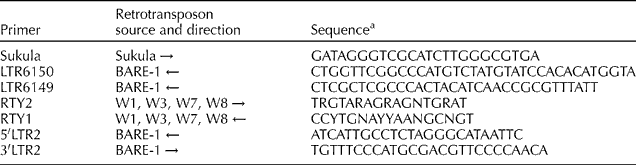
Bulked genomic DNA was isolated from leaves of –five to eight individual culms (0.1 g of dried leaves of each individual culm) of each accession following the method developed by Gawel and Jarret (Reference Gawel and Jarret1991). In order to perform IRAP analysis, 52 combinations of IRAP primers, originally derived from barley (Kalendar et al., Reference Kalendar, Grab, Regina, Suoniemi and Schulman1999, Reference Kalendar, Tanskanen, Immonen, Nevo and Schulman2000; Manninen et al., Reference Manninen, Kalendar, Robinson and Schulman2000; Boyko et al., Reference Boyko, Kalendar, Korzun, Gill and Schulman2002) and banana (Reverse Ty1 (RTY1) and RTY2; Teo et al., Reference Teo, Tan, Ho, Faridah, Othman, Heslop-Harrison, Kalendar and Schulman2005), were tested, of which eight primer combinations that amplified scorable polymorphic DNA bands were selected for analysis. The primer sequences, retrotransposon source, orientation and other details related to the primers are given in Table 1.
Table 1 Sequences of primers used for IRAP, their retrotransposon source and direction (→ or ←)

a Y = C or T; N = A, G, C or T; R = A or G.
Polymerase chain reactions (PCRs) were performed in a total volume of 15 μl reaction volume, containing approximately 50 ng genomic DNA, 1 × PCR buffer, 1.5-mM MgCl2, 10 pmol of each primer, 200 μm dNTP mix and 1 U Taq polymerase. The PCR parameters are as follows: at 94°C for 4 min, followed by 35 cycles of 94°C for 1 min, T a (Table 2) for 1 min, ramped 0.5°C/s, 72°C for 2 min +3 second per cycle, with an final extension at 72°C for 10 min. PCR products were separated on 2% (w/v) agarose gel and detected by ethidium bromide staining.
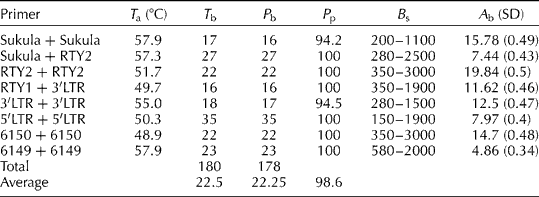
Table 2 Primer combinations, annealing temperature (T a), percentage of polymorphism (P p), and total and average number of bands (A b) produced by primers used for IRAP
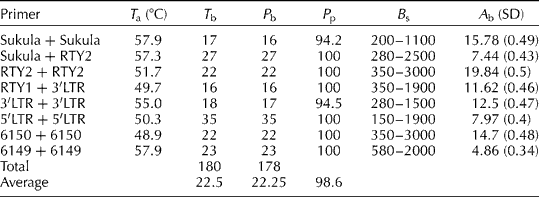
T b, total number of bands; P b, number of polymorphic bands; B s, band size range (bp).
Data analysis
The presence (1) or absence (0) of clear and distinguishable bands with the same migration rate on gel was scored for each accession. Data were entered into a raw data matrix, and genetic similarities were calculated based on the procedures of Jaccard (Reference Jaccard1908), simple matching and Dice (Nei and Li, Reference Nei and Li1979) similarity coefficients using the NTSYSpc software (version 2.02e; Rohlf, Reference Rohlf2000) and PowerMarker (version 3.25e; Liu and Muse, Reference Liu and Muse2005). Genetic similarity-based neighbour-joining (NJ) dendrograms were constructed using different similarity coefficients. The accuracy and stability of branches were measured through bootstrapping.
Analysis of molecular variances (AMOVA) was also performed to calculate the proportion of variation within and between the populations using the GenAlex software (version 6.5; Peakall and Smouse, Reference Peakall and Smouse2006). The Shannon diversity index was calculated via the Shannon-Pairwise option implemented in the GenAlex software, as
![]() $$H _{o} = - \sum P _{i}\,log\,2 P _{i} $$
(Sherwin et al., Reference Sherwin, Jabot, Rush and Rossetto2006), in which P
i represents the frequency of the given IRAP fragments.
$$H _{o} = - \sum P _{i}\,log\,2 P _{i} $$
(Sherwin et al., Reference Sherwin, Jabot, Rush and Rossetto2006), in which P
i represents the frequency of the given IRAP fragments.
As principal coordinate analysis (PCoA) could reveal more dimensions of relationships that may not be well reflected in cluster analysis, this was also performed. The genetic linkages between accessions were then shown by superimposing a Minimum Spanning Tree (MST) on the 2D plot of PCoA (Fig. 3).
Correlation between geographical distances and genetic distances (r), which is a measure of population isolation by distance and level of gene flow, was calculated using the Mantel test (Peakall and Smouse, Reference Peakall and Smouse2006) implemented in GenAlex software version 6.5.
Results
In this study, genetic diversity was examined in S. halepense based on IRAP fingerprinting. In total, eight IRAP primer combinations amplified 180 multiple DNA fragments from genomic DNA of all the 38 accessions of S. halepense and the two accessions of S. bicolor, with a high level of polymorphism (average = 98.9%). The number of bands ranged from 16 to 35, averaging 22.5 bands per primer combination. The highest and the lowest numbers of bands were produced from the primer combinations 5′LTR/5′LTR (35 bands) and RTY1/3′LTR (16 bands, Table 2), respectively. There were no significant differences between IRAP dendrograms generated based on the Jaccard (Reference Jaccard1908), Simple Matching and Dice (Nei and Li, Reference Nei and Li1979) coefficients or different clustering methods. However, the results were interpreted as revealed in NJ dendrogram generated based on Jaccard's coefficient, which showed the highest correlation value (r= 87%) between cophenetic matrix and its corresponding similarity matrix. The highest genetic similarity (72.46%) among accessions was observed between Sh-W-88 and Sh-W-56, both collected from the west, and the lowest one (10.63%) was found between Sh-C-82, collected from the centre, and Sh-N.WC-16, collected from the western coast of Caspian Sea in the north.
In the IRAP similarity-based dendrogram (Fig. 2), the two accessions of S. bicolor, used as outgroups, were grouped well apart from the accessions of S. halepense. The close genetic similarity between these two accessions of S. bicolor was shown by 93% bootstrap support. Based on the topology of the dendrogram, accessions were grouped in seven distinct clusters (Fig. 2). Except for western accessions, which were divided into two clusters (groups B and D, Fig. 2), other groups mainly corresponded to their geographical origin (groups A–G, Fig. 2). The groups were as follows: (1) group A included all accessions collected from the centre of Iran (Fig. 1), which showed more advanced position compared with other accessions; (2) four accessions collected from the west were clustered in group B; (3) the accessions collected from the north-west (Sh-NW-68 and Sh-NW-64) were clustered in group C among the western accessions (groups B and D); (4) six accessions collected from the western region were clustered in group D; (5) accessions collected from the northern region along the south-eastern coast of Caspian Sea were clustered in group E; (6) the accessions collected from the south-west (SW) of the country were included in group F and (7) the accessions collected from the northern region along the south-western coast of Caspian Sea were clustered in group G. Exceptions were the positions of Sh-N.WC-20 (collected from the south-western coast of Caspian Sea), Sh-C-82 (collected from Yazd in the centre) and Sh-C-78 (collected from Natanz in the centre). The two former accessions showed high genetic distance from others and were placed in an intermediate position between S. bicolor and other accessions of S. halepense. Sh-C-78 was unusually placed among the northern accessions.

Fig. 2 NJ dendrogram generated based on IRAP data and Jaccard's similarity coefficient showing relationships among 38 accessions of S. halepense and two accessions of S. bicolor used as outgroups. Accession numbers are shown with their geographical grouping (SW = south-west, W = west, NW = north-west, C = centre, N.EC= north, eastern coast of Caspian Sea, N.WC = north, western coast of Caspian Sea).
In PCoA 2D plot and MST (Fig. 3), grouping of S. halepense accessions mainly followed their geographical origin as shown in NJ dendrogram.

Fig. 3 Minimum Spanning Tree (MST) and 2D plot generated by principal coordinate analysis based on IRAPs of 38 S. halepense accessions. (W = west, NW = north-west, N.EC = northern region eastern coast of Caspian Sea, N.WC = northern region western coast of Caspian Sea, C = centre, SW = south-west).
In AMOVA, 16% of the total genetic variation was attributed to the between-population differentiations and 84% to the within-population differentiations and the amount of PhiPT was 0.161. The amount of gene flow among geographical regions was indirectly calculated from PhiPT as
![]() $$N _{m} = 0.25\,[(1/PhiPT) - 1] = 1.3. $$
$$N _{m} = 0.25\,[(1/PhiPT) - 1] = 1.3. $$
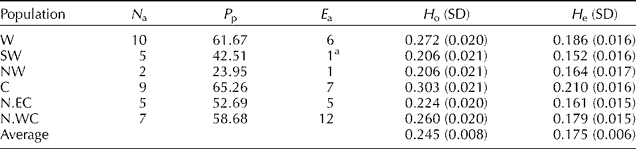
While calculating the Shannon Index, which readily translates into heterozygosity, the highest observed heterozygosity (0.303) was calculated within central population and the lowest one (0.206) within germplasms collected from SW and NW. The highest polymorphism ratio was calculated among germplasms collected from the centre of the country (65.25%) and the lowest polymorphism ratio (23.95%) in germplasms from north-western regions (Table 3).
Table 3 Genetic variability within populations of Sorghum halepense in Iran as revealed by IRAP analysis
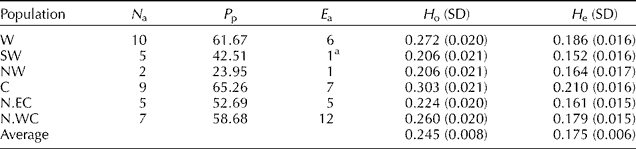
N a, number of accessions; P p, percentage of polymorphism at population level; E a, number of exclusive alleles; H o, observed heterozygosity (Shannon Index); H e, expected heterozygosity (unbiased).
a This exclusive allele was found in all accessions of the region.
In the Mantel test, the correlation between genetic distance and geographical distance was measured as r= 0.07 (P= 0.1).
The unique alleles (alleles exclusive to one or more accessions in a particular region) as an alternative approach to estimate gene flow or differentiation among groups were also calculated. There were 32 unique alleles exclusive to geographical regions (Table 3). The accessions collected from the northern region along the Southwest Caspian Sea coast had the highest number of unique alleles (12) and the accessions collected from SW and NW each with one allele had the lowest number of unique alleles. From 32 exclusive alleles, there were 18 alleles each unique to one accession.
Discussion
World distribution of S. halepense spanned from Mediterranean region to Southeast Asia (De Wet and Huckabay, Reference De Wet and Huckabay1967) and they entered into other regions as exotic plants. S. halepense is believed to be of Mediterranean origin (Meredith, Reference Meredith1955), but was introduced very early in India (Bor, Reference Bor1960). However, as the greatest morphological diversity of S. halepense is in Southeast Asia and its distribution overlaps the distribution of parental species (S. propinquum and S. bicolor) in this region, the species is more likely to be originated from Southeast Asia. Regardless of which opinion we accept, Iran is situated in the path of the distribution of the species. Regarding the vast ecological heterogeneity in Iran, S. halepense has probably been subjected to different adaptation-based genetic variation through its path in this region.
Genetic relationships and grouping resulted from IRAP data following an ecogeographical pattern. In an analysis of genetic diversity of Johnsongrass accessions collected from Argentina by Fernández et al. (Reference Fernández, De Haro, Distefano, Martínez, Lía, Papa, Olea, Tosto and Hopp2013) using simple sequence repeat markers, clustering according to geographical origin was evident. This pattern of diversity can be interpreted as an indication of adaptation-based genetic diversity in Johnsongrass germplasm.
Accessions collected from along the Zagros Mountains (from the SW to the NW of the country) showed higher genetic diversity compared with those collected from other regions. Accessions collected from Chaharmahal va Bakhtiari and Lorestan provinces (SW), despite being geographically close to other accessions collected along the west, were grouped apart. This group indicates the presence of genetically related colonies in the SW of Iran. Among accessions collected from the western region of the country, accessions collected from Kordestan, Ilam and Hamedan were genetically close to each other and partially differentiated from those collected from the NW (Azerbaijan Gharbi and Azerbaijan Sharghi). This grouping in the western accessions indicated the existence of three differentiated groups of colonies along the Zagros Mountains in Iran. Previous investigations into genetic diversity of other Poaceae taxa such as wild barley (Khodayari et al., Reference Khodayari, Saeidi, Akhavan, Pourkheirandish and Komatsuda2014), Hordeum murinum (Sharifi-Rigi et al., Reference Sharifi-Rigi, Saeidi and Rahiminejad2014), diploid wheats (Naghavi et al., Reference Naghavi, Malaki, Alizadeh, Pirseiedi and Mardi2009; Mousavifard et al., Reference Mousavifard, Saeidi, Rahiminejad and Shamsadini2015) and Secale spp. (Akhavan et al., Reference Akhavan, Saeidi and Rahiminejad2010; Jenabi et al., Reference Jenabi, Saeidi and Rahiminejad2011) also showed high variability in germplasms that originated from the Zagros Mountains in the west of Iran. This high variability could be related to the high ecological variation present in this region, which changes even in very short distances.
All accessions collected from the centre of the country were grouped. This can be regarded as an indication of existence of another genetically related group of colonies in this region. Exceptions were for accessions Sh-C-78 (collected from Natanz), Sh-C-82 (collected from Yazd) and Sh-N.WC-20 (collected from the south-western coast of Caspian Sea in the north). The two later accessions showed an intermediate position between S. bicolor and other accessions of S. halepense. Morrell et al. (Reference Morrell, Williams-Coplin, Lattu, Bowers, Chandler and Paterson2005) demonstrated the occurrence of active gene flow from cultivated sorghum to Johnsongrass. Therefore, gene flow occurring between cultivated sorghum and S. halepense in mentioned localities may have resulted in such a situation. Sh-C-82 was collected from urban green space that assuredly is not the natural habitat of the species. Sh-C-78 was clustered within the accessions that originated from eastern coast of Caspian Sea. These can be caused more likely by introduction of the seeds with cereals by farmers from the northern region. Interestingly, in MST, accessions Sh-C-78 and Sh-C-82 showed genetic linkages with the accessions collected from the central region while Sh-N.WC-20 was clustered among SW accessions. These cases can be correlated with the impact of analysis method on the results and on the interpretation of data.
In the northern region of the country along the Caspian Sea shore, despite being ecologically uniform, accessions were divided into two distinct clusters. One included all accessions collected from the eastern coast of Caspian Sea and the other contained accessions collected from the western coast of Caspian Sea. In MST, the accessions collected from the western coast of Caspian Sea (N.WC) showed considerable distance from others. These accessions had different morphological characteristics such as higher length, larger and wider leaves, stout rhizomes versus creeping rhizomes, boated shape of upper glumes versus flat ones, obscurely denticulate apex in lower glumes versus distinctly 3-tooth ones and larger inflorescences. De Wet and Huckabay (Reference De Wet and Huckabay1967) considered two morphologically distinct ecotypes for S. halepense as; Mediterranean ecotype that are smaller plants with narrow leaves and 2n= 40, distributed from Pakistan to the Mediterranean region, and a tropical ecotype described as stronger plants with 2n= 20, which occurs in the tropical regions in the Southeast Asia. Later, the tropical ecotype was recognized as a separate species, S. propinquum L. Piper (Harlan and De Wet, Reference Harlan and De Wet1972). Many of the morphological characters observed in accessions collected from the south-western coast of Caspian Sea in Iran resembled the morphological characteristics of S. propinquum. Pakistan is considered to be the western border of distribution of S. propinquum and there is no report indicating the presence of this species in Iran. The climate in the northern region of Iran along the Caspian Sea coast is very similar to that of the Southeast Asia. Moreover, the northern region is the main rice cultivation centre in Iran, where rice seeds have probably been introduced through past decades from Southeast Asia. Therefore, introduction of the seeds of S. propinquum from the Southeast Asia to this region was not unexpected. The presence of high unique alleles indicates that the Johnsongrass populations in this region are genetically highly dynamic, and presumably some reproductive or genetic barriers prevent genetic exchanges between these populations and those of neighbouring regions. Interestingly, Paterson et al. (Reference Paterson, Schertz, Lin, Liu and Chang1995) in a molecular analysis of S. halepense and related species, using restriction fragment length polymorphism, indicated that the only two accessions of S. propinquum examined were remarkably diverse. Therefore, we recommend that the future efforts should be focused on cytology and genetics of the Johnsongrass in the western coast of Caspian Sea in Iran.
The main strategy for reproduction of Johnsongrass is either by clonal dispersal of rhizome fragments through cultural practices or by delivery of seeds after self-pollination (Warwick and Black, Reference Warwick and Black1983). It has been reported that more than 95% of self-fertilization occurs even in places where plants are closely spaced (Warwick and Black, Reference Warwick and Black1983). This reproduction strategy would theoretically lead, in the long run, to a decrease in genetic diversity in each colony. Nevertheless, the results of this study demonstrated considerable genetic diversity in Iranian S. halepense germplasm. Our accessions were collected from distant localities, inasmuch as each accession can be considered as representative of one isolated colony. Therefore, each colony owed its own history of adaptation and evolution, which led to high genetic differentiations among accessions. The reproductive behaviour of the species limits the genetic exchanges among colonies leading to the increase in genetic diversity. No significant correlation between genetic distance and geographical distances, and the amount of N m (1.33) indicate the occurrence of gene flow among geographical regions, which has probably occurred through delivery of seeds with cereals by farmers or dispersion of seeds by birds, but the nature of reproductive strategy of the species has prevented the distribution of the introduced genes among colonies. The similar level of heterozygosity calculated within geographical regions can be considered as an indication for the occurrence of gene flow among regions, which balanced the level of diversity in different regions.
The presence of high unique alleles in many accessions is another indication of high genetic dynamism in Iranian germplasm of Johnsongrass. Two measures of genetic diversity, the polymorphism ratio and the number of unique alleles, are largely influenced by sample size (number of accessions; Lowe et al., Reference Lowe, Harris and Ashton2004). In this study, aside from the NW accessions (two accessions) with the lowest polymorphism ratio and unique alleles, the SW accessions showed the lowest genetic diversity considering both measures. There was only one regional exclusive allele shared between all SW accessions, and they also showed high genetic distance with accessions from other regions. These results indicated that a particular genotype of the species is established in this region with very low genetic exchanges with other regions. The observed heterozygosity in the SW and NW was also lower than that in other regions.
The results of this study demonstrated high genetic variability in the Iranian germplasm of Johnsongrass and indicated that there is value in sampling the germplasm for useful alleles for crop improvement. Information on genetic diversity of S. halepense in other regions is significantly lacking. Morden et al. (Reference Morden, Doebley and Schertz1990) surveyed allozyme variation among spontaneous species of Sorghum section Sorghum. Their study mainly focused on the analysis of the variation within S. bicolor subsp. arundinaceum (Desv.) de Wet et Harlan ex Davidse and did not demonstrate anything about the within-species variation of S. halepense to be compared with our results. Fernández et al. (Reference Fernández, De Haro, Distefano, Martínez, Lía, Papa, Olea, Tosto and Hopp2013) indicated considerable genetic diversity in Argentinean germplasm of S. halepense, but due to the different molecular markers used, we cannot compare the level of genetic diversity of that germplasm with that of Iranian germplasm assessed in this study. Therefore, we were unable to compare the level of genetic diversity in Iranian germplasm of the species with that of other regions. A broad genetic diversity analysis covering all natural habitats of S. halepense from Mediterranean region to the Southeast Asia may clarify the centre of origin, centre/centres of diversity and species migration pathways, and provide useful information regarding the origin of exotic Johnsongrasses in other areas.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S147926115000167
Acknowledgements
The authors thank the Office of Graduate Studies of the University of Isfahan (Iran) for their support.