Introduction
Palmer amaranth, tall waterhemp, horseweed, and giant ragweed (Behrens et al. Reference Behrens, Mutlu, Chakraborty, Dumitru, Jiang, Lavallee, Herman, Clemente and Weeks2007; Johnson et al. Reference Johnson, Young, Matthews, Marquardt, Slack, Bradley, York, Culpepper, Hager, Al-Khatib, Steckel, Moechnig, Loux, Bernards and Smeda2010) are four dicot weeds that are ranked among the most troublesome weeds in the United States and the state of Indiana (Gibson et al. Reference Gibson, Johnson and Hillger2005; Van Wychen Reference Van Wychen2016). Palmer amaranth and tall waterhemp are problematic in row-cropping systems due to their rapid growth rates, prolific seed production, season-long emergence pattern, and a wide genetic diversity due to obligate outcrossing (Franssen et al. Reference Franssen, Skinner, Al-khatib, Horak, Peter and Kulakow2001; Schwartz et al. Reference Schwartz, Norsworthy, Young, Bradley, Kruger, Davis, Steckel and Walsh2016; Sellers et al. Reference Sellers, Smeda, Johnson and Ellersieck2003). Horseweed is problematic in soybean due to its high seed production, long-distance seed dispersal, and variable emergence pattern (Davis and Johnson Reference Davis and Johnson2008). Its competitiveness for light and ability to emerge in a wide range of environments make giant ragweed troublesome in soybean (Abul-Fatih and Bazzaz Reference Abul-Fatih and Bazzaz1979; Baysinger and Sims Reference Baysinger and Sims1991; Webster et al. Reference Webster, Loux, Regnier and Harrison1994). In addition to the troublesome biology of these four dicot weeds, all four species have herbicide-resistant biotypes in multiple sites of action in many grain-producing states in the United States (Heap Reference Heap2017). Resistance to glyphosate and acetolactate synthase inhibitors in all four weed species and resistance to protoporphyrinogen oxidase (PPO) inhibitors in waterhemp and Palmer amaranth have severely limited the POST herbicide options in soybean.
Growers in the state of Indiana have traditionally dealt with horseweed and giant ragweed as the predominant troublesome weeds in soybean. More recently, Palmer amaranth and tall waterhemp have become more widespread across the state of Indiana. Palmer amaranth has been confirmed in more than half of Indiana’s counties, while 26 counties have confirmed glyphosate-resistant tall waterhemp, and 10 counties have confirmed PPO-resistant tall waterhemp (TRL and WGJ, personal observation). The introduction of dicamba-resistant soybean provides an additional tool for Indiana farmers and farmers across the United States to control these troublesome broadleaf weeds at planting and in season while providing an additional site of action to the soybean rotation.
While the commercialization of dicamba-resistant soybean will bring additional weed control options to farmers, there is concern that the introduction of this technology will increase the occurrence of off-site movement of dicamba onto susceptible vegetation (Johnson et al. Reference Johnson, Whitford, Weller and Legleiter2012). The movement of dicamba from application sites is of special concern due to the low dosages that cause damage to susceptible dicot plants and the likely presence of economically important susceptible crops such as tomatoes (Solanum lycopersicum L.) and sensitive soybean in nearby locations during POST application timings (Chang and Vanden Born Reference Chang and Vanden Born1971). Tomatoes and sensitive soybean are susceptible to yield-reducing dicamba damage from a drift or volatility event (Kruger et al. Reference Kruger, Johnson, Doohan and Weller2012; Robinson et al. Reference Robinson, Simpson and Johnson2013). Concerns of widespread, economically damaging dicamba movement have recently increased, with multiple cases of off-site movement in the delta regions of Missouri, where unlabeled dicamba products were applied to dicamba-resistant soybean and cotton (Bradley Reference Bradley2016). The ultimate success of this new herbicide-resistant soybean technology will hinge largely on the success in minimizing off-site movement events.
Movement of herbicides by particle drift or volatilization is influenced by a number of factors, including meteorological conditions, herbicide formulations, sprayer setup, and droplet spectra size (Carlsen et al. Reference Carlsen, Spliid and Svensmark2006; Combellack Reference Combellack1982). While the meteorological conditions that affect herbicide movement cannot be controlled, the other factors can be controlled or manipulated by the applicator to mitigate off-site movement. Three EPA-approved dicamba products, EngeniaTM (BASF Corporation, 26 Davis Drive, Research Triangle Park, NC 27709 ), XtendimaxTM (Monsanto, 800 N. Lindbergh Boulevard, St Louis, MO 63167), and FeXapan™ (DuPont Crop Protection, P.O. Box 80705 CRP 705/L1S11, Wilmington, DE19880-0705) are registered for application to dicamba-resistant soybean and contain label language that restricts applications to specific broadcast nozzle types, operating pressures, and orifice sizes to minimize the risk of off-site movement. The labels specifically restrict users to large-orifice nozzles that contain pre-orifice, air-induction, or turbulence chamber designs that produce extremely coarse to ultra-coarse droplet spectra and minimize the number of driftable fines. The use of nozzles that produce these larger droplet spectra will reduce horizontal movement of spray particles or drift due to their increased mass and reduced time in the state of fall when used in combination with the other labeled application parameters (Bode Reference Bode1987).
Extremely coarse to ultra-coarse droplet spectra not only reduce off-site movement, but can also reduce herbicide spray coverage, which can reduce herbicide performance (Knoche Reference Knoche1994). While droplet size is largely influential on herbicide coverage and performance, the type of herbicide, target species, and interfering crop canopies must also be considered (Knoche Reference Knoche1994). Performance of contact herbicides is much more influenced by droplet size and coverage than systemic herbicides, such as dicamba and glyphosate, that can perform over a wider range of coverages and droplet sizes (Ramsdale and Messersmith Reference Ramsdale and Messersmith2001). The architecture of the target weed also influences the overall deposition of herbicide solution, because dicots with greater leaf areas are likely to receive greater coverage than monocot species that have a smaller leaf surface to capture droplets (Dorr et al. Reference Dorr, Hanan, Adkins, Hewitt, O’Donnell and Noller2008). The interference of a crop canopy can also alter herbicide coverage, because droplets can be filtered and coverage reduced at lower levels in the canopy, where target weed(s) may exist (Bradley and Sweets Reference Bradley and Sweets2008; Legleiter and Johnson Reference Legleiter and Johnson2016).
POST applications of dicamba in dicamba-resistant soybean are most likely to be targeted toward troublesome and herbicide-resistant broadleaf weeds such as Palmer amaranth, tall waterhemp, giant ragweed, and horseweed (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012). These applications are likely to occur with interference from a crop canopy if they following a PRE application or interference from other weeds in a total POST application system (Legleiter et al. Reference Legleiter, Bradley and Massey2009).
The objective of this experiment was to evaluate (1) the influence of droplet spectra produced by two traditional flat-fan nozzles and two drift-reduction air-induction nozzles on deposition of a POST glyphosate plus dicamba application on glyphosate-resistant Palmer amaranth, tall waterhemp, giant ragweed, and horseweed and (2) the resulting absorption of the herbicides into each weed species and any differences in efficacy on those species.
Materials and Methods
Field Sites
The experimental data set is represented by 2 site-years per weed species or 8 total site-years. Field experiments were conducted at locations with populations of glyphosate-resistant Palmer amaranth, tall waterhemp, horseweed, and giant ragweed during the 2015 and 2016 growing seasons. Locations of experiments can be found in Table 1. Vegetation was terminated before planting either with tillage or a paraquat treatment, with the exception of the Brookston 2016 site, which was planted into an existing stand of horseweed due to delays in planting and spray applications due to weather. A glyphosate-resistant soybean variety (Asgrow® 2933, Monsanto) was planted at all sites in 38-cm row spacing at rate of 312,000 to 370,000 seeds ha−1. A PRE application of acetochlor at 840 g ai ha−1 (Warrant®, Monsanto) was applied to the Medaryville 2016 Palmer amaranth and Meigs 2016 tall waterhemp experiment sites to suppress high-density populations of each respective weed species and allow for soybean emergence and development before the POST application. Planting dates for each location can be found in Table 1.
Table 1 Planting date, date of herbicide application, and application parameters for the Palmer amaranth, tall waterhemp, giant ragweed, and horseweed site-years.Footnote a

a Abbreviations: BRK, Brookston, IN; MDV, Medaryville, IN; MGS, Meigs South Research Facility; WL, West Lafayette, IN; AMAPA, Palmer amaranth; AMATA, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed.
Herbicide Application and Experimental Design
Plots were arranged in a randomized complete block design with six replications, and measured 3-m wide by 8-m long. An all-terrain vehicle with a 2-m side boom with four nozzles spaced on 50-cm centers was used to apply treatments. Treatments were applied using nozzle orifices rated for a 1.5 L min−1 output, pressurized at 276 kPa at a travel speed of 19 km h−1 in an effort to replicate commercial field applications. Total output of the spray application was 94 L ha−1. Crop stage, weed height and density, and weather conditions at the time of application are listed in Table 1.
Four 110° broadcast flat-fan TeeJet® (TeeJet Technologies, 200 W. North Avenue, Glendale Heights, IL 60139) nozzles were selected for evaluation due to the following design attributes: the XR11004 represents a traditional, single-stage, flat-fan nozzle without any drift-reduction attributes; the TT11004 represents a two-stage plus turbulence chamber nozzle design; the AIXR11004 represents a two-stage air-induction nozzle design; and the TTI11004 represents a two-stage, air-induction, and turbulence chamber nozzle design. The AIXR11004 and TTI11004 nozzles would both represent drift-reduction technology nozzles, although only the TTI11004 is currently labeled for applications of the approved dicamba formulations.
The herbicide solution was 280 g ha−1 glyphosate (Roundup PowerMax®, Monsanto) plus 140 g ha−1 dicamba (EngeniaTM, BASF) and a nonionic surfactant (NIS) at 0.25% v/v. The lower than labeled rates of herbicide were used to maximize any differences in efficacy that might occur between application treatments. A visual pink foam marker dye (Vision PinkTM, Garrco Products, P.O. Box 619, Converse, IN 46919-0619) and a fluorescent 1,3,6,8 pyrene tetra sulfonic acid (PTSA) dye (Spectra Trace SH-P, Spectra Colors, 25 Rizzolo Road, Kearny, NJ 07032) were also included in the spray mixture at 0.25% v/v and 600 µg ml−1, respectively.
Data Collection and Analysis
Spray Solution Coverage and Deposition Density
Spray solution coverage and deposition density were evaluated using 5 cm by 7.6 cm cardstock coated with Kromekote that shows defined marking when contacted with spray solutions containing the Vision PinkTM foam marker dye. Five cards were placed parallel to the ground in each plot using metal holders just before each application. Cards were placed at the height of the target weeds and were arranged in a diagonal pattern between two soybean rows to capture droplets at all positions. Cards were allowed to dry after application and were then placed in plastic bags for storage until further analysis.
A duplex scanner (Image CenterTM ADS-2000, Brother International, 200 Crossing Boulevard, Bridgewater, NJ 08807-0911) was used to convert the cards into 600 by 600 dpi, 24-bit color digital images. The pink droplet depositions were separated from the white background of the image using Assess 2.0 Image Analysis Software for Plant Disease Quantification, (American Phytopathological Society, 3340 Pilot Knob Road, St Paul, MN 55121). The Assess data output included the area of droplet depositions (mm2) and droplet deposition counts from within the area of the card. Using the known size of the cards, the droplet deposition area was converted to percent coverage, and droplet deposition counts were converted to deposition density.
The five individual cards from each plot were treated as subsamples of the whole plot. Differences in percent coverage and deposition density were analyzed using ANOVA in SAS v. 9.4 PROC MIXED (SAS Institute, Cary, NC 27513) with replication as a random factor. Means separation occurred at alpha=0.05 adjusted for Tukey honest significant difference (HSD). Means were pooled across site-years within a species when differences between site-years did not occur.
Herbicide Solution Deposition on Target Weeds
Herbicide deposition data were collected using a fluorescent tracer dye and methods developed based on Fritz et al. (Reference Fritz, Hoffmann and Jank2011). One plant of the target weed that represented the average weed height of the plot was harvested and washed with 200 ml of an NIS (TritonTM X-100, EMD Chemicals, 480 South Democrat Road, Gibbstown, NJ 08027) and water (1:1000) solution immediately following herbicide application. The representative target weed height for each site can be found in Table 1; crew members were instructed to collect 10- to 15-cm-tall weeds with minimal overhead interference at the Brookston 2016 site, which had a large variation in weed heights. Plants were washed in the solution using the following method: a syringe was used to pull wash solution from the clean vial before any introduction of plant material; the target plant was then cut at the soil surface while being grasped with a set of forceps and was then placed into and agitated in the wash solution for 30 s; and the plant was then rinsed with the solution from the syringe as it was removed from the wash vial. Washed plants were then placed in envelopes for transportation back to the campus laboratory for whole-plant leaf-area analysis using a leaf-area meter (LI-3100, LI-COR, 4647 Superior Street, Lincoln, NE 68504-5000). Between treatments, the forceps were washed with a 1:1 water and methanol solution to avoid cross contamination; if used properly, the syringes were only exposed to uncontaminated solution and were only replaced between treatments if a contamination event occurred.
Wash solutions were transported to the campus laboratory and quantified for raw fluorescence with a laboratory fluorimeter (Trilogy Laboratory Fluorometer, Turner Designs, 1995 N. 1st Street, San Jose, CA 95112) equipped with a PTSA-specific module. The PTSA concentration (µg ml−1) in the wash solution, known volume of the wash solution (200 ml), known rate of PTSA in the spray-tank solution (600 µg ml−1), and leaf area of the plant (cm2) were all used to calculate the final values of wash solution deposited onto the target plant surface (µl cm−2).
ANOVA of spray solution deposition onto target plants was conducted using SAS v. 9.4 PROC MIXED with means separation using Tukey HSD at alpha=0.05. Herbicide solution deposition means within a species were pooled across site-years when differences between years were not significant.
Dicamba Concentration on Leaf Surface
Dicamba concentration on the leaf surface was taken immediately following herbicide application and at 2, 4, 6, and 24 h after herbicide application. One leaf from a target species plant was harvested from each replication at each timing. The leaf was selected from a plant of the target height as described in the previous section; the harvested leaf was at the node below the newest fully expanded leaf. The selected leaf was washed in 50 ml of 1:1 water and high-performance liquid chromatography–grade methanol solution. The wash procedure consisted of using a syringe to extract 10 ml of clean solution from the 50 ml vial before introducing any leaf material and then agitating the leaf in the remaining solution in the vial for 30 s. The leaf was then rinsed with the 10-ml solution from the syringe as it was pulled from the wash solution vial. Wash solutions were stored at room temperature in closed boxes until preparation and analysis. The leaf area and the biomass of the leaves were taken from the washed leaves in the lab following collection in the field. Leaf wash solutions were collected from all six replications, although only three replications were analyzed for dicamba concentration in the procedures described in the following paragraphs.
Wash solutions were prepared for analytical analysis by taking a 1-ml aliquot of wash solution and adding 500 ng of d3-dicamba (CDN Isotopes, Pointe-Claire, QC, Canada) as an internal standard. Samples were dried in a vacuum concentrator, then derivatized by adding 40 µl anhydrous pyridine and 60 µl MSTFA, and finally heated for 1 h at 60 C.
Levels of dicamba were determined using a gas chromatograph/mass spectrometer–mass spectrometer analysis. The gas chromatograph was a 1310 Thermo Trace using a Thermo TG-SQC column (15 m by 0.25 mm by 0.25 µm). A 1-µl injection volume was used with an inlet temperature of 250 C with a 10:1 inlet split ratio and column flow of 1.5 ml min−1. The thermal gradient had an initial temperature of 120 C, held for 1 min, a 20 C min−1 increase until 320 C, then held for 3 min. The retention time for dicamba was 3.6 min.
Analytes were then quantified with a Thermos TSQ Evo 8000 triple-quadrupole mass spectrometer. Positive chemical ionization mode was used, with a methane flow rate of 1.0 ml min−1. Quantitation was based on multiple reaction monitoring. A transition of 292 to 202 was used for dicamba and 295 to 204 for d3-dicamba. A collision energy of 5 V was used for all transitions. Data were collected and analyzed with Thermo Chromeleon v. 7.2 SR4 software (Thermo Fisher Scientific, Waltham, MA). Responses for dicamba were normalized and quantitated against the internal standard.
Quantities of dicamba from the wash samples were then converted to nanograms of dicamba per square centimeter of leaf surface area using the previously measured leaf areas. A two-factor ANOVA was used to evaluate differences in nanograms of dicamba per square centimeter between nozzles and collection times. Analysis was conducted using SAS v. 9.4 PROC MIXED with means pooled across years for each species to increase the power of statistical analysis. Means separation was performed with Tukey HSD, α=0.05.
Herbicide Efficacy
Plots were evaluated for herbicide efficacy 21 d after application. A 0% to 100% visual evaluation was taken for control of the target weed species with 0% representing no control and 100% representing complete control. Height measurements were taken for three randomly selected plants per plot and for three plants within untreated strips in each replication block at the Palmer amaranth, tall waterhemp, and horseweed sites. Heights of giant ragweed were not taken due to the overall high efficacy of dicamba on the species and a lack of measurable plants. Height measurements of the three plants per plot were treated as subsamples of the whole plot and converted to percent height reduction using the weed heights in the untreated strips.
Differences in visual evaluations and percent height reduction were determined using ANOVA in SAS v. 9.4 PROC MIXED with means separation using Tukey HSD at alpha=0.05. Replications were considered a random factor. Visual evaluation means for Palmer amaranth, giant ragweed, and horseweed were pooled across years due to a lack in differences between years. Visual evaluation means for tall waterhemp and plant height reduction means for all species were pooled across years to maximize statistical power due to similarities in means differences.
Droplet Spectrum Analysis
Spray droplet spectrum analysis was conducted on one randomly selected nozzle of the four used for each nozzle type in the field study in an effort to broaden the applicability of the data to droplet categories rather than just the four specific nozzles evaluated. Analysis was conducted using the discriminating spray tank mix from the field experiments (280 g ha−1 glyphosate plus 140 g ha−1 dicamba plus 0.25% v/v NIS) and the labeled rates of the products (1,120 g ha−1 glyphosate plus 560 g ha−1 dicamba plus 0.25% v/v NIS).
The Pesticide Application Technology Laboratory at the University of Nebraska–Lincoln West Central Research and Extension Center (UNL PAT) conducted spray droplet spectrum analysis. Before analysis, each nozzle tip was flow rated to determine that wear or damage had not occurred to the orifice during field experiment applications. The spray droplet spectrum was analyzed using laser diffraction with a Sympatec Helos Vario KR particle-size analyzer equipped with an R7 lens. Analysis was conducted within a low-speed wind tunnel with an air velocity of 24 km h−1 to aid in evacuation of spray droplets from the laser path after analysis. The spray plume of each nozzle was analyzed three times by traversing the entire plume vertically through the laser path. The Dv10, Dv50, and Dv90, which represents the percentage (10, 50, and 90 respectively) of droplets within the spray volume that are at or below the reported diameter, were output from the analysis. Each nozzle and herbicide rate combination was classified into a droplet category based on the Dv10, Dv50, and Dv90 values using an established reference curve from the UNL PAT lab per ASABE S572.1.
Results and Discussion
Droplet Spectrum Analysis
The spray droplet sizes produced by the nozzle types were as expected, with a Dv50 of 234 to 276 µm occurring with the single-stage XR11004 nozzle and Dv50 values of 337 to 763 µm provided by the TT11004, AIXR11004, and TTI11004 two-stage nozzles (Table 2). The two air-induction nozzles, AIXR11004 and TTI11004, had Dv50 values of 432 to 763 µm; they also both had Dv10 values greater than 200 µm, indicating spray volume contained less than 10% driftable fines (Table 2). Despite the low percentage of driftable fines for both air-induction nozzles, only the TTI11004 nozzle with the greatest volume mean diameter is allowed for applications of the approved dicamba formulations. When comparing the Dv50 values between the herbicide rates, the XR11004 and AIXR11004 nozzles had a smaller Dv50 value at the full rate compared with the reduced rate. The smaller Dv50 at the full rate would be expected with the increased amount of glyphosate formulation and surfactant load which is known to decrease droplet size (Hilz and Vermeer Reference Hilz and Vermeer2013). Conversely, the two nozzles with turbulence chambers (TT11004 and TTI1104) produced smaller Dv50 values at the reduced rate, which may be an indication of an interaction of the turbulence chamber design.
Table 2 Dv10, Dv50, Dv90, and spray classification category for each nozzle at a discriminating and full rate of glyphosate plus dicamba.Footnote a

a Dv10, Dv50, and Dv90: the percentage (10, 50, and 90 respectively) of droplets in the spray volume that are at or below the reported diameter.
b Discriminating rate: 280 g ha−1 glyphosate plus 140 g ha−1 dicamba; full rate: 1,120 g ha−1 glyphosate plus 560 g ha−1 dicamba.
c Spray classification categories assigned using reference curve generated at the University of Nebraska–Lincoln Pesticide Application Technology laboratory in accordance to ASABE S542.1.
The Dv10, Dv50, and Dv90 values of each nozzle and herbicide rate were plotted on an established standard curve and placed into droplet categories in accordance to ASABE S572.1. The TT11004 produced medium droplets at both herbicide rates, the AIXR11004 produced a very coarse droplet spectrum for both rates, and the TTI11004 produced the largest droplet spectrum of ultra-coarse for both herbicide rates (Table 2). The XR11004 nozzle was the only nozzle to differ in droplet spectrum categories between the two herbicide rates, with the full rate producing a fine spectrum and the reduced rate producing a medium droplet spectrum. The two reduced-drift nozzles of interest (AIXR11004 and TTI11004) provided similar droplet categories between rates, despite differences in Dv50 values between rates, allowing for evaluation of the data in the following sections without regard to the rate. The two non–air induction nozzles (TT11004 and XR11004) will be considered as medium droplet–producing nozzles, as this is the category that both fell within at the discriminating rate.
Spray Solution Coverage and Deposition Density
Spray solution coverage was reduced by the TTI11004 nozzle (10.7% to 20.1%) compared with the AIXR11004 (11.1% to 24.6 %), TT11004 (16.1% to 25.9 %), and XR11004 (16.6% to 26.6%) nozzles in the tall waterhemp, horseweed, and giant ragweed experiments (Table 3). The TTI11004 nozzle also reduced spray solution coverage compared with the XR11004 and TT11004 nozzles in the Palmer amaranth experiments and was similar to the AIXR11004 (Table 3). The other drift-reduction nozzle, AIXR11004, was similar to the two smaller droplet–producing nozzles in all experiments, with the exception of the 2015 Palmer amaranth site. The decrease in spray coverage with the ultra-coarse droplet produced by the TTI11004 nozzle was expected, as previous work has also shown a decrease in coverage with increasing droplet size (Knoche Reference Knoche1994).
Table 3 Glyphosate plus dicamba solution coverage on spray cards placed at the height of Palmer amaranth, tall waterhemp, giant ragweed, and horseweed at a POST application.

a Abbreviations: AMAPA, Palmer amaranth; AMATA, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed
b Means pooled across site-years.
c Means within a column followed by a different letter are significantly different. Tukey HSD at α=0.05.
Deposition density was the greatest with the two non–drift reduction nozzles and ranged from 33 to 53 deposits cm−2 across all sites (Table 4). The drift-reduction AIXR11004 nozzle produced 19 to 31 deposits cm−2, which was lower than the two non–drift reduction nozzles, but greater than the TTI11004 nozzle (9 to 15 deposits cm−2) (Table 4). The decrease in deposition density with an increase in droplet size would be expected with a set carrier volume and may be a concern if approaching a minimum threshold for deposition.
Table 4 Glyphosate plus dicamba deposition counts per square centimeter on spray cards placed at the height of Palmer amaranth, tall waterhemp, giant ragweed, and horseweed at a POST application.

a Abbreviations: AMAPA, Palmer amaranth; AMATA, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed.
b Means pooled across site-years.
c Means within a column followed by a different letter are significantly different. Tukey HSD at α=0.05.
The use of spray cards gives an effective estimate of solution coverage of a surface as well as density of depositions onto a surface. The data collected by the cards in this study across multiple species were as expected in comparison to previous work (Knoche Reference Knoche1994). However, due to differences in target plant leaf surface angles and composition, investigating the actual deposition onto our target weed leaf surfaces is warranted.
Herbicide Solution Deposition on Weed Species
Means of herbicide solution deposition onto Palmer amaranth, giant ragweed, and horseweed were pooled across years, while tall waterhemp means were separated. Deposition of herbicide solution on Palmer amaranth was 0.41 to 0.52 µl cm−2, giant ragweed deposition was 0.49 to 0.58 µl cm−2, and horseweed deposition was 0.38 to 0.41 µl cm−2 (Table 5). Herbicide solution deposition onto tall waterhemp was 0.55 to 0.62 µl cm−2 in 2015 and 0.78 to 0.87 µl cm−2 in 2016. Differences between tall waterhemp site-years likely occurred due to differences in weed density, because the density was higher in 2015 than in 2016 (Table 1), and a depleted soybean canopy in 2016. The lower level of soybean canopy and lower densities of tall waterhemp plants in 2016 likely reduced the amount of droplet filtering and thus increased overall herbicide solution deposition onto the target plants. The lowest solution deposition occurred on horseweed, which can be attributed to the vertical architecture of smaller leaves stacked along the bolting stem on horseweed that are less exposed to a broadcast application than the other three weed species, which have more horizontally spread leaf architectures.
Table 5 Deposition of glyphosate plus dicamba spray solution on target plants of Palmer amaranth, tall waterhemp, giant ragweed, and horseweed.

a Abbreviations: AMAPA, Palmer amaranth; AMAT, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed.
b Means pooled across site-years.
The theoretical maximum herbicide solution deposition on the leaf surfaces would be 0.935 µl cm−2, which is simply a conversion of the field application rate (0.94 l ha−1) to microliters per square centimeter. In comparison to a theoretical maximum deposition, the depositions in these studies were 41% to 93% of that value. A reduction in the deposition compared with the theoretical maximum would be expected, because the application was not made to a flat surface in a vacuum, but in the field, with environmental conditions that effect deposition and highly variable deposit surfaces of multiple plant species that occur at varying angles and heights.
There were no differences in herbicide solution deposition between nozzle types within each species, despite differences in site-years and variability among weed species. The two air-induction nozzles (AIXR11004 and TTI11004) both provided equivalent herbicide solution deposition on the four target species compared with the two traditional medium droplet–producing nozzles.
The deposition of herbicide solution from a broadcast application onto these four broadleaf weeds was not affected by the nozzle design or droplet spectrum, but rather was more likely affected by filtering from the soybean canopy and other weeds, and the overall weed leaf architecture.
Dicamba Concentration on Leaf Surface
The level of dicamba on the leaf surface was influenced by time after application (P<0.0001); the influence of nozzle and the interaction of nozzle and time after application was insignificant for all four weed species (Figure 1). Dicamba levels on the leaf surface were greatest at the 0-h time for all four weed species and ranged from 707 to 1,083 ng dicamba cm−2 leaf surface area (Table 6). These concentrations of dicamba are 50% to 77% of the field application target of 1,400 ng cm−2 or 140 g ha−1. The recovery efficiency of dicamba from the leaf surface following application was similar to the recovery efficiency of the fluorescent dye. Dicamba levels on the leaf surface were reduced by 2 h after application and continued to drop until 24 h after application for all four weed species (Table 6).
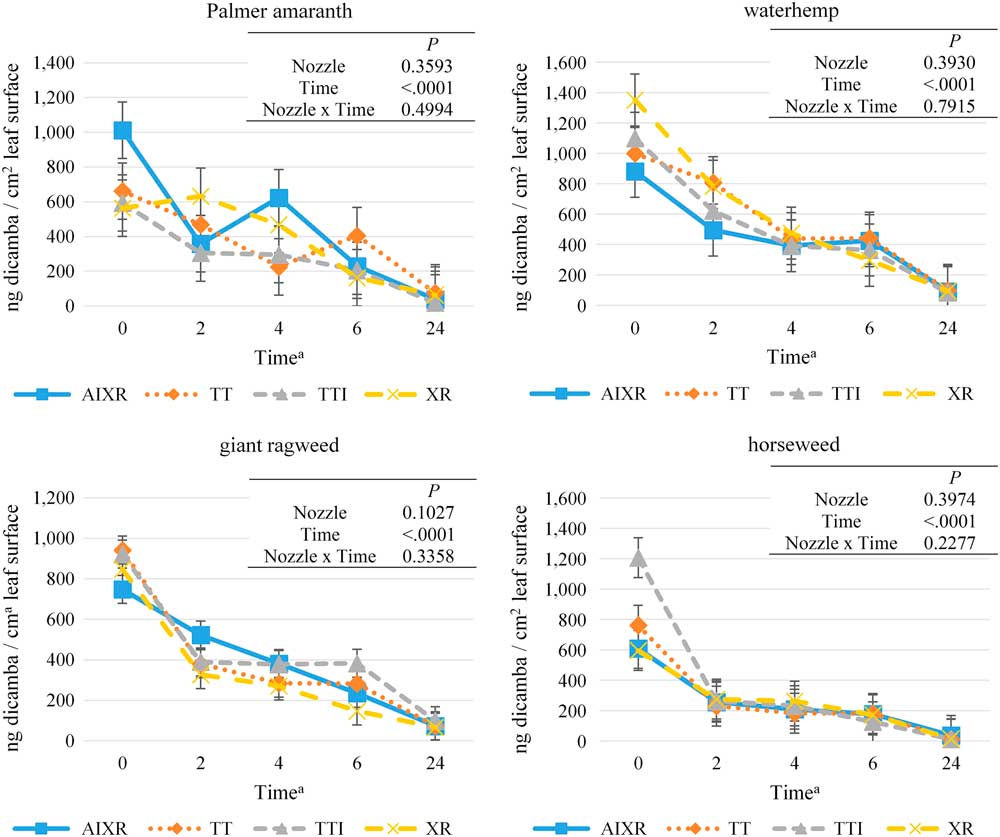
Figure 1 Concentration of dicamba on the leaf surface of Palmer amaranth, waterhemp, giant ragweed, and horseweed leaves over a 24-h period after herbicide application as influenced by broadcast spray nozzle design. Abbreviations: XR, XR11004 nozzle; TT, TT11004 nozzle; TTI, TTI11004 nozzle; AIXR, AIXR11004 nozzle (TeeJet Technologies, 200 W. North Avenue, Glendale Heights, IL 60139); Time=hours after application.
Table 6 Concentration of dicamba on the leaf surface of Palmer amaranth, waterhemp, giant ragweed, and horseweed leaves over a 24-h period following herbicide application.

a Abbreviations: AMAPA, Palmer amaranth; AMATA, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed.
b Means within a column followed by a different letter are significantly different. Tukey HSD at α=0.05.
Dicamba concentration analysis from the surface of the leaf following herbicide application revealed that likely maximum absorption of the herbicide occurs 2 h after application for waterhemp, giant ragweed, and horseweed and at 4 h for Palmer amaranth, with significant decreases on the leaf surfaces occurring at these times (Table 6). The droplet spectrum size of the nozzle used to make the application did not influence the absorption of dicamba in Palmer amaranth, waterhemp, giant ragweed, and horseweed, because the concentrations on the leaf surfaces were similar between all four nozzles at 0, 2, 4, 6, and 24 h after application (Figure 1). Results from the methods used in this study indicate that the use of drift-reduction nozzles that produce very coarse and ultra-coarse droplet spectra did not influence the absorption of dicamba in four glyphosate-resistant dicot species.
Herbicide Efficacy
Control and height reduction means were pooled across site-years for each species due to similarities in mean differences. Visual control evaluations were 16% to 17%, 22% to 24%, 37% to 40%, and 77% to 85% for Palmer amaranth, tall waterhemp, horseweed, and giant ragweed, respectively (Table 7). Height reduction was 69% to 72%, 64% to 67%, and 63% to 69% for Palmer amaranth, tall waterhemp, and horseweed, respectively (Table 8). Height reduction was not taken for giant ragweed due to the high levels of efficacy and lack of measurable plants.
Table 7 Control of Palmer amaranth, tall waterhemp, giant ragweed, and horseweed 21 d after treatment with glyphosate (280 g ha−1) plus dicamba (140 g ha−1) as influenced by broadcast nozzle.

a Abbreviations: AMAPA, Palmer amaranth; AMATA, tall waterhemp; AMBTR, giant ragweed; ERICA, horseweed.
b Means within a column followed by a different letter are significantly different. Tukey HSD at α=0.05.
Table 8 Percent height reduction of Palmer amaranth, tall waterhemp, and horseweed 21 d after treatment with glyphosate (280 g ha−1) plus dicamba (140 g ha−1) as influenced by broadcast nozzle.

a Abbreviations: AMAPA, Palmer amaranth; AMATA, tall waterhemp; ERICA, horseweed.
b Means within a column followed by a different letter are significantly different. Tukey HSD at α=0.05.
There were no differences in control and height reduction as influenced by the nozzle design for any of the weed species (Tables 7 and 8). This was similar to the data on herbicide spray deposition, in that nozzle designs and droplet spectra did not have an influence on deposition of the herbicide solution or the resulting efficacy.
In conclusion, the results from the 2 site-years and four weed species indicate that the use of drift-reduction nozzles that produce very coarse to ultra-coarse droplets did not influence the deposition, absorption, or efficacy of a POST application of glyphosate plus dicamba, despite reductions in coverage on spray cards. It should be noted that in the majority of site-years, applications were applied to low-density weed populations and relatively small plants (5 to 15 cm), whereas commercial applications may occur on larger plants and higher densities. The four broadleaf weeds evaluated are likely to be the targets of POST applications of dicamba in dicamba-resistant soybean hectares. The success of dicamba-resistant soybean systems will hinge on the ability to keep dicamba applications on-site and from drifting onto sensitive non-target crops. The use of the approved DRT nozzles for POST applications will allow for lower drift-risk applications while achieving acceptable efficacy when applied in the appropriate conditions of smaller weeds and lower-density populations.
Acknowledgments
The authors would like to acknowledge Greg Kruger, Ryan Henry, and Annah Geyer of the UNL PAT lab in North Platte, NE, for the use of their low-speed wind tunnel and laser-diffraction system and assistance in analyzing the spray droplet spectra of the broadcast nozzles used in this study.











