Introduction
Woolly apple aphid (WAA), Eriosoma lanigerum (Hausmann), is native to North America and a worldwide and quarantine pest of apple, Malus domestica in China (Long et al., Reference Long, Wang and Tang1960; Lordan et al., Reference Lordan, Alegre, Gatius, Sarasúa and Alins2015). Since this aphid established in Shandong, Liaoning, and Yunnan provinces between 1914 and 1930, it has had a serious impact on production and acceptance of fruit for export (He et al., Reference He, Tian and Mao2004). However, recent studies have found that WAA has continued to expand in China (Lu et al., Reference Lu, Liu, Ran, Qu and Li2013). Surveys in Rizhao, Shandong Province from 2000 to 2002, found WAA in about 8000 hectares of orchard, with 10–20% of the trees being infested, causing an annual loss of 5 × 106 kg of apples (Wang et al., Reference Wang, Jiang and Xu2011). In 2007, WAA spread to Hebei Province where it has caused significant crop damage in several regions (Qinhuangdao, Tangshan, and Shijiazhuang) (Wu et al., Reference Wu, Wan and Li2009).
Aphelinus mali (Haldeman), a special parasitoid of WAA, has been introduced several times into China from the 1940s to the 1960s, and plays an important role in controlling WAA in China (Zhou et al., Reference Zhou, Zhang, Tan, Tao, Wan, Wu and Chu2015). A. mali would have had little influence on WAA spread, but may well influence population abundance and severity of damage.
In previous work, we found that A. mali is represented in China by two different genetic clades based on mitochondrial COI sequences and PCR-RFLP: a Shandong clade and a Liaoning clade (Zhang et al., Reference Zhang, Zhou, Guo, Tao, Wan, Wu and Chu2014; Zhou et al., Reference Zhou, Zhang, Guo, Tao, Wan, Wu and Chu2014). We studied the differences in the control ability between these two clades on WAA from Shandong (Qingdao) Province, and found the developmental threshold temperature of the Shandong clade to be lower than that of the Liaoning clade, while the fecundity, longevity, and pest control ability of the Shandong clade were all significantly better than that of the Liaoning clade (Su et al., Reference Su, Tan, Yang, Zhou and Wan2016). However, these differences may have been due to the fact that the WAA used were from Shandong, to which the Shandong parasitoid clade may have become locally adapted. To exclude this possibility, here we report experiments in which we assess the same biological characteristics and control ability of the same two clades of A. mali but with WAA collected from Hebei Province (Qinhuangdao), the area in which only the Liaoning clade of parasitoids in present.
Materials and methods
Test insects
WAA used in this study were collected from the apple orchards of Qinhuangdao in Hebei Province. A. mali of the two clades used in this study were collected from apple orchards in several locations in each of the two provinces specifically for this study: (1) Shandong Province (Qingdao) (=the Shandong clade) and (2) Hebei Province (Qinhuangdao) (=the Liaoning clade). We have distinguished the A. mali to two clades based on mitochondrial COI sequences and PCR–RFLP, in order to make clear whether A. mali in our research are two different species, we are doing the hybridization test between two clades. To collect parasitoids, we cut apple branches bearing WAA and then collected and placed the mummified aphids (indicating parasitism) into 1.5 ml centrifuge tubes, where they were held until A. mali emergence. A. mali and WAA were held at 25°C, 70% RH, and a 16:8 h L:D photoperiod.
Comparison of reproductive biology between the two clades of A. mali
To measure the total oviposition by parasitoids of each clade, we modified the sentence as we used the method described by Su et al. (Reference Su, Tan, Yang, Zhou and Wan2016). Mated females were placed individually with an excess of aphids on apple twig (ca 50 aphids) in Petri dishes (13. 5 cm diam) and held for 24 h, after which the female was transferred to a new batch of aphids. Such transfers continued until the female died. Aphids exposed to parasitoids were dissected under the dissecting microscope at the end of their 1-day exposure to determine the total number of eggs laid by each parasitoid. This process was continued until all parasitoids died. This process was repeated for eight females of each parasitoid clade.
To determine the lifespan, aphids were reared rather than being dissected as above, and were checked twice daily to detect parasitism until adult parasitoid emergence. Newly emerged adults of A. mali were held separately under the same environment conditions and provided with 10% honey water. We recorded the sex and date of death of each A. mali individual. Each treatment was repeated for 30 times for males and females of each clade.
Comparison of parasitic behaviors between the two clades of A. mali
Behavioral traits associated with host attack and oviposition were determined for adult parasitoids of each of the two clades as described by Su et al. (Reference Su, Tan, Yang, Zhou and Wan2016). Behaviors (host searching, oviposition, grooming, and resting) and their duration were recorded using a computer video monitor and classified into the defined behaviors mentioned. This was done for 13 newly emerged and mated females of each clade.
Comparison of oviposition to eclosion durations between two clades of A. mali
Fifteen pairs for newly emerged A. mali of each clade were placed together with an excess of hosts on apple twig (ca 200 aphids) in Petri dishes (13.5 cm diameter). The parasitoids were removed after 24 h, and the exposed WAA were held at 70% RH and a 16:8 h L:D photoperiod and allowed to develop under one of five temperatures (18, 20, 23, 25, and 28°C). The aphids were observed daily to record when aphids turned black and eclosion of each parasitoid. Newly emerged adults of A. mali were removed and held separately in 1.5 ml centrifuge tubes under the same environment conditions and provided with 10% honey water. We recorded the sex and date of death of each A. mali individual, and determined the number of day degrees for each stage of development and the longevity of adult of each clade at each temperature.
Comparison of effective accumulated temperature and developmental threshold temperatures between the two clades of A. mali
Using the same approach as above, the effective accumulated temperature (K) and the developmental threshold temperature (C) were calculated according to Ma (Reference Ma2009).
 $$C = \displaystyle{{\sum {\mathop V\nolimits^2 \sum {T - \sum {V\sum {VT}}}}} \over {n\sum {\mathop V\nolimits^2 - \mathop {(\sum V )}\nolimits^2}}}, $$
$$C = \displaystyle{{\sum {\mathop V\nolimits^2 \sum {T - \sum {V\sum {VT}}}}} \over {n\sum {\mathop V\nolimits^2 - \mathop {(\sum V )}\nolimits^2}}}, $$where n is the number of groups in every experiment, T is the constant temperature, and V is the average development rate.
Comparison of searching efficiency and control ability between the two clades of A. mali
Searching efficiency is E = Na/(N 0 × P), where E is the searching efficiency, Na is the number of prey consumed by the predator, N 0 is the initial prey density, and P is the density of the natural enemies (Zhang et al., Reference Zhang, Hua and Xu2005). In our study, searching time was modeled based on a searching efficiency (E) and prey density (N 0) model of E = a/(1+a × Th × N 0), in which all parameters of the formula are functional response parameters. To determine the searching efficiency of A. mali from each clade, a prey group of approximately 50 WAA with mixed life stages were exposed in a Petri dish (13.5 cm diameter) to various numbers of newly mated pairs of A. mali (1, 2, 3, 4, or 5). Parasitoids were removed after 24 h, and the exposed WAA allowed to continue to develop normally to detect parasitism. Each density of parasitoids was replicated four times for each clade, and each group was held at 25°C, 70% RH, and L:D = 16:8.
To determine the ability of A. mali from different clades to attack woolly apple aphid under laboratory conditions, one male and one female of A. mali were placed in a petri dish with either 50, 100, 150, 200, or 250 woolly apple aphids. A. mali adults were removed after 24 h, and the aphids allowed to develop naturally at 25°C, 70% RH, and L:D = 16:8. Aphids were observed each day, and the number of black aphids (indicating parasitization) recorded. Each treatment was repeated four times for each genetic clade.
Statistical analyses
Developmental rates at different temperature conditions [mean ± standard deviation (SD)] were calculated with Statistical Product and Service Solutions (SPSS) 20.0 (Su et al., Reference Su, Tan, Yang, Zhou and Wan2016). Significant differences in oviposition and lifespan, parasitic behaviors, duration times, effective accumulated temperature and developmental threshold temperatures, and searching efficiency and control ability were tested and analyzed using one-way analysis of variance by SPSS 20.0. Independent-sample t tests were used to analyze the developmental duration of each clade by SPSS 20.0.
Results
Comparison of reproductive biology between the two clades of A. mali
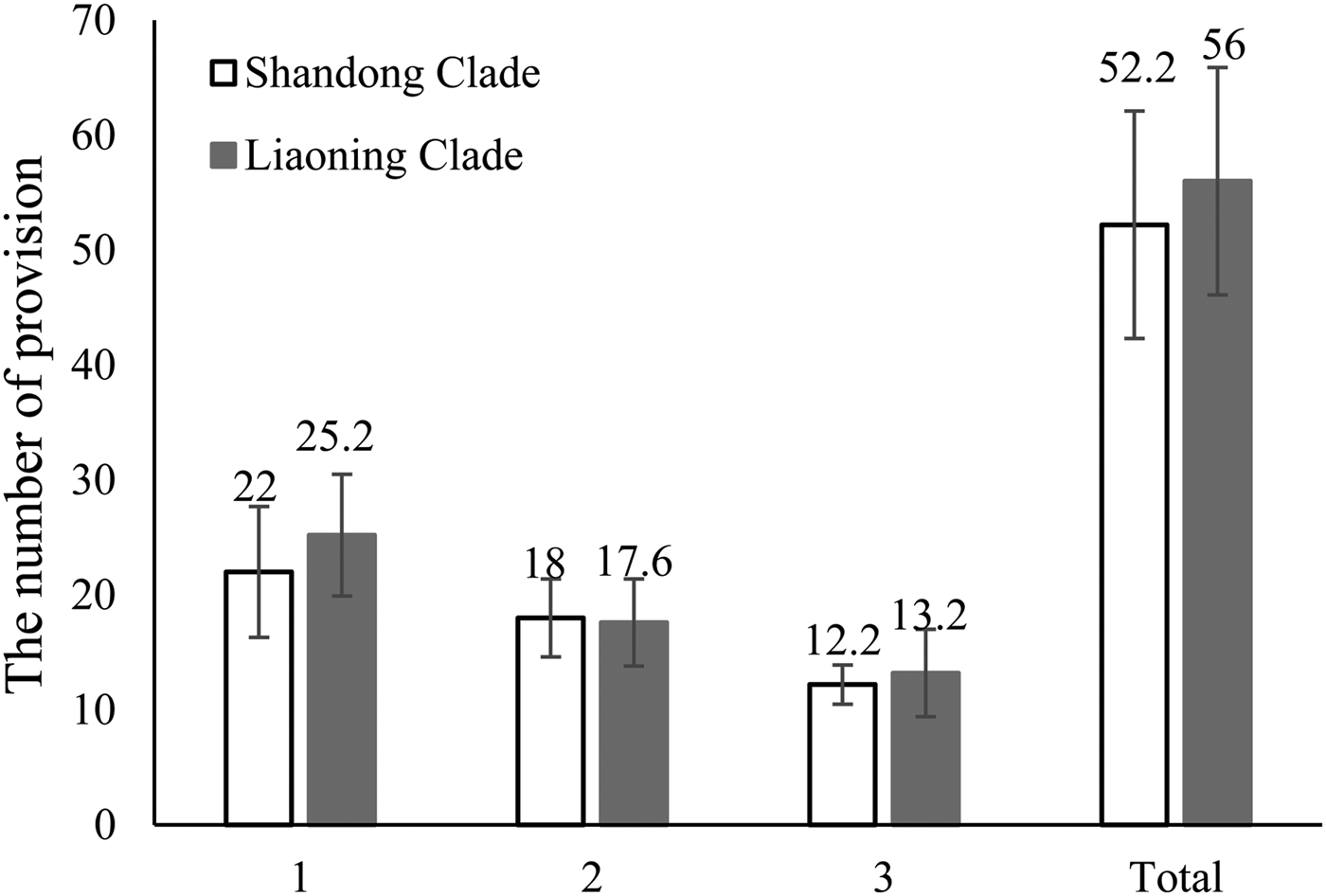
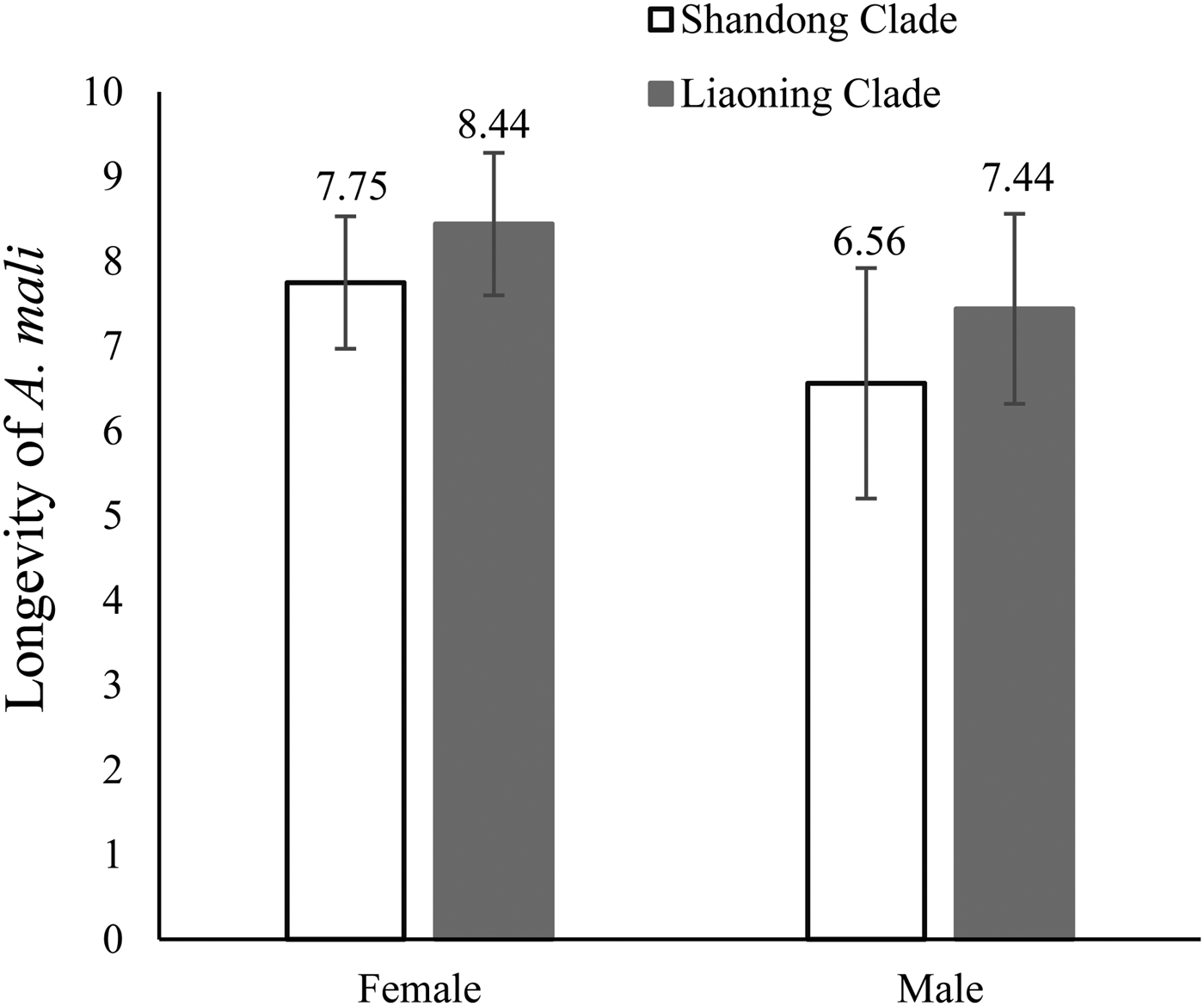
No significant difference was observed between the average daily fecundity of the Shandong clade vs. the Liaoning clade (F = 0.003, df = 8, P = 0.793) (fig. 1), nor between the longevity of the Shandong clade vs. the Liaoning clade (female: F = 2.386, df = 23, P = 0.417; male: F = 0.143, df = 23, P = 0.486) (fig. 2).

Fig. 1. Mean number of woolly apple aphid (Eriosoma lanigerum) parasitized by Aphelinus mali, with daily provision with new hosts.

Fig. 2. Longevity of Aphelinus mali from two genetic clades, as mean (of 50), maximum, and minimum values in days.
Comparison of parasitic behaviors between the two clades of A. mali
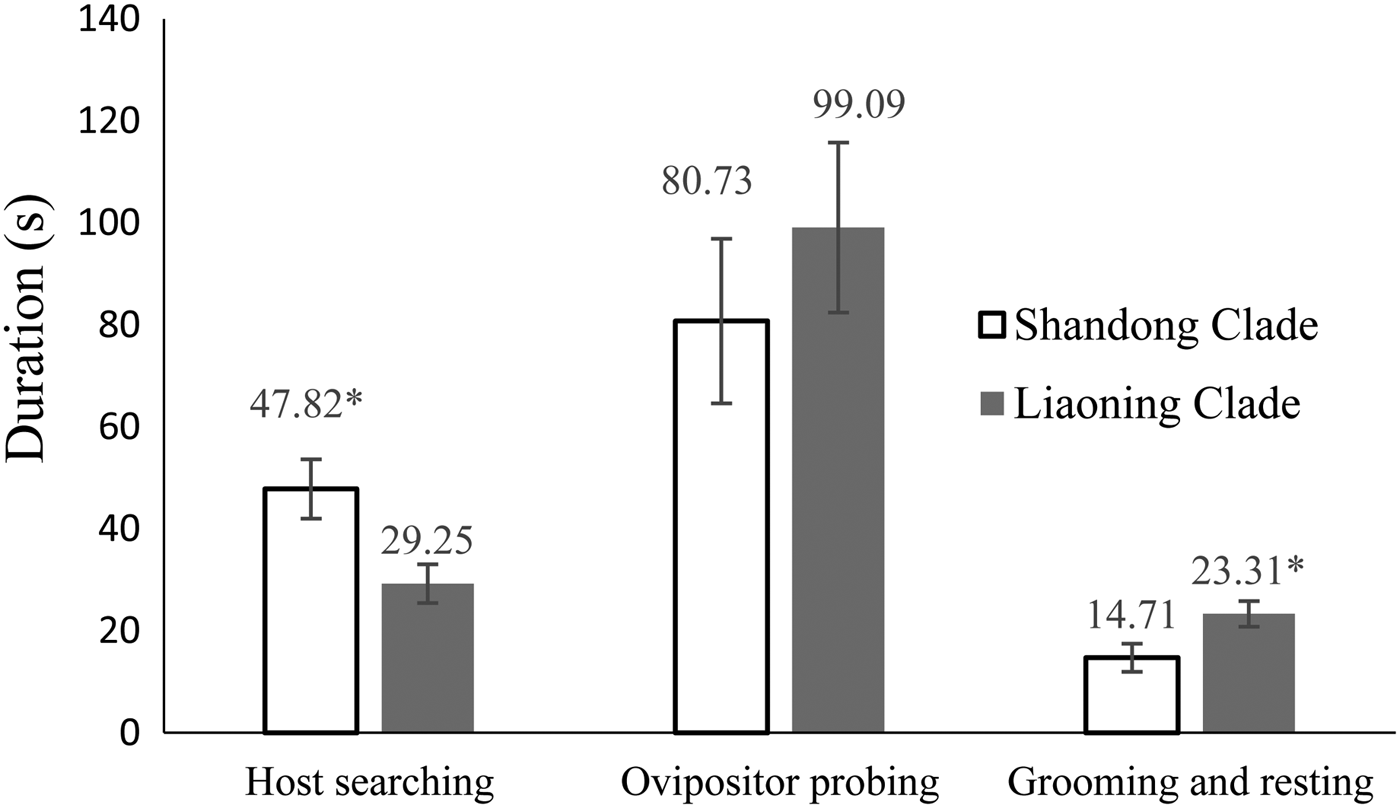
When we divided the time spent by A. mali into four behaviors (host searching, oviposition, grooming, and resting), we found that the host searching time of the Shandong clade (47.8 s per host encounter) was significantly longer than that of the Liaoning clade (29.3 s per host encounter) (F = 4.883, df = 27, P = 0.013), while the time spent grooming or resting by the Shandong clade was significantly shorter than that of the Liaoning clade (F = 0.062, df = 18, P = 0.037). No significant differences were observed in times actually used for oviposition between the two clades (fig. 3).

Fig. 3. Durations (in seconds per host encounter) of behaviors of Aphelinus mali from two genetic clades in China.
Comparison of oviposition to eclosion durations between two clades of A. mali
There were no significant differences between the two clades of A. mali in the stage duration from oviposition to adult eclosion (table 1).
Table 1. Stage duration (D, in days) and developmental rate (V as proportion total development per day) of non-overwintering generations of Aphelinus mali from two genetic clades in China, from oviposition to adult eclosion, for both sexes at five temperatures.

Values in the table are means ± SD; different lowercase letters indicate significant differences between different temperatures in males and females.
Comparison of effective accumulated temperature and developmental threshold temperatures between the two clades of A. mali
We found the lower developmental thresholds of both males (F = 1.929, df = 4, P = 0.054) and females (F = 1.231, df = 4, P = 0.516) of the Shandong clade did not differ significantly to that of the Liaoning clade, and the total number of degree-day units needed for stage completion of the Shandong clade was not significantly different to the Liaoning clade (males: F = 0.789, df = 4, P = 0.085 and females: F = 2.146, df = 4, P = 0.472) (table 2).
Table 2. Lower developmental thresholds (LDT) and total degree-days (TDD) of non-overwintering generation of Aphelinus mali from two genetic clades in China from oviposition to adult parasitoid emergence.

Comparison of searching efficiency and control ability between the two clades of A. mali
Attack rates
As WAA density in the test arena increased, the number of aphids parasitized per female within the 24 h period increased, being greatest for both clades when WAA density was 200 aphids per parasitoid. At this aphid density, the Shandong clade of A. mali parasitized more hosts (6.50 ± 2.20 aphids/female parasitoid/day) than the Liaoning clade (5.67 ± 0.33), but this difference was not significant (table 3), and numbers of hosts attacked per day then decreased for both clades at the last and highest host density.
Table 3. Number of aphids parasitized by females of two genetic clades of Aphelinus mali on different densities of the aphid Eriosoma lanigerum in China.

Because the data fitted a type II functional response, we calculated handling times and attack coefficients using Holling's disc equation (Holling, Reference Holling1959), modified by reciprocal linear transformation Na = a × T × N 0/(1 + a × Th × N 0), where Na is the number of prey consumed by a predator, N 0 is the initial prey density, a is the attack rate, T is the time that predator and prey are exposed to each other, and Th is the handling time associated with each prey consumed. The parameters were obtained by fitting the data to the least square regression (Omkar, Reference Omkar2005), given the following relationships:
The instantaneous attack rate of the Shandong clade of A. mali on WAA (0.0946) was not significantly higher that of the Liaoning clade (0.0713). Similarly, there was no significant difference in handling time of the Shandong clade (0.1855 s) from that of the Liaoning clade (0.2051 s), nor was the control ability of the Shandong clade (a/Th = 0.543) significantly different from that of the Liaoning clade (a/Th = 0.382).
Searching efficiency of the two parasitoid clades
The mathematical model of the searching efficiency of A. mali was as follows: Shandong clade:
As the density of WAA increased from 50 to 250 aphids per female wasp, the searching efficiency of both the Shandong and Liaoning clades decreased (table 4). Over this range of host density, the searching efficiency of Shandong clade A. mali dropped from a maximum of 0.043 ± 0.003 to 0.019 ± 0.006, and that of the Liaoning clade dropped from 0.040 ± 0.001 ± 0.004 to 0.016 ± 0.003. However, at any given WAA density, the Shandong clade of A. mali showed consistently higher searching efficiency than the Liaoning clade (table 4).
Table 4. Host-searching efficiency of Aphelinus mali at different host densities.

As A. mali density increased relative to a fixed group of 50 hosts, mutual interference affected searching efficiency (E) and natural enemy density (P) as estimated by the model E = Q × P −m (Hassell & Varley, Reference Hassell and Varley1969). Specifically, we found that this effect varied between the two parasitoid clades:
In this model, Q is the search parameter (the effect of natural enemies in the absence of competition) and m is the mutual interference parameter (estimation of intraspecific competition). The significant χ2 test value indicates the model fit the relationship between the parasitoid density and the searching efficiency well.
As A. mali density increased, the number of aphids parasitized by individual A. mali females increased and then decreased, the attack rates of the Shandong clade are consistently higher than that of the Liaoning clade but not significant (table 5).
Table 5. Numbers of woolly apple aphids parasitized by two genetic clades of Aphelinus mali when parasitoid number varied.

We found that the search parameter (Q) of the Shandong clade (0.0725) was not significantly different from that of the Liaoning clade (0.0381) (table 6). The searching efficiency of both clades decreased with increasing parasitoid density, but at the parasitoid densities of three (F = 1.500, df = 3, P = 0.010), four (F = 0.162, df = 3, P = 0.010), and five (F = 22.164, df = 3, P = 0.030) females, the searching efficiency of the Shandong clade was significantly higher than that of the Liaoning clade (table 6). The interaction parameter of the natural enemy (m) for the Shandong clade (1.042) was not significantly lower than that of the Liaoning clade (1.045), even though the intraspecific competition parameter for the Shandong clade was consistently lower than that for the Liaoning clade.
Table 6. Host-searching efficiency of Aphelinus mali for woolly apple aphid at different parasitoid densities.

Different lowercase letters within a row represent significant differences (P<0.05).
Discussion
Adaptations by parasitoids to variations in potential hosts are essential for parasitoid survival and reproduction (Wang & Yang, Reference Wang and Yang2010). To adapt to variation in host resources and their environment, parasitoids often evolve locally adapted races adjusted to their dominant hosts. Such parasitoid clades may differ in their genetics, morphology, or ecology (Xu & Hua, Reference Xu and Hua2004). We found that the host searching time of the Shandong clade (47.8 s per host encounter) was significantly longer than that of the Liaoning clade (29.3 s per host encounter), while the grooming + resting time of the Shandong clade was also significantly shorter than that of Liaoning clade, suggesting that it takes parasitoids of the Shandong clade longer to find an appropriate host and to re-initiate host searching afterwards. This suggests that the Shandong clade may have a lower responsiveness to WAA from Hebei (i.e., requiring longer stimulation before acceptance) in contrast to earlier reciprocal work with WAA from Shandong that showed no such drop in responsiveness of the Liaoning clade originating from Hebei Province (Su et al., Reference Su, Tan, Yang, Zhou and Wan2016).
With Heibei WAA as host, although Shandong clade A. mali showed less adaptability and we found the search parameters (Q) of Shandong clade A. mali to be larger than for the Liaoning clade, the ability of Shandong clade A. mali to search for a host in the petri dish arena was actually better than that of the Liaoning clade. In the interaction parameters of natural enemies (m), the Shandong clade was lower than that of the Liaoning clade, and the intraspecific competition of the Shandong clade was lower than that of the Liaoning clade, which improves the parasitism rate by Shandong clade. The control abilities a/Th of the Shandong clade is higher than that of Liaoning clade, and different host densities, the searching efficiency of the Shandong clade was higher than that of the Liaoning clade. So, we speculate that, when the host is the WAA in Hebei, the control ability of Shandong clade is better than that of Liaoning clade. In a study (Su et al., Reference Su, Tan, Yang, Zhou and Wan2016), we found the ecological adaptability and control ability of the Shandong clade of A. mali to be better than that of the Liaoning clade, a finding further confirmed by our results here. Further research is now needed to screen Shandong clade A. mali for biological control of WAA, for example, screening A. mali with lower developmental temperature thresholds that can occur earlier in the spring to control the WAA. In small arenas, A. mali can sometimes parasitize the same host several times, and while we observed up to six eggs on the same host, only one A. mali can develop in each host (Long et al., Reference Long, Wang and Tang1960). So, there are some differences like oviposition between the test in the Petri dish and environment.
Acknowledgements
Su Min and Tan Xiumei each contributed equally to this work. The authors would like to thank Dong Guangjin, Liu Xukun, and Yan Hao of the College of Agronomy and Plant Protection, Qingdao Agricultural University, China, for their help. The English editing was done by Van Driesche Scientific Editing, and the authors thank Roy Van Driesche for suggestions and advice. This work was supported by the National Key Research and Development Plan (2016YFC1201200), the National Key Basic Research Development Plan Project (2013CB127600), the National Natural Science Foundation (31371994), Major Scientific and Technological Innovation Project of Shandong Province (2017CXGC0214), and the Taishan Mountain Scholar Constructive Engineering Foundation of Shandong, China.