INTRODUCTION
Some tropical octopods, such as Octopus chierchiae, have been observed to alternate several episodes of egg laying with mating activity (Rodaniche, Reference Rodaniche1984). These examples have led some authors (Rodaniche, Reference Rodaniche1984; Harman et al., Reference Harman, Young, Reid, Mangold, Suzuki and Hixon1989; Lewis & Choat, Reference Lewis and Choat1993) to propose iteroparity in cephalopods. If these arguments were accepted, then it would still be the case that this version of ‘iteroparity’ occurs over a short period of time within a single spawning annual cycle (Boyle & Rodhouse, Reference Boyle and Rodhouse2005). However, similar to many incirrate octopods, Octopus vulgaris females confine their egg laying to a period of only a few days or weeks and have a monocyclic gonadic maturation; thus, this species should be considered a semelparous (breeding once) organism (Mangold, Reference Mangold and Boyle1983, Reference Mangold and Boyle1987). The eggs are elongated (2.2 × 0.9 mm), and the chorion is drawn out into a shell. Secretions result in the stalks of the eggs adhering together and forming a string that the female attaches to the roof of the den. The eggs are brooded, aerated and cleaned by the female, which brooding can last as long as 5 months (Mangold, Reference Mangold and Boyle1983).
The costs of brooding to the female include enhanced vulnerability during the brooding period and increased metabolic effort, which leads to reduced post-reproductive survival (Calow, Reference Calow and Boyle1987). The first danger was reduced by female O. vulgaris laying their eggs in dens, which are primarily located in crevices or caves in a rock wall, and blocking up their den opening with stones (Hanlon & Messenger Reference Hanlon and Messenger1996). The second effect of brooding leads a female of this species to die when most of her well-cared-for eggs have hatched (Calow, Reference Calow and Boyle1987). Hidden dens make it difficult for scientists to find nesting female octopuses and study this important part of their life cycle (Anderson et al., Reference Anderson, Mather and Wood2010).
Although observations made in the sea have revealed some interesting aspects of the spawning behaviour of this shallow-water species, the studies that have provided most such information were primarily carried out under confinement (Hanlon & Messenger, Reference Hanlon and Messenger1996; Mather et al., Reference Mather, Anderson and Wood2010). In terms of the mode of spawning, Gonçalves et al. (Reference Gonçalves, Sendao and Borges2002) defined O. vulgaris as an intermittent spawner because mature females expel egg strings intermittently and without long pauses as the ovary becomes full. Nevertheless, Mangold et al. (Reference Mangold, Young, Nixon, Okutani, O'Dor and Kubodera1993) and Rocha et al. (Reference Rocha, Guerra and González2001) classified this species as a simultaneous terminal spawner. The term ‘simultaneous terminal spawner’ means that ovulation is synchronous and that there is no oocyte maturation during the spawning period (Rocha et al., Reference Rocha, Guerra and González2001). The egg-spawning pattern is monocyclic, and egg laying occurs within a very short period at the end of the animal's life (Rocha et al., Reference Rocha, Guerra and González2001). Khallahi & Inejih (Reference Khallahi and Inejih2002) suggested that the gonadal development of O. vulgaris was asynchronous but that ovulation and spawning were synchronous. Therefore, whether the spawning strategy is simultaneous or intermittent in this species is a debatable question.
The embryonic development of O. vulgaris was described in detail by Naef in 1928 (see Naef Reference Naef2000) based on several large egg masses that were spawned in the large tanks of the public Aquarium of the Zoological Station of Naples. Since Naef's study, a considerable body of literature has developed about the embryonic development of O. vulgaris at different temperatures (Boletzky, Reference Boletzky and Boyle1987; Iglesias & Fuentes, Reference Iglesias, Fuentes, Iglesias, Fuentes and Villanueva2014).
Most of the information presented in the former publications and the subsequent reproductive studies on this species come from research that was conducted under controlled conditions. The fact that O. vulgaris has been brought into captivity and successfully bred and grown in experimental tanks has allowed new insights to be gained into aspects of their biology and ecology that cannot be gained in fieldwork. However, the contrary is also true: there are some types of information that can only be gained from wild observations, and one can never be fully confident that the experimental conditions have not introduced biases into the results. Because the literature on cephalopod behaviour is often strongly biased towards rearing experiences, additional field studies are needed (Boal, Reference Boal2011), which is also true for other aspects of the animal life cycle.
The main objective of this paper was to report on a complete series of events that included the spawning, brooding and the embryonic development of O. vulgaris in the wild.
MATERIALS AND METHODS
A spawning den of O. vulgaris was monitored from 15 March until 13 September 2013. The den was located in Salaiños (Ría de Vigo, Galician waters, NW Spain; 42°06′2N 08°53′8W) at a depth of 12.5 m. A brooding female occupied the spawning den on 8 May. Table 1 details the 14 visual censuses performed on this individual during the study period.
Table 1. Observations from the spawning den monitored in Salaiños (Ría de Vigo, NW Spain, 42°06′2N 08°53′8W). Depth: 12.5 m. DE, days elapsed since female occupied the den; M, mean, SD, standard deviation, n, number; T, temperature (°C). EG, egg clusters. SS, egg strings sampled. SL, string length (mm); ENS, egg numbers per string. EDS, embryo development stages after Naef (Reference Naef2000). KSC, key stages characteristics (Naef, Reference Naef2000). PS, paralarvae sampled. YSP, yolk sac present; YSA, external yolk sac absent or very small.

The temperature and salinity were automatically recorded every 20 min for 24 h for the whole observation by two long-battery-life mini-loggers (DST Star-Odi's CTD, Star-Oddi, Gardabaer, Iceland). One of the devices was placed within the den, and the other was positioned 1 m outside the den at 13.5 m depth. A two-tailed variance test ratio was used to test whether records from in and out mini-data loggers were significantly different (Zar, Reference Zar1999). Video recordings were made by scuba divers using a SONY HDR-CX700 camera.
In order to determine fecundity, 5 to 10 egg strings were collected on each visit to the nesting female. They were transported to the laboratory in plastic vials. Four strings were randomly selected from the total sample of strings. They were measured in a fresh condition, and the total number of eggs on each string was counted. The embryos were euthanized using over-anaesthesia of increasing concentrations of ethanol, ranging from 1 to 5%, over a period of 2 h (Anderson, Reference Anderson1996). The procedures that were followed conformed to the Spanish regulations for studies on wild animals (BOE No 34, 8 February 2013: 11370–11421). The embryos were examined and their stages of development were classified according to the 20 stages described by Naef (Reference Naef2000). A total of 306 newly hatched specimens was collected from both hatched egg capsules in the surroundings of the den with a small net and transported to the laboratory in plastic vials, and from hatched egg capsules in the laboratory. These hatchlings were euthanized following the Anderson's technique (1996) and then frozen at −20°C. Dorsal mantle length (ML) of 25 randomly selected hatchlings was measured using an image analysis system (NIS Elements D 3.0) after defrosting at room temperature. The purpose of the measurements was to compare them with those obtained in previous studies (Mangold, Reference Mangold and Boyle1983; Villanueva, Reference Villanueva1995; Iglesias et al., Reference Iglesias, Otero, Moxica, Fuentes and Sánchez2004).
RESULTS
The female started to spawn on 8 May (Figure 1A). Figure 2 illustrates the principal reproductive events observed in relation to the temperature inside the den. The den temperature ranged from 12.9 to 19.3°C. The salinity ranged between 33.69 and 35.30 throughout the observation period. No significant differences (F = 1.42; P > 0.001) were found between the mini-loggers in the recorded salinity and temperature measurements.
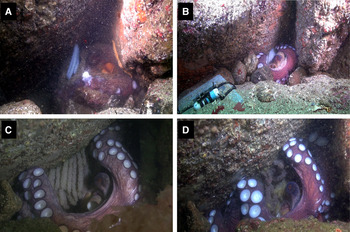
Fig. 1. Octopus vulgaris female observed at Salaiños. (A) with four strings of white eggs, 8 May 2013; (B) with approximately 160 strings of white eggs, 12 June. View of the mini-data logger; (C) dark-coloured eggs near hatching, 14 August; (D) Many strings showing hatching eggs, 26 August.

Fig. 2. Laying interval, hatching and brooding periods of the Octopus vulgaris female monitored at Salaiños in relation to temperature (T°C) within the den and the number of days elapsed since spawning (DESE).
The laying interval lasted for 35 days (Figure 2). The brooding and the hatching periods lasted 129 and 43 days, respectively. The time lasted for the embryonic development varied from 85 to 128 days. The first hatching observed occurred after a progressive and gradual increase of the temperature from 14.9 to 19.3°C (Table 1; Figure 2). The female died the day that it was collected by the divers (Table 1).
The total number of egg strings (estimated from the images) was 160 (Table 1; Figure 1C). The mean length and standard deviation of the length of strings were 92.9 and 13.3 mm, respectively (Table 1). The egg strings became increasingly dark as embryonic development progressed (Figure 1B, C &D). Embryos were found in different Naef stages of embryonic development (IX–XII) within a temperature range from 12.9 to 14.9°C. The embryonic stage of development was different in distinct egg strings as well as inside the same egg string (Table 1). Egg developing at different rates in different egg strings and in the same egg string were also found at 98 days from sampling, when the eggs had passed through a temperature range of 14.9 to 19.3°C. Thus, the embryos were at development stages from XVII to XX (Table 1). The embryos in the basal and in the upper middle parts of egg string were generally less developed that those of the lower middle and distal parts of the egg string. It was also observed that the embryos at stage XVII to XX ecloded at the same time, both in the wild and in the laboratory.
The average number of eggs per string and its standard deviation were 1190 and 331, respectively (Table 1). The total number of eggs spawned, assuming a total of 160 strings, ranged between 139,040 and 241,760 (mean 190,400). The body and ovary weight of the spent female were 1326 g and 6.9 g, respectively. A total of 16 eggs remained in the spent ovary.
Overall, 54.9% of the 306 paralarvae sampled showed an external yolk sac (YSP), whereas the yolk was entirely absorbed in the remaining paralarvae (YSA) (Table 1). Mean and standard deviation of the mantle length of 25 randomly selected YSP paralarvae were 2.38 and 0.11, whereas these measures were 0.84 and 0.05 to the 25 randomly selected YSA paralarvae.
We observed behaviour by the female that has not been previously described in the wild: (1) initially, when the eggs were still immature, the entry of the den was completely occluded by small stones (Figure 3A); and (2) when the female had newly hatched eggs (Figure 3B) she opened a small window in the wall of stones (Figure 3C). The divers always closed that window after the visual census was finished (Figure 3D). Nevertheless, during the next visual census, the divers found that the female had opened the window. Our observations showed that the brooding female constantly remained in her den.
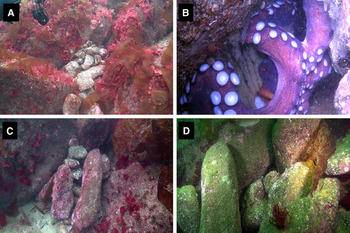
Fig. 3. Octopus vulgaris female observed at Salaiños. (A) Spawning den totally closed with small stones, 11 July 2013; (B) brooding female with hatching eggs. The stones were removed by the scuba divers, 14 August. (C) window opened by the female, 14 August. (D) den totally closed by the female, 29 August.
DISCUSSION
The brooding period (128 days) found in the Salaiños female agrees with Mangold & Boletzky (Reference Mangold and Boletzky1973) results, which showed that egg care may take as long as 5 months, depending on the length of embryonic development. Assuming a life span of 12–18 months for O. vulgaris (Mangold, Reference Mangold and Boyle1983), this brooding period would represent between 24.25 and 36.39% of the species’ total life cycle. In contrast, the hatching period (43 days) would represent from 8 to 12% of the species’ total life cycle, also assuming the above lifespan. The observed hatching period was not defined taking into account only the single major hatching event but from the first egg string hatching to the last one, as suggested by Villanueva & Norman (Reference Villanueva and Norman2008).
Our results showed that the mode of spawning of the monitored female was non-synchronous or intermittent, which agrees with the existence of an asynchronous oogenesis in the species (Gonçalves et al., Reference Gonçalves, Sendao and Borges2002; Cuccu et al., Reference Cuccu, Mereu, Porcu, Follesa, Cau and Cau2013; Sieiro et al., Reference Sieiro, Otero and Guerra2014). Egg laying lasted 35 d in the monitored brooding female, which also concurs with the period of 4 or 6 weeks indicated by Mangold (Reference Mangold and Boyle1983).
As in all cephalopods the duration of O. vulgaris embryonic development is related to temperature (Mangold, Reference Mangold and Boyle1983). We found that embryonic development lasted from 85 to 128 days in a range of temperatures between 12.9 and 19.3°C. The shorter embryonic development duration agrees with that indicated by Naef (Reference Naef2000) at 15°C and the one found by Caverivière et al. (Reference Caverivière, Domain and Diallo1999) rearing the species at 17°C, whereas the longest embryonic development duration was similar to that of 4 months in Galician waters (Otero et al., Reference Otero, González, Sieiro and Guerra2007). During the spawning peak found in June, July and early August in the eastern Mediterranean, embryonic development is completed in about 1 month (Katsanevakis & Verriopoulus, Reference Katsanevakis and Verriopoulus2006). That duration of embryonic development is 3–10 days less than the one obtained when the species is reared in experimental tanks at a constant temperature ranging from 20 to 24.7°C and feeding ad libitum (Itami et al., Reference Itami, Izawa, Maeda and Nakai1963; Mangold & Boletzky, Reference Mangold and Boletzky1973; Iglesias et al., Reference Iglesias, Otero, Moxica, Fuentes and Sánchez2004). The difference of duration in the embryonic development could be due to the fact that the sea surface summer temperature in the eastern Mediterranean was higher in the previous cases, ranging from 24 to 29°C (www.meteoprog.com/en/water/).
Octopus vulgaris is a stenohaline species and rainfall and consequent salinity changes are factors affecting common octopus abundance (e.g. Sobrino et al., Reference Sobrino, Silva, Bellido and Ramos2002; Sonderblohm et al., Reference Sonderblohm, Pereira and Erzini2014). Nevertheless, the species studied tolerate salinity ranging from 29 (Delgado et al., Reference Delgado, Gairin, Carbo and Aguilera2011) to 40 (Mangold, Reference Mangold and Boyle1983), which are values within the salinity range (33.69–35.50) found during the period in which the female was monitored. Salinity change variation could not be related with the development success of egg strings due to some fouling issues in the salinity sensor. We managed to gather a good quality salinity range but not a time series long enough to verify any salinity influence on development success of egg strings.
We found that the eggs develop at different rates in different egg strings and in the same egg string. This could be due to a number of factors. Although female O. vulgaris guard and care for the eggs (Hanlon & Messenger, Reference Hanlon and Messenger1996), the embryos develop within the perivitelline fluid, and the capsules and chorions act as a barrier to the diffusion of dissolved gases and other substances (Iglesias & Fuentes, Reference Iglesias, Fuentes, Iglesias, Fuentes and Villanueva2014; Robin et al., Reference Robin, Roberts, Zeidberg, Bloor, Rodriguez, Briceño, Downey, Mascaró, Navarro, Guerra, Hofmeister, Barcellos, Lourenço, Roper, Moltschaniwskyj, Green and Mather2014), several environmental factors affect the duration and success of embryonic development. One of these factors is dissolved oxygen, which accounted for differences in the embryonic development rates within single egg strands of some loliginid species (Chung, Reference Chung2003; Steer et al., Reference Steer, Moltschaniwskyj and Jordan2003). Strathmann & Strathmann (Reference Strathmann and Strathmann1995) suggested that the proximal embryos were not sufficiently oxygenated, and Chung (Reference Chung2003) observed that large egg clusters tended to obstruct the current, which causes less circulation within the egg masses. We lack reliable data to indicate in which position within the mass of egg the sampled strings were found. We observed that there were small differences in the basal, middle and distal part of egg strings. However, within the recorded temperature range, this could mean 2 or 3 days delay in some embryos relative to others. It was also noted that the ML of the newly hatched paralarvae in Naef's stages XIX–XX, which have a small external yolk sac (Table 1), was similar to that indicated in previous papers (Mangold, Reference Mangold and Boyle1983; Villanueva, Reference Villanueva1995; Iglesias et al., Reference Iglesias, Otero, Moxica, Fuentes and Sánchez2004). When the egg strings are disturbed, the young octopuses may hatch before Naef's stage XX is reached (Boletzky, Reference Boletzky and Boyle1987), i.e. embryos at Naef's stages XVII and XVIII (still with a large external yolk sac) hatch and are viable, as noted by Mangold & Boletzky (Reference Mangold and Boletzky1973). Specific studies would be required to elucidate the effect on survival of the presence of a still large yolk sac, since, theoretically, at least two hypotheses are possible: (1) that the paralarvae born with a good deal of external yolk, i.e. with more food reserves, have less possibility of being detected by predators as they do not need to move to find prey; and (2) that said paralarvae are more vulnerable to their predators by having less capacity to move than those born with no external yolk.
The mean length of the egg strings was within the range (36–114 mm) found by Oosthuizen & Smale (Reference Oosthuizen and Smale2003) in seven egg masses in the field. This also agrees with the figures found by other authors (Mangold-Wirz, Reference Mangold-Wirz1963; Mangold, Reference Mangold and Boyle1983). The range of the number of eggs per string (1190 ± 321) was slightly lower than that found by Oosthuizen & Smale (Reference Oosthuizen and Smale2003) in South Africa (602–2133), but was within the range of values (100 eggs per 1 cm egg string) observed in reared animals (Mangold-Wirz, Reference Mangold-Wirz1963) and in those obtained from the adjacent areas of the Ría de Vigo (Guerra, unpublished data).
The fecundity estimated for our female agrees with the one obtained in the wild in the same and other geographic areas. Mean (±SD) potential fecundity was estimated at 221,447 ± 116,031 in Galician waters, and these values were significantly correlated with length and weight of the female (Otero et al., Reference Otero, González, Sieiro and Guerra2007). The total number of eggs produced by a wild female O. vulgaris on the south-eastern coast of South Africa ranged from 42,133 to 789,111 (Oosthuizen & Smale, Reference Oosthuizen and Smale2003).
The behavioural patterns of this brooding female were similar to the ones described by Hanlon & Messenger (Reference Hanlon and Messenger1996). The occlusion and opening of the den entrance was observed in the wild Enteroctopus dofleini (Cosgrove, Reference Cosgrove1993). However, it seems that the latter species moved the whole obstruction when their eggs were about to hatch, whereas the monitored O. vulgaris female opened and closed a small window to release a batch of hatchlings, which would be the first observation of this aspect of brooding behaviour in the field. Our video recordings showed that this behaviour was performed also by four observed brooding females which were not manipulated, therefore we can conclude that it is a common behaviour.
ACKNOWLEDGEMENTS
We would like to thank ECIMAT (University of Vigo) and José Castro for his help during scuba diving operations. The authors would also like to thank Dr Francisco Rocha for kindly assisting with the paralarvae and to two anonymous referees whose comments helped to improve the original manuscript.
FINANCIAL SUPPORT
This study was conducted by CSIC, within the project 458/2011 (CEFAPARQUES), financed by the Organismo Autónomo de Parques Nacionales de España, Ministerio de Agricultura, Alimentación y Medio Ambiente, LARECO (CTM2011-25929) and FEDER Funds. Miguel Gilcoto's collaboration was supported by the project CMT 2012-35155 (STRAMIX) financed by the Ministerio de Economía y Competitividad (Spain).






