Introduction
The family Lecideaceae Chevall., erected in 1826 as ‘Lecideae’ (Chevallier Reference Chevallier1826), originally included all crustose lecideoid genera but now consists of relatively few genera with lecideine apothecia, simple hyaline ascospores, and a Lecidea or Porpidia-type ascus structure. Many of these genera were formerly included in the family Porpidiaceae Hertel & Hafellner (Hafellner Reference Hafellner1984), the genera of which differed from those of the Lecideaceae in having an ascus with an amyloid tube structure (Porpidia-type), halonate ascospores, and branched and anastomosing paraphyses. However, Buschbom & Mueller (Reference Buschbom and Mueller2004) showed that ‘Porpidiaceae’ was not monophyletic unless the Lecideaceae was also included. This was confirmed by Miądłikowska et al. (Reference Miądłikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006), who also demonstrated that the family should be transferred from Lecanorales to Lecanoromycetidae inc. sed.
Lecideaceae has been widely studied in the Northern Hemisphere (e.g., Hertel Reference Hertel1977a , Reference Hertel b , Reference Hertel1981, Reference Hertel1991, Reference Hertel1995, Reference Hertel2009; Inoue Reference Inoue1982, Reference Inoue1983; Brodo & Hertel Reference Brodo and Hertel1987; Gowan Reference Gowan1989; Andreev et al. Reference Andreev, Kotlov and Makarova1998; Buschbom & Mueller Reference Buschbom and Mueller2004; Hertel & Printzen Reference Hertel, Printzen, Nash, Ryan, Diederich, Gries and Bungartz2004; Fryday Reference Fryday2005; Gowan & Ahti Reference Gowan and Ahti1993; Schmull et al. Reference Schmull, Miądlikowska, Pelzer, Stocker-Wörgötter, Hofstetter, Fraker, Hodkinson, Reeb, Kukwa and Lumbsch2011), but less so in the Southern Hemisphere (e.g., Hertel Reference Hertel1984, Reference Hertel1997, Reference Hertel2007; Rambold Reference Rambold1989; Inoue Reference Inoue1991; Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010). Here, eight species in six genera are described as new to science, from various islands in the southern subpolar region, and a further species of Lecidea is resurrected from the synonymy of a Northern Hemisphere species. In addition, two genera are reduced to synonomy with other genera and the necessary new combinations made.
Materials & Methods
The study is based chiefly upon collections made by Henry Imshaug and co-workers during fieldwork in the southern subpolar region between 1968 and 1973 and now held in the herbarium of Michigan State University (MSC; Fryday & Prather Reference Fryday and Prather2001). Comparative collections were obtained on loan from COLO, F, GZU, M, UCR and W.
Apothecial characteristics were examined by light microscopy on hand-cut or freezing-microtome sections mounted in water, 10% KOH (K), 15% HCl (H), 50% HNO3 (N) or 0·15% aqueous IKI. Thallus sections were investigated in water and 10% KOH. Ascus structure was studied in 0·15% aqueous IKI, both without prior treatment and after pretreatment with 10% KOH. Anatomical measurements were made in 10% KOH.
Thin-layer chromatography followed the methods of Orange et al. (Reference Orange, James and White2001). Nomenclature for apothecial pigments follows Meyer & Printzen (Reference Meyer and Printzen2000).
Selected additional comparative material examined (all MSC unless otherwise stated).
Catarrhospora mira Brusse. South Africa: Cape Province: Ladismith, Muriskrall, Langeberg Range, 1994, F. Brusse 3627 (COLO).
Immersaria athroocarpa (Ach.) Rambold & Pietschm. New Zealand: South Island: Canterbury, Banks Peninsula, summit of Mt. Herbert, 1973, Imshaug 58184.
‘Labyrintha’ implexa Malcolm et al. New Zealand: Campbell Island: west end of Lyall Ridge, 1969, Imshaug 46173. South Island: Mt. Cook NP, summit of Mt. Sebastopol, 1970, Imshaug 47519; ibid., Arthur's Pass NP, south side of road at Arthur's Pass, 1971, Imshaug 47880. Auckland Islands: Adams Island, NNW of Mt. Dick, summit of peak on central ridge, 1972, Imshaug 56938.
Lecidea mannii Tuck. USA: California: Mariposa Co., Yosemite National Park, mouth of Pigeon Gulch, 2009, Fryday 9319; Orange Co., Santa Ana Mountains, near Main Divide Truck Trail, 2004, K. Knudsen 1543 (UCR); Riverside Co., Wildomar, Menifee Hills, 2003, K. Knudsen 20 (UCR); ibid., San Jacinto Mountains, Idyllwild, near Inspiration Point, 2005, K. Knudsen 3462 (UCR); San Diego Co., Peninsular Range, Palomar Mountain State Park, Palomar Mountain, 2005, K. Knudsen (3002) & M. Knudsen (UCR).
Melanolecia transitoria (Arnold) Hertel. Austria: Steiermark: Dachsteingruppe, Ramsau, Weg von der Dachsteinsüdwandhütte in Richtung Hunerscharte, 7 viii 1993, J. Poelt & M. Grube (GZU); Dachstein-Gruppe, Steiermark/Oberösterreich: Gipfel des Hohen Dachstein, 29 vii 1990, J. Poelt (GZU).
‘Notolecidea’ subcontinua (Nyl.) Hertel. Îles Kerguelen: Presqu'ille Jeanne d'Arc, W edge of Ravin du Charbon, 1971, G. C. Bratt 71/186; Péninusule Courbet, 1·5 km N of Port-aux-Français, 1971, R. C. Harris 6884; ibid., just below summit of Mt. Crozier, R. C. Harris 7356; Ile Haute, SE edge of table des Mouflons, 1971, R. C. Harris 6964.
Paraporpidia leptocarpa (Nyl.) Rambold & Hertel. Australia: Victoria: Northern Plains Region, Euroa—Strathbogie Road, 9 km E of Euroa, 1999, H. Streimann 53502 (MSC, unseen duplicates in CANB, B, TU, NY, MUB & MHA).
Poeltiaria coromandelica (Zahlbr.) Rambold & Hertel. Australia: Victoria: Mt. William, Grampians National Park, 29 km W of Ararat, 1994, H. Streimann 55155.
Poeltiaria corralensis (Räsänen) Hertel. Chile: Magallanes and Antártica Chilena Region: S shore B. Pond, 1969, Imshaug (45335) & K. Ohlsson.
Poeltiaria urbanskyana (Zahlbr.) Hertel. Îles Kerguelen: Île Haute, north side of isthmus east of cabin, 1971, R. C. Harris 6959.
Poeltidea perusta (Nyl.) Hertel & Hafellner. Argentina: Tierra del Fuego Province: Isla Grande de Tierra del Fuego, Bahia Valentin, 1971, Imshaug (50282) & K. Ohlsson.—Falkland Islands: West Falkland: Weddell Island, on summit of peak NE of Mt. Weddell, 1968, Imshaug (41971 A) & R. C. Harris.—Îles Kerguelen: Plateau des Lacs, cliffs at N end of plateau, 140 m, 1971, G. C. Bratt 71/275.
Rhizolecia hybrida (Zahlbr.) Hertel. New Zealand: South Island: Canterbury, Castle Hill Basin, 1935, H. H. Allan A 18 (W—holotype).
Schizodiscus afroalpinus Brusse. South Africa: Natal: 65 km N of Maclear, summit of Naudé's Nek, 1986, F. Brusse 4593 (COLO).
Stenhammarella turgida (Ach.) Hertel. Austria: Nordtirol, Tuxer Alpen: Gipfel des Bentelsteins bei Steinach, ix 1958, M. Steiner (Lichens Alpinum # 123). Salzburg: Hohe Tauern, Glockner Gruppe, ridge NW of Großer Magrötzenkopf, W of Hochtor pass, 1996, F. Lutzoni 96.8.30-1 (F).
New Taxa
Bryobilimbia austrosaxicola Fryday & Coppins sp. nov.
MycoBank No.: MB805056
Similar to Bryobilimbia australis but saxicolous, with a brown, not violaceous, epihymenium and 1-septate ascospores.
Type: New Zealand, Campbell Island, rock outcrops at summit of Mt. Azimuth [52°30·1′S 169°9·9′E], 1600 ft. [488 m], 3 January 1970, H. A. Imshaug 46535, (MSC—holotype).
(Fig. 1)

Fig. 1. Bryobilimbia austrosaxicola, thallus and apothecia (Imshaug 46535—holotype). Scale=1 mm. In colour online.
Thallus effuse, to 5 cm across, areolate, composed of thin, angular, flat to slightly convex, pale grey areoles, 0·2–0·3 mm across; medulla I−. Primary photobiont chlorococcoid, cells 5–9 µm diam. Secondary photobiont comprised of numerous small (up to 0·25 mm across) clumps of cyanobacteria scattered over the surface, cells of two types; short, branched, yellow-brown filaments, 15–20 µm across, 1–2 cells wide (Stigonema), plus groups of 2–4 cells in a reddish (K+ purple) sheath, cells 5 µm diam. (Gloeocapsa).
Apothecia frequent, adnate to sessile, black, flat to convex, lecideine, 1·0–1·5 mm diam., ±orbicular but often confluent and then angular; proper margin rarely discernible, even in young apothecia. Hymenium 50–65 µm, streaked with red-brown pigment (K+ olivaceous-brown); paraphyses rather sparse and inconspicuous, simple, 1·0–1·5 µm thick, apices not pigmented or capitate. Asci cylindrical to somewhat clavate, 40–45×12–15 µm, tholus I+ pale blue with a darker axial tube (?Porpidia-type); ascospores hyaline, (0–)1-septate, 10–12×3·5–4·0 µm. Hypothecium massively developed and carbonaceous, dark red-brown (K+ olivaceous-brown), internal structure not discernible.
Pycnidia uncommon, black, 0·10–0·15 mm wide; ostiole gaping at maturity; wall in section dark red-brown (K+ olivaceous-brown); conidia bacilliform, 7–8×1 µm.
Chemistry
K−, C−, Pd−. No substances detected by TLC.
Distribution and ecology
Known from only three collections from mountainous sites on Campbell Island, New Zealand. Associated species are few but include Placopsis sp. (with a lichenicolous Cercidospora sp.) and Steinera radiata subsp. aucklandica P. James & Henssen.
Remarks
The new species is included in the recently described genus Bryobilimbia Fryday et al. (Fryday et al. Reference Fryday, Printzen and Ekman2014) because of its similarity to B. australis (Kantvilas & Messuti) Fryday et al. (syn. Mycobilimbia australis Kantvilas & Messuti), which grouped with the type species, B. hypnorum (Lib.) Fryday et al. , in that study. The bacilliform conidia are also consistent with a placement in Bryobilimbia. It is separated from B. australis, and all other species of the genus, by a combination of its saxicolous habit and consistently 1-septate ascospores.
Brian Coppins is included as an author of this species because it was his suggestion that this taxon may be referable to the Lecidea hypnorum group.
Additional specimens examined (all MSC). New Zealand: Campbell Island: north-west slope of Mt. Honey, 1969, Imshaug 46430; ibid., summit and summit ridge of Mt. Honey, 1800–1867 ft., 1969, R. C. Harris 4898; ibid., cliffs and shingle feldmark at summit of Mt. Fizeau, 1655 ft., 1970, Imshaug 46791.
Immersaria fuliginosa Fryday sp. nov.
MycoBank No.: MB805057
Separated from all other species of the genus (and the Lecideaceae) by the presence of thalloconidia.
Type: Falkland Islands, West Falkland, pass SW of Mt. Maria summit, UTM 21F UC 2078 [51°36·630′S, 59°35·910′W], 2000 ft. [610 m], rock outcrops, 28 January 1968, H. A. Imshaug (41296) & R. C. Harris (MSC—holotype; M—isotype).
(Fig. 2)
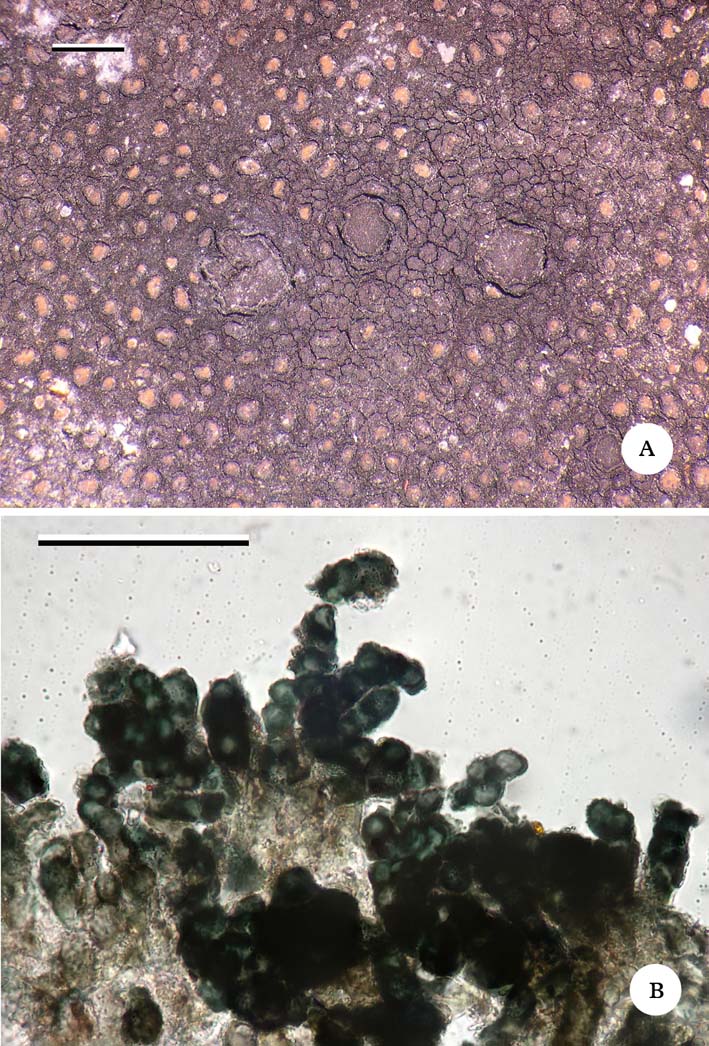
Fig. 2. Immersaria fuliginosa (Imshaug 41296—holotype); A, thallus and apothecia; B, thalloconidia. Scales: A=1 mm; B=50 µm. In colour online.
Thallus consisting of concave to flat, pale to red-brown areoles, 0·1–0·4 mm across, dispersed on a black prothallus that is completely dissolved into thalloconidia; prothallus forming a dark margin to each areole; areoles often larger and forming a ± contiguous crust at the thallus margin; upper cortex poorly developed, composed of a diffuse layer of dilute brown pigmented cells, c. 10–20 µm thick; epinecral layer 70–120(–150) µm; medulla I+ violet. Photobiont chlorococcoid, forming a well-defined horizontal layer near the base of the areoles; cells thick-walled, 7–12 µm diam.
Apothecia infrequent, lecideine, immersed; disc concave, dark brown to black, epruinose, brownish when wet, 0·8–1·5 mm across; proper margin thin (0·05 mm), slightly raised, poorly developed in section. Hymenium 140–160 µm tall; paraphyses 1·0–1·5 µm wide, branched and anastomosing, not widening at apex; epihymenium brownish. Asci clavate, 50–60×25–30 µm, Porpidia-type; ascospores simple, hyaline, with thin halo, 16–18×6–8 µm. Hypothecium brown (K+ golden brown).
Conidia: thalloconidia, blue-black, c. 6 µm diam.
Chemistry
2′-O-methylperlatolic and ±confluentic acids by TLC.
Distribution and ecology
Known from only two collections from mountainous sites on the Falkland Islands. Associated species include Rhizocarpon geographicum (L.) DC. aggr., Lecidea sp., Pertusaria spegazzinii Müll. Arg., and Poeltidea perusta (Nyl.) Hertel & Hafellner.
Remarks
Similar in gross morphology to Poeltidea perusta, with which it occurs in the same habitat, but separated from that species by the rough prothallus consisting of thalloconidia, and its smaller, hyaline ascospores. It differs from all other species of Immersaria Rambold & Pietschm. by having an extensive hypothallus composed of thalloconidia.
The production of thallocondia is a rare phenomenon in lichenized fungi. They are most frequently found in the genus Umbilicaria Hoffm. (Hestmark Reference Hestmark1990) but have also been reported in Protoparmelia M. Choisy, Rhizoplaca Zopf and Sporastatia A. Massal. (Poelt & Obermayer Reference Poelt and Obermayer1990). It has also been suggested that the “pearl-string hairy tomentum” of some species of Leptogium (Ach.) Gray may act as vegetative propagules (Bjelland Reference Bjelland2001). This is the first report of thallocondia in the Lecideaceae.
Additional specimen examined. Falkland Islands: West Falkland: summit ridge of Mt. Adam, UTM 21F TC 8781, 2200–2297 ft., feldmark, 1968, Imshaug (41057) & R.C. Harris (MSC, BCRU).
Lecidea aurantia Fryday sp. nov.
MycoBank No.: MB805058
Similar to L. lygomma but with an orange thallus lacking lichen substances.
Typus: New Zealand, Auckland Islands, Auckland Island, tussock and rock outcrop on summit of Hooker Hills [50°32·7′S 166°8·9′E)] 1435 ft. [435 m], 22 December 1972, H. A. Imshaug 56650 (MSC—holotype; CHR—isotype).
(Fig. 3A)

Fig. 3. A, Lecidea aurantia (Imshaug 56650—holotype); B, Lecidea campbellensis (Imshaug 46583—holotype). Scales=1 mm. In colour online.
Thallus orange, wide spreading, 0·3–0·4 mm thick, cracked-areolate; areoles angular, 0·5–0·7 mm across; prothallus thin, black, present at the margin; medulla and cortex I+ violet. Photobiont chlorococcoid, arranged in loose vertical bands c. 50 µm wide; cells thick-walled, 12–15 µm diam.
Apothecia black, immersed, 0·5–0·7 mm diam.; disc 0·2–0·4 mm diam., flat to concave, somewhat ridged or gyrose; proper margin black, thick, 0·1–0·2 mm across, usually separated from the disc by a wide crack, in section cupular, inner region thin, dark brown, composed of vertical, cellular hyphae 3·5–5·0 µm wide, cells 5·0–8·5 µm long, outer region to 400 µm wide, completely carbonaceous, K+ purple at edges. Hymenium hyaline, 130–150 µm tall, I+ blue; paraphyses 1·5–2·0 µm thick, branched and anastomosing, apically scarcely up to 3·0 µm, without pigmented cap; epihymenium 30–40 µm thick, pale orange-brown (K+ paler) with darker, K+ purple patches towards exciple/umbo. Asci cylindrical, 85–100×20–25 µm, Lecidea-type; ascospores hyaline, often pseudodiblastic and appearing spuriously 1-sepate, 12–15×7·5–8·5 µm. Hypothecium hyaline, 70–80 µm thick, of vertically arranged hyphae.
Conidiomata not observed.
Chemistry
K−, C−, KC−, Pd−, UV+ pale yellowish. No substances detected by TLC.
Distribution and ecology
Six collections from mountain summits or ridges on the Auckland Islands. Associated species include Fuscidea subasbolodes Kantvilas, Miriquidica effigurata Fryday and Rimularia maculata Fryday.
Remarks
This species has the general appearance of a member of the L. lygomma group but the I+ violet medulla indicates otherwise. Lecidea lapicida, which can have a reddish thallus due to incorporation of iron oxides, differs in having more sessile apothecia and the thallus always containing stictic or norstictic acid. It is further distinguished from all species of the genus by its orange thallus and thick proper apothecial margin. The presence of branched and anastomosing paraphyses are anomalous for a species of Lecidea and the systematic placement of this species deserves further investigation.
Additional specimens examined. New Zealand: Auckland Islands: Adams Island, end of ridge from Magnetic Station leading to central ridge, 1600 ft. [488 m], 1972, Imshaug 56928; Auckland Island, tussock and rock outcrop on summit of Hooker Hills, 1435 ft. [435 m], 1972, Imshaug 56658 (topotype); ibid., summit of Mt. D'Urville, 2099 ft. [640 m], 1973, Imshaug 57368; ibid., summit of Mt. Eden, 1380 ft. [420 m], 1973, Imshaug 57533, 57536; ibid., summit of Cloudy Peak, 1529 ft. [566 m], 1973, Imshaug 57545.
Lecidea campbellensis Fryday sp. nov.
MycoBank No.: MB805059
Similar to L. lygomma but with sessile apothecia with a well-developed exciple, and a thallus lacking norstictic acid.
Type: New Zealand, Campbell Island, rock outcrops at the summit of Mt. Azimuth [52°30·1′S 169°9·9′E], 1600 ft. [488 m], 1970, H. A. Imshaug 46583 (MSC—holotype).
(Fig. 3B)
Thallus effuse, immarginate, grey, thin and scant, areoles ± dispersed on a black hypothallus; rarely contiguous and then becoming cracked-areolate; medulla I−. Photobiont chlorococcoid; cells 7–15 µm diam. arranged in poorly-defined vertical bands, 2–5 cells wide.
Apothecia numerous, sessile but not constricted at the base, 0·6–0·8(–1·0) mm. diam.; proper margin thick (0·15 mm) and barely raised, in mature apothecia often separated from the disc by a crack, in section surface cells dark brown to carbonaceous, pale brown within and composed of hyphae c. 3 µm wide. Hymenium 100–150 µm tall, pale aeruginose; paraphyses simple, 1·5–2·0 µm thick, gradually expanding apically to 3–5 µm, with a dark brown cap; epihymenium dilute olivaceous. Asci cylindrical, c. 50×15 µm, Lecidea-type; ascospores simple, non-halonate, hyaline, (12·0–)12·5–13·5(–15·0)×(5·5–)6·0–7·0(–8·0) µm. Hypothecium brown above, composed of vertically arranged hyphae, dark brown below, mostly K−, but basal region K+ purple.
Pycnidia usually present, sometimes frequent, slightly raised, orbicular to somewhat elongate, 0·2–0·4 mm diam., black, sometimes with a white centre, sometimes with an additional white pseudothalline margin; apparently developing into apothecia; conidia bacilliform, 8–10 µm long.
Chemistry
Stictic acid by TLC.
Distribution and ecology
Known from five collections from siliceous rocks on three mountain summits on Campbell Island. Associated species include Lecidea medusula (C.W. Dodge) Hertel, Placopsis sp. and Rhizocarpon reductun Th. Fr.
Remarks
The sessile apothecia with a thick proper margin give this species the superficial appearance of a species of Porpidia, but the simple paraphyses, non-halonate ascospores and Lecidea-type asci indicate that it should be placed in Lecidea. The I− medulla suggests that it is referable to the Lecidea lygomma group but it is distinguished from other members of that group by its superficial, adnate apothecia, well-developed exciple and thalline chemistry.
Additional specimens examined (all MSC). New Zealand: Campbell Island: rock outcrops and feldmark at summit of Mt. Honey, 1867 ft. [570 m], 1969, Imshaug 46360; cliffs around Mt. Lyall pyramid, 1300 ft. [396 m], 1970, Imshaug 46435, 46480, 46511.
Poeltiaria coppinsiana Hertel sp. nov.
MycoBank No.: MB805060
Similar to Poeltiara subcontinua, but thallus bullate-areolate instead of continous-rimose.
Type: Antarctica, South Shetland Islands, King George Island, Admiralty Bay area, unnamed hills between Italia Valley and Ornithologists Creek, W of Arctowski Station, 170 m, with accompanying Carbonea vorticosa, on andesite rock on an eastern slope, 3 January 1980, R. Ochyra 97/80a (M—holotype).
(Fig. 4A)

Fig. 4. A, Poeltiaria coppinsiana (Ochyra 97/80a—holotype), thallus with apothecia. B, Poeltiaria tasmanica (Kantvilas 302/09—holotype), thallus with apothecia and pycnidia. Scales=1 mm. In colour online.
Thallus bullate-areolate, consisting of pale brown to grey, convex, basally constricted areoles, 0·2–1·5 mm broad, older areoles sometimes somewhat sunken and darker towards the centre; medulla and upper algal-free layer I−, or locally I+ pale violet. Photobiont chlorococcoid.
Apothecia black when dry and dark brown when wet, soon convex, up to 1·7 mm diam; proper margin only apparent in young apothecia, in section cupular, unpigmented, except for a narrow, epihymenium-like brown rim. Hymenium 75–110 µm tall, upper 15–20 µm with brown pigment; paraphyses c. 2·0 µm wide, branched and anastomosing, apically up to 3–5 µm; epihymenium olive-brown. Asci clavate-cylindrical, 55–70×12–17 µm, Porpidia-type; ascospores simple, hyaline, ellipsoid, with a thin halo, (10–)12–15(–18)× (5·0–)6·0–6·5(–8·0) µm. Hypothecium hyaline.
Conidiomata not detected.
Chemistry
No substances detected by TLC.
Etymology
Named after Brian Coppins, in admiration of his outstanding contributions to the knowledge of crustose lichens.
Distribution and ecology
Known only from siliceous rocks in maritime Antarctica.
Remarks
Closely related to Poeltiaria subcontinua (Nyl.) Hertel & Fryday (see below) and largely consistent with the anatomical characters of its ascocarps. Differing in thallus structure and perhaps in its maritime Antarctic distribution, for it is still unknown from those subantarctic regions (South Georgia, Prince Edward Islands, Îles Kerguelen) where P. subcontinua is known to occur.
Additional collections examined. South Shetland Islands: Livingston Island: South Bay, Caleta Espanola, Peak of Reina Sofia, 62°40′S, 60°23′W, W-exposed seepage rock (sedimentary bedrock), 277 m, 1998, U. Søchting US 7018 (hb. Søchting).—South Orkney Islands: Signy Island: Moraine Valley fellfield, 60°43′S, 45°37′W, 1981, R. I. L. Smith 7101 (AAS).
Poeltiaria tasmanica Fryday sp. nov.
MycoBank No.: MB805061
Similar to Poeltiara urbanskyana but with much smaller ascospores.
Type: Australia, Tasmania, Bisdee Tier, 42°26′S, 147°17′E, 640 m, on moist dolerite seepage rocks in rough pasture, 17 June 2009, G. Kantvilas 302/09 (HO—holotype; MSC—isotype).
(Fig. 4B)
Thallus effuse, consisting of contiguous, grey to ochraceus, flat to convex areoles; medulla and upper algal-free layer I+ violet. Photobiont chlorococcoid; cells 9–12 µm diam., arranged in a discontinuous layer composed of vertically aligned columns c. 5 cells wide.
Apothecia black, 0·4–0·8 mm diam., immersed in the thallus; disc convex when young becoming gyrose when mature; proper margin striate, in section cupular, carbonaceous, c. 50 µm wide laterally, 20 µm at base. Hymenium 170–180 µm tall, upper 15–20 µm with brown pigment; paraphyses richly branched and anastomosing, 1·0–1·5 µm wide, apically to 2·0–2·5 µm; epihymenium brownish. Asci clavate-cylindrical, c. 60×15 µm, Porpidia-type; ascospores simple, hyaline, subglobose to ellipsoid, with a thin halo, 12–15× 6–8 µm. Hypothecium hyaline.
Pycnidia slit-like, 10–20 µm long, simple or branched, black with a white pseudomargin; conidia bacilliform, 6–7 µm long.
Chemistry
No substances detected by TLC.
Distribution and ecology
Known from a single collection from damp siliceous rocks in Tasmania. Associated species include Collema durietzii Degel., Xanthoparmelia mougeotina (Nyl.) D. J. Galloway, and unidentified species of Aspicilia and Placopsis.
Remarks
This species appears to closely resemble P. urbanskyana (Zahlbr.) Hertel, from which it differs in its much smaller ascospores and cool-temperate, as opposed to subantarctic, locality.
Poeltidea inspersa Fryday sp. nov.
MycoBank No.: MB805062
Separated from P. perusta by its ±endolithic thallus and inspersed hymenium.
Type: Falkland Islands, West Falkland, Mt. Adam, 21F TC 8781 [51°35′S, 60°2′W], 2200–2297 ft. [670–700 m], feldmark on summit ridge, 25 January 1968, H. A. Imshaug 41054 (MSC—holotype).
(Fig. 5)

Fig. 5. Poeltidea inspersa (Imshaug 41054—holotype); A, apothecia; B, hymenium with oil globules. Scales: A=1 mm; B=20 µm. In colour online.
Thallus mostly immersed in the substratum, where present reduced to the immediate vicinity of apothecia and sheltered spots (underside of the rock), creamy white, smooth, cracked-areolate; epinecral layer often present, 5–10 µm thick; medulla I+ violet. Photobiont chlorococcoid; cells 9–12 µm diam.
Apothecia black, lecideine, 0·8–1·5(–2·0) mm diam., ±flat, orbicular; disc ± flat, becoming convex and irregularly fissured when overmature; proper margin thin (0·05 mm wide), barely raised, becoming excluded in mature apothecia, in section poorly developed; pseudothalline margin usually present, thick (up to 0·2 mm wide) appearing pruinose due to thin (5–10 µm thick) epinecral layer. Hymenium 170–200 µm tall, with large oil globules [10–30(–40)×10(–15) µm] in the upper hymenium that dissolve in K; paraphyses 1·0–1·5 µm wide, branched and anastomosing, with distinctly swollen apices (up to 5 µm) and pigmented caps; epihymenium 25–50 µm tall, dilute brown to olivaceous-brown (K± olivaceous or blue-black in patches). Asci cylindrical, c. 100×30 µm, Porpidia-type, no mature asci seen; ascospores simple, pigmented, 8 per ascus, 25–30× 12–15 µm. Hypothecium hyaline to pale straw coloured.
Conidiomata not observed.
Chemistry
No substances by TLC.
Distribution and ecology
Known only from the type collection, which is from an apparently loose, angular piece of crystalline granite from the summit of Mt Adam on West Falkland. Dactylospora australis Triebel & Hertel occurs on the apothecia of the type collection but no other species are present on the piece of rock. However, other species collected from the vicinity include Lecanora xantholeuca (Müll. Arg.) Hertel, Lithographa graphidioides (Cromb.) Imshaug ex Coppins & Fryday, Micarea incrassata Hedl., Poeltidea perusta and Thamnolia vermicularis (Sw.) Schaer.
Remarks
In apothecial characters, this species closely resembles the type species of the genus, P. perusta. However, it differs from that species in the lack of a well-developed thallus, and the apothecia with a hymenium containing large oil globules.
Porpidia vulcanoides Hertel & Fryday sp. nov.
MycoBank No.: MB805063
Separated from most other members of the genus by its tall hymenium and large ascospores. Porpidia stephanodes from Îles Kerguelen, with even taller hymenia (170–230 µm) and larger ascospores (35–60 µm), differs by its exciple having a pale brown medulla (similar to that of P. macrocarpa).
Type: Chile, Magallanes and Antártica Chilena Region, Isla Madre de Dios, gap at head of fiord E of Mte Roberto, 50°20′S, 67°21′W, moorland, 29 September 1969, H. A. Imshaug (44145) & K. Ohlsson (MSC—holotype; M, CONC—isotypes).
(Fig. 6)

Fig. 6. Porpidia vulcanoides (Imshaug 44145), thallus with apothecia. Scale=1 mm. In colour online.
Thallus thin and effuse, creamy white to grey, composed of dispersed areoles, 0·5–1·5 mm across, on a dispersed black prothallus; medulla I−. Photobiont chlorococcoid; cells 9–12 µm diam.
Apothecia black, lecideine, 0·7–1·2 mm diam. ±immersed in the thallus; disc concave; proper margin thick (0·2 mm), raised and persistent, in section thick and carbonaceous, K+ magenta solution at edge. Hymenium c. 170 µm tall; paraphyses slender, branched and anastomosing, not swollen or pigmented at tips; epihymenium dilute brownish; subhymenium hyaline 45–50 µm tall. Asci Porpidia-type; ascospores simple, halonate, 20–24–28×9·0–10·0–12·5 µm. Hypothecium brown.
Conidiomata not observed.
Chemistry
Stictic acid by TLC.
Distribution and ecology
Known from the type and one other collection on siliceous rocks on Isle Madre de Dios in SW Chile. No other identifiable lichen species are present on the collections.
Remarks
The thick carbonaceous margin and concave disc give this species a distinctive appearance, but the massively developed exciple is possibly an environmental modification and may not be a good species-level character. The exciple structure and chemistry suggest this species belongs to the P. macrocarpa group (Haplocarpon M. Choisy).
Porpidia vulcanoides is unusual in the genus because of its large ascospores. The Northern Hemisphere species P. superba differs in having a thick, bullate, white thallus, whereas P. stephanodes from Îles Kerguelen has an even taller hymenium (170–230 µm), larger ascospores (35–60 µm) and an exciple having a pale brown medulla [similar to that of P. macrocarpa (DC.) Hertel & A. J. Schwab].
Additional specimen examined. Chile: Magallanes and Antártica Chilena Region: Isla Madre de Dios, gap at head of fiord E of Mte Roberto, 50°20′S, 67°21′W, moorland, 1969, Imshaug (44162) & K. Ohlsson (MSC—topotype).
Other taxonomic changes
Labyrintha Malcolm et al.
The genus Labyrintha Malcolm et al. was erected by Malcolm et al. (Reference Malcolm, Elix and Owe-Larsson1995) for the single species L. implexa Malcolm et al. which is endemic to New Zealand. They characterized their new genus by its unusual thalline structure in that it lacked the well-defined, horizontal photobiont layer present in most lichenized fungi, having instead the photobiont cells distributed throughout the thallus in vertically aligned columns interspersed with columns of fungal cells. Further diagnostic traits of the new genus were the large, pigmented ascospores, and the presence of cephalodia.
However, a similar thallus organization is displayed by some of the species described here (i.e., Lecidea aurantia, L. campbellensis, Poeltiaria tasmanica, P. subcontinua), and has also been reported in the genus Orceolina (Poulsen et al. Reference Poulsen, Schmitt, Søchting and Lumbsch2001) endemic to the Prince Edward Islands, Crozet Island and Îles Kerguelen, although in these taxa the columns rarely reach the upper surface of the thallus and, consequently, there is no resultant distinctive surface marking as in L. implexa. This thalline organization is also not restricted to the Lecideaceae, or to the southern subpolar region. Indeed, it was reported as widespread in various unrelated species in the xeric conditions of central Asia by Vondrák & Kubásek (Reference Vondrák and Kubásek2013) who observed the phenomenon in several, not closely related species of Caloplaca (Teloschistaceae): C. variabilis (Pers.) Müll. Arg. coll., C. elegantissima (Nyl.) Zahlbr., C. gomerana J. Steiner, C. scrobiculata H. Magn. and C. trachyphylla (Tuck.) Zahlbr.; as well as Acarospora (Acarosporaceae), thick-crusted Aspicilia (Megasporaceae), and alpine collections of Miriquidica complanata (Körb.) Hertel & Rambold and Psorinia (both Lecanoraceae). They also reported literature references to its occurrence in xeric conditions from Acarospora in North America (Knudsen Reference Knudsen, Nash, Gries and Bungartz2007), various vagrant species of Aspicilia (Owe-Larsson et al. Reference Owe-Larsson, Nordin, Tibell, Nash, Gries and Bungartz2007; Sohrabi et al. Reference Sohrabi, Ahti and Litterski2011a , Reference Sohrabi, Stenroos, Högnabba, Nordin and Owe-Larsson b ), Diplotomma (Physciaceae) in South America (Follmann Reference Follmann1965) and Psora crystallifera (Taylor) Müll. Arg. (Psoraceae) in South Africa (Vogel Reference Vogel1955). The phenomenon is, therefore, widespread both geographically and systematically, and Vogel, in particular, emphasized and illustrated (Vogel Reference Vogel1955: Fig. 15) the unusual arrangement of the photobiont cells. Vondrák & Kubásek (Reference Vondrák and Kubásek2013) showed that species with this thalline structure had a higher area-based light-saturated photosynthetic rate, and explained the phenomenon as an adaptation to take advantage of high insolation levels. They also suggested it was an adaptation to xeric conditions, but its occurrence in various species of Lecideaceae from the hydric conditions of the southern subpolar region suggests that this assumption merits further investigation.
The presence of cephalodia is also not a good generic character, especially in nutrient-poor habitats in humid regions. There are several examples of a single cephalodiate species in a genus that typically does not have cephalodia, and these invariably occur in humid, oceanic areas. For example, Rhizocarpon hensseniae Brodo known from only the Queen Charlotte Islands and SE Alaska (Brodo Reference Brodo1990), Carbonea gallowayi Hertel known only from south-west Chile (Hertel Reference Hertel2007), and Pertusaria stellata Fryday known only from Campbell Island/Auckland Islands and south-west Chile (Fryday Reference Fryday2008). The last mentioned is an especially good example because the cephalodia are well-formed, discrete structures immersed in the thallus of the lichen. The occasional collections of L. implexa lacking cephalodia (i.e., Imshaug 47880; see Materials and Methods) also cast doubt on the validity of the presence of cephalodia as a genus-level character.
Because thalline organization and the presence of cephalodia are not good characters at the generic level, and all other characters (including large and pigmented ascospores) are consistent with the genus Poeltidea Hertel, we here reduce Labyrintha to synonymy with Poeltidea and make the necessary new combination for L. implexa.
Poeltidea Hertel
=Labyrintha Malcolm et al., Lichenologist 27: 241 (Reference Malcolm, Elix and Owe-Larsson1995) syn. nov.
Poeltidea implexa (Malcolm et al.) Hertel & Fryday comb. nov.
MycoBank No.: MB805064
Basionym: Labyrintha implexa Malcolm et al., Lichenologist 27: 242 (Reference Malcolm, Elix and Owe-Larsson1995).
Notolecidea Hertel
Notolecidea Hertel was described by Hertel (Reference Hertel1984) as a genus close to the Porpidiaceae for the single species Notolecidea subcontinua (Nyl.) Hertel. Because of its unpigmented hypothecium it was regarded as close to Poeltiaria, from which it was said to differ in often containing algal cells in the basal parts of the exciple and the occurrence of atranorin in the thallus. However, chemical examination by TLC of four specimens (see Materials & Methods) as part of the present study failed to detect this substance and revealed all four specimens to contain either a series of triterpenes (Rf 3, 3–4, 4–5, 5 in solvent C) or a lack of substances. Atranorin here appears to be a sporadically occurring accessory substance of no taxonomic significance, as it is, for instance, in various species of Lecidea (see e.g. Culberson & Hertel Reference Culberson and Hertel1972). The presence of algal cells in the unpigmented excipula is also not a constant character. On comparing Notolecidea subcontinua with taxa currently included in Poeltiaria, a close similarity is evident with, for example, P. corralensis (Räsänen) Hertel (bacilliform pycnidia, non-carbonaceous exciple, hymenium height, ascospore size). Therefore it seems unnecessary to separate these taxa at the generic level and we consequently include Notolecidea in the synonymy of Poeltiaria and make the necessary new combination for N. subcontinua. However, as currently circumscribed, there is considerable infrageneric variation in Poeltiaria with respect to length of conidia, exciple pigmentation, and thallus chemistry (Table 1), and it is possible that Notolecidea may have to be resurrected for some of these species. However, molecular data is required to fully elucidate these relationships and no such changes are proposed here.
Table 1. Characters of species included in Poeltiaria (* = type species)
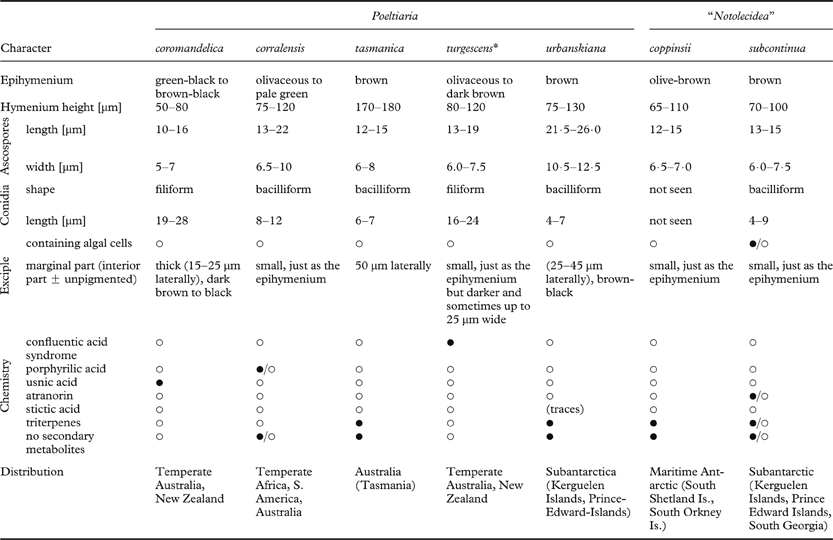
Poeltiaria
=Notolecidea Hertel, Nova Hedwigia, Beih. 79: 440 (Reference Hertel1984), syn. nov.
Poeltiaria subcontinua (Nyl.) Hertel & Fryday comb. nov.
MycoBank No.: MB805065
Basionym: Lecidea subcontinua Nyl. in Crombie, J. Linn. Soc. London, Bot. 15: 189 (1877).
Lecidea kalbii Hertel/L. mannii Tuck.
Hertel (Reference Hertel1984) described the new species L. kalbii Hertel from a single collection from southern Chile, distinguishing it by its atrobrunnea-type thallus, hyaline hypothecium, C+ red exciple, and wide ascospores (5·0–5·7 µm). Later (Hertel Reference Hertel1997), the same author reduced L. kalbii to synonymy with L. mannii Tuck., a species described from southern California that also possessed these characters. However, the two species are entirely different in gross morphology. Whereas the thallus of L. kalbii consists of angular, concave, red-brown areoles with a grey pruinose margin (Fig. 7A), those of L. mannii are regularly convex, pale fawn-coloured and lack grey pruina (Fig. 7B). The hypothecium of L. mannii is also often pale brown, whereas the hypothecium of L. kalbii is always completely hyaline. The two species also differ in thalline chemistry: whereas both contain gyrophoric acid, the Californian collections additionally always contain schizopeltic acid and two unidentified substances (Rf 1: UV+ yellow; Rf 2: UV+ blue; both in solvent C). The two species also occur in different climatic zones: L kalbii is a species of the high-rainfall, southern temperate region, whereas L. mannii occurs in the xeric Mediterranean climate of southern California. Given these differences, we have no hesitation in resurrecting L. kalbii from the synonymy of L. mannii.

Fig. 7. A, Lecidea kalbii (Imshaug 49521); B, Lecidea mannii (Fryday 9337). Scales=1 mm. In colour online.
Hertel (Reference Hertel1997) also reported L. mannii from northern Chile (Santiago). These collections have a similar gross morphology to that of L. kalbii and in addition to gyrophoric acid also contain a substance that is possibly 2′-O-methylperlatolic acid (orange spot at Rf 5.5 in solvent C). They are here referred to L. kalbii pending further investigation.
Lecidea kalbii is here reported for the first time from Argentina.
Specimens examined. Chile: Magallanes and Antártica Chilena Region: Brunswick Peninsula, near Hotel Cabeza del Mar, 52°48′S, 71°00′W, 1971, H. A. Imshaug (49521, 49540) & K. Ohlsson (MSC). Región Metropolotana de Santiago: Prov. Santiago, Quebrada de Peñalolén, 33°28′S, 70°32′W, al E de Santiago, 1962, Mahu 78 (M); ibid., Prov. Santiago, 8 km W of Tiltil, shrub Caparral on east slope of Cuesta de la Dormida, 1000–1300 m alt., 1976, W. A. Weber & B. Johnston (COLO L-64162).—Argentina: Tierra del Fuego: Isla Grande, Bahia San Sebastian, Cabo San Sebastian, 53°19′S, 68°13′W, 1971, H. A. Imshaug (54099) & K. Ohlsson (MSC).
Discussion
Biogeography
Although many genera of Lecideaceae s. lat. occur in both hemispheres, some have been reported from only one or the other (Table 2). This distribution is probably subject to erroneous generic and familial circumscription, but the family does appear to be more diverse in the Southern Hemisphere, with a number of genera having characters that are unknown in Northern Hemisphere genera. Two examples are Catarrhospora Brusse with submuriform ascospores and Poeltidea with pigmented ascospores, although it is probable that these genera do not belong in Lecideaceae s. str.
Table 2. Comparative characters of the genera of the Lecideaceae s. lat. (Lecideaceae s. str in bold)
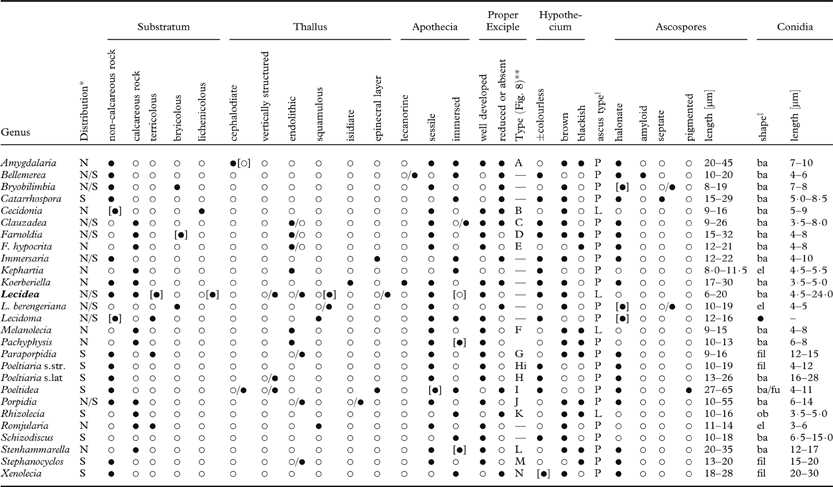
* N=Northern Hemisphere, S=Southern Hemisphere, N/S=both hemispheres; **, excipulum type: numbering refers to Fig. 8; †, L=Lecidea-type, P=Porpidia-type; ‡, ba=bacilliform; el=ellipsoid; fu=fusiform; ob=oblong.
Interestingly, most of the new species described here are island endemics: Auckland Islands (Lecidea aurantia), Campbell Island (Bryobilimbia austrosaxicola, Lecidea campbellensis), Tasmania (Poeltiaria tasmanica), Falkland Islands (Immersaria fuliginosa, Poeltidea inspersa), Isla Madre de Dios (Porpidia vulcanoides). Only Poeltiaria coppinsiana is known from more than one island, and even here the two groups (South Orkney and South Shetland) are separated by only c. 500 km and could be considered as one archipelago. This apparent endemism could reflect lack of collecting, but there are almost 20 000 collections from the region in MSC and at least some of the species (e.g., I. fuliginosa, L. aurantia) are distinctive and unlikely to have been overlooked elsewhere. It is unclear whether the new species described here are neo- or palaeoendemics. The closest relatives of the two Lecidea species and Porpidia vulcanoides are unclear, but Poeltiaria tasmanica appears to be most closely related to P. urbanskyana, which is known only from the Prince Edward Islands and Îles Kerguelen, whereas of the two other species of Poeltidea, P. perusta is widespread in the southern subpolar region including the Falkland Islands, and P. implexa is a New Zealand endemic. The only other species of Immersaria reported from the Southern Hemisphere, the cosmopolitan I. athroocarpa (Ach.) Rambold & Pietschm., is known from Antarctica, Australasia and South Africa, but not from South America.
Genera circumscribed within Lecideaceae
As currently circumscribed (Lumbsch & Huhndorf Reference Lumbsch and Huhndorf2010), the Lecideaceae contains a number of genera that are not closely related to Lecidea. A molecular phylogeny by Buschbom & Mueller (Reference Buschbom and Mueller2004) revealed a core Lecideaceae consisting of the genera Amygdalaria Norman, Cecidonia Triebel & Rambold, Immersaria Rambold & Pietschm., Lecidea Ach., Porpidia Körb., and Stenhammarella Hertel, with Porpidia stephanodes (Stirt.) Hertel and Stephanocyclos Hertel as a sister clade, and Bellemerea Hafellner & Cl. Roux and Koerberiella Stein as sister to this larger group (Table 2). Distant to Lecideaceae s. str. was a clade, possibly related to Psoraceae Zahlbr., consisting of Clauzadea Hafellner & Bellem., Farnoldia Hertel, Melanolecia Hertel, Bryobilimbia (as Mycobilimbia Rehm), Poeltiaria (as Notolecidea), and Pachyphysis R. C. Harris. Unfortunately, most of the genera described from the southern subpolar region were not included in the analysis of Buschbom & Mueller (Reference Buschbom and Mueller2004) and so their systematic position is unclear.
Lumbsch & Huhndorf (Reference Lumbsch and Huhndorf2010) also included a number of other genera that were historically included in Lecideaceae but clearly do not belong there. These are two neotropical genera; Bahianora Kalb, which has Lecanora-type asci (Sipman Reference Sipman2007), and Lopacidia Kalb, which is included in the synonymy of Bapalmuia Sérus. (Pilocarpaceae Zahlbr.) by Kalb et al. (Reference Kalb, Lücking and Sérusiaux2000); the corticolous/terricolous Steinia Körb., which has multispored asci and is included in the family Aphanopsidaceae Printzen & Rambold by Kantvilas & McCarthy (Reference Kantvilas and McCarthy1999); Pseudopannaria (B. de Lesd.) Zahlbr., which has curved, fusiform, multiseptate ascospores (65–88×6–5 µm, 7–10 septate; Bouly de Lesdain Reference Bouly de Lesdain1906); and Cryptodictyon A. Massal., which apparently refers to a corticolous species from Java that has ascospores with lenticular cells.
Infrageneric relationships within the Lecideaceae are also unclear. The analysis by Buschbom & Mueller (Reference Buschbom and Mueller2004) showed that Porpidia consisted of several distinct clades with some species [e.g., P. cinereoatra (Ach.) Hertel & Knoph, P. contraponenda (Arnold) Knoph & Hertel] closer to Amygdalaria than the type species of Porpidia, and Stenhammarella nesting close to the P. macrocarpa group. Elsewhere, the relationship between Bryobilimbia, Clauzadea, Romjularia Timdal and the L. berengeriana group is in need of further investigation. The genus Farnoldia is heterogeneous, with the position of F. muscigena (Vězda) Clauzade & Cl. Roux and, in particular F. hypocrita (A. Massal.) Fröberg, also in need of investigation. In the genera described from the southern subpolar region, Poeltiaria appears to consist of two disparate elements united by a pale hypothecium but differing in conidia and exciple structure. However, no further taxonomic novelties are proposed here pending a full molecular/morphological investigation of the species assigned to this genus and the family in general.
Key to genera of Lecideaceae
This key includes all saxicolous genera of Lecideaceae that have been reported from the southern subpolar region, plus those reported only from the Northern Hemisphere, along with some other genera with crustose taxa and a Porpidia-type ascus structure.
Notes on interpretation of the Key and Character Table (Table 2)
Ascospore dimensions: many dimensions are taken from the literature and these often do not distinguish between mean and extreme values.
Apothecial sections (Fig. 8): for the key and table, it is important to study young apothecia where exciple reduction has not taken place.Very thin (preferably microtome) sections are necessary to interpret the pigmentation of dark pigmented hypothecia and excipula. Especially in some taxa with a pigmented exciple, the exciple spreads far below the hymenium, and to determine whether the exciple forms a closed cup (cupular) or is open towards the centre of the ascocarp (annular), it is necessary to study only sections through the central part of the apothecium.
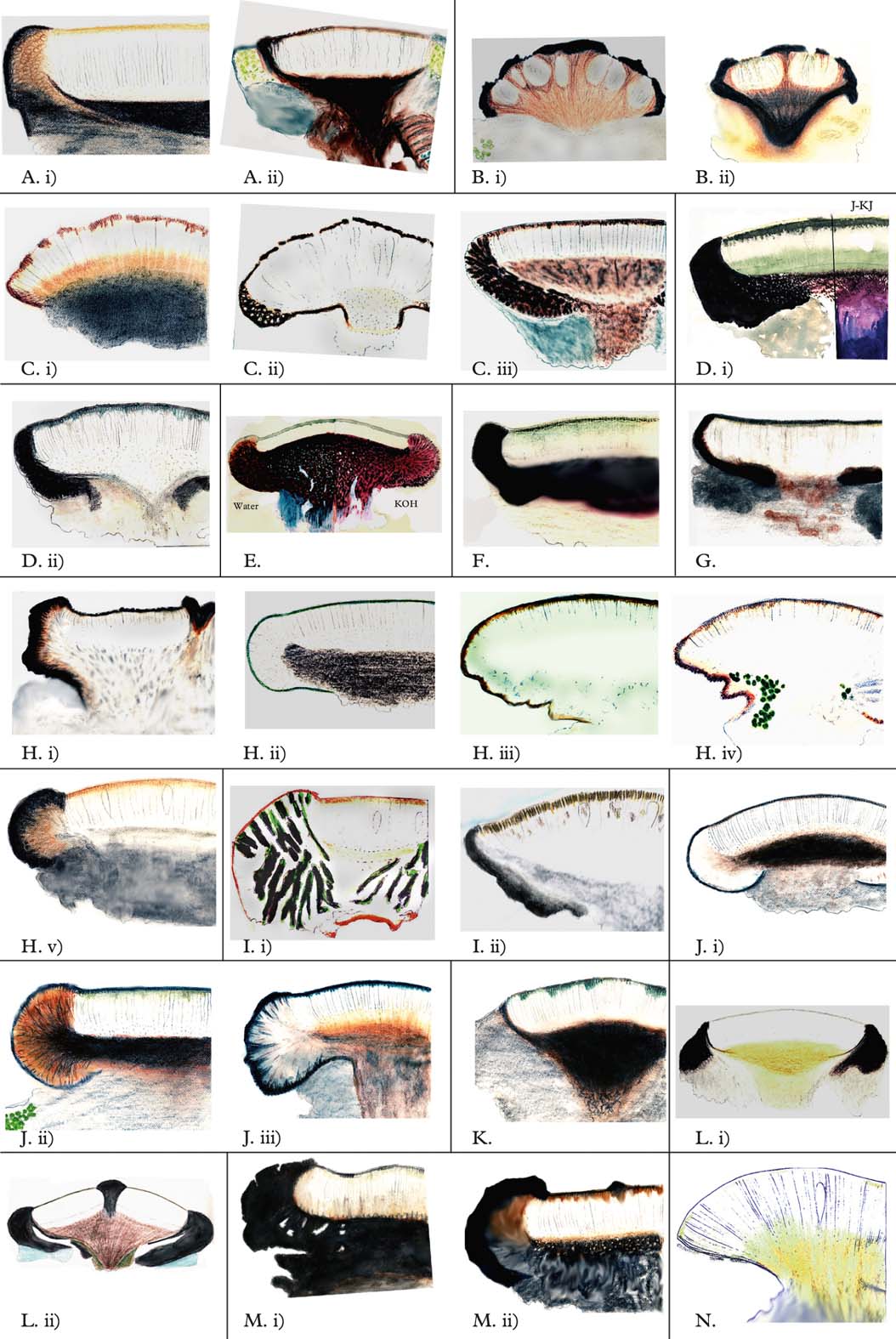
Fig. 8. Lecideaceae s. lat., thin vertical sections through central parts of young apothecia. A, Amygdalaria; i) A. aeolotera (Luzon, TUR-Vain 25.079, holotype—HH-1507); ii), A. pelobotryon (Sweden, Härjedalen, 1867, Hellbom, UPS—HH-1908). B, Cecidonia; i), C. umbonella (Sweden, Torne Lappmark, Nissuntjårro, 1967, Poelt, GZU—HH-0749); ii), C. xenophana (Ireland, holotype of Lecidea alumnula, H-Nyl 16238—HH-0819). C, Clauzadea; i), C. immersa (Bavaria, Körb.: Lich. Sel. Germ. 111, M—HH-1915); ii), C. metzleri (Tunisia, Ain Oktor, 1968, Hertel 8743, M—HH-0863); iii), C. monticola (USA, N. Alaska, Mancha Creek, 1958, Sharp 10700, WIS—HH-1847). D, Farnoldia; i), F. jurana (Austria, Tirol, Feuerspitze, 1963, Hertel 90, M, HH-0184); ii), F. micropsis (Novaya Zemlya, 1921, Lynge, holotype of Lecidea macrospora, O—HH-1842). E, “Farnoldia”; F. hypocrita (Italy, Trento, M. Castellazzo above Paneveggio, 1868, Arnold, M—HH-0195). F, Melanolecia; M. transitoria (Austria, Tirol, Mt. Wasenwand, 1965, Hertel 5607, M—HH-0429). G, Paraporpidia; P. leptocarpa (Tasmania, Snug Falls, 1981, Tibell 11277, UPS—HH-2595). H, Poeltiaria; i), P. coromandelica (Tasmania, Ben Lomond, 1907, Rodway, HO 69371—HH-2811); ii), P. corralensis (Argentina, Lake Nahuel Huapí, 1950, Lamb 5818, UPS—HH-1176); iii), P. coppinsiana (South Shetlands, Livingston Island, 1998, Søchting US 7018—HH-3681); iv), P. subcontinua (South Georgia, Lindsay 3024, AAS—HH-2557); v), P. urbanskyana (Marion Island, 1982, Hertel 23420, M—HH-2415). I, Poeltidea; i), P. implexa (New Zealand, Nelson, Malcolm 707, fragmentum typi)—HH-2975); ii), P. perusta (Chile, Magallanes, B. Borja, 1969, H. A. Imshaug 45213 & K. Ohlsson, MSC—HH-3779). J, Porpidia; i), P. austroshetlandica (South Shetland Isl., Isla Greenwich, Follmann 14064, holotype, M—HH-2311); ii), P. crustulata (China, Jilin, Changbai Shan, 1980, Hertel 22609, M—HH-2131); iii), P. skottsbergiana (South Georgia, Skottsberg 92, S, holotype—HH-2284). K, Rhizolecia; R. hybrida (New Zealand, holotype, W—HH-2372). L, Stenhammarella; i), S. turgida (Switzerland, Bern, summit of Mt. Niesen, 1965, Hertel 6240, M—HH-0453); ii), S. turgida (Austria, Salzburg, Hohe Tauern, Schmiedinger Scharte, 1963, Hertel 2256, M—HH-0241). M, Stephanocyclos; i), S. henssenianus (Marion Island, 1982, Hertel, Hertel: Lecideac. Exs. 95, M—HH-2227); ii), S. henssenianus (Marion Island, 1982, Hertel 24575, M—HH-2274). N, Xenolecia; X. spadicomma (Chile, Eden Harbour, holotype, BM—HH-2360).
Ascus type: in the key and table, ‘Porpidia-type’ means only the presence of a distinct amyloid tube-like structure in the ascus tholus to distinguish it from ‘Lecidea-type’ where no such tube exists. Hafellner (Reference Hafellner1984) uses a more detailed scheme, where he differentiates between pale and bluish tholi.
We have not seen material of the recently described genus Kephartia R. C. Harris & Lendemer (Lendemer et al. Reference Lendemer, Harris and Tripp2013), and so all data are taken from the protologue.
-
1 Ascospores 3-septate to submuriform ... 2
Ascospores simple (rarely 1-septate) ... 3
-
2(1) Thallus of flat areoles dispersed on a black hypothallus; ascospores with rounded apices. Atranorin absent (K−). Known only from South Africa ... Catarrhospora
Thallus of ± peltate or convex areoles, hypothallus absent; ascospores apically attenuated. Atranorin present (K+ yellow) ... Stereocaulon
-
3(1) Epihymenium K+ red (anthraquinones); apothecia yellow to orange or dark brown ... 4
Epihymenium K− or K+ violet; apothecia brown to black, sometimes grey pruinose ... 5
-
4(3) Thallus crustose; apothecia yellow to orange ... Protoblastenia
Thallus squamulose; apothecia brown to black ... Psora
-
5(3) Ascospores pigmented ... Poeltidea
Ascospores hyaline. ... 6
-
6(5) Ascospores with an amyloid (I+ blue) wall; apothecia ±immersed with thalline margin ... Bellemerea
Ascospores without an amyloid wall ... 7
-
7(6) Apothecia sessile with well-developed thalline margin; thallus with papillate isidia. Usually in damp habitats. Not yet reported from the Southern Hemisphere ... Koerberiella
Apothecia lacking well-developed thalline margin, ±immersed or sessile ... 8
-
8(7) Thallus with a distinct epinecral layer in section ... 9
Thallus lacking a distinct epinecral layer ... 10
-
9(8) Apothecia immersed; ascus Porpidia-type ... Immersaria
Apothecia ± sessile, rarely immersed; ascus Lecidea-type ... Lecidea p.p.
-
10(8) Hypothecium either orange K+ purple or with orange crystals reacting K+ purple ... Kephartia
Hypothecium not K+ purple ... 11
-
11(10) Hypothecium hyaline ... 12
Hypothecium pigmented ... 15
-
12(11) Ascospores with distict gelatinous coat (halonate) ... 13
Ascospores with indistinct gelatinous coat or gelatinous coat absent ... 14
-
13(12) Growing on limestone. [C. immersa with an endolithic thallus and slightly ornamented ascospores; C. chondrodes with smooth and large ascospores (16–26×7–12 µm)] ... Clauzadea p.p.
Growing on acid rocks. Ascospores smooth. Southern Hemisphere genus ... Poeltiaria
-
14(12) Ascus Porpidia-type, apothecia immersed, pruinose. On calcareous rocks. Known only from South Africa ... Schizodiscus
Ascus Lecidea-type, ascospores with a central plasma-bridge (‘pseudodiblastic spores’) ... Lecidea
-
15(11) Mature ascospores lacking distinct gelatinous coat, but with a thin gelatinous coat sometimes evident in young ascospores ... 16
Mature ascospores with a distinct gelatinous coat ... 26
-
16(15) Asci Lecidea-type ... 17
Asci Porpidia-type ... 20
-
17(16) Exciple carbonaceous. Not yet reported from the Southern Hemisphere ... 18
Exciple not carbonaceous ... 19
-
18(17) Lichenicolous fungus, forming white galls on Lecideaceae species; host species occurring on siliceous rocks. Paraphyses branched and anastomosing ... Cecidonia
Autonomous lichen, thallus not forming white galls, endolithic on calcareous, alpine rocks. Paraphyses ± simple. Northern Hemisphere only ... Melanolecia
-
19(17) Thallus cretaceous; apothecia blue-black, ±immersed; paraphyses branched and anastomosing. On calcareous rocks. Known only from New Zealand. Resembles Porpidia speirea, but with non-halonate ascospores and Lecidea-type ascus ... Rhizolecia
Thallus otherwise; paraphyses ±unbranched. The Southern Hemisphere species occur exclusively on siliceous rocks ... Lecidea
-
20(16) Conidia filiform, mostly >15 µm long. Known only from Australasia ... Paraporpidia
Conidia usually bacilliform or ellipsoid, <12 µm long ... 21
-
21(20) Paraphyses with gelatinous coat (up to 10 µm thick); hymenium with red-purple (K+ bright purple) pigment; ascospores ±globose. Known only from North America ... Pachyphysis
Paraphyses lacking gelatinous coat (<5 µm thick); hymenium ±hyaline ... 22
-
22(21) Terricolous. Thallus either distinctly squamulose or areolate-squamulose with distinct marginal lobes ... 23
Saxicolous, or bryicolous on rocks, trees or soil (rarely corticolous). Thallus crustose or minutely squamulose without distinct marginal lobes ... 24
-
23(22) On acid alpine soils. Thallus areolate-squamulose with wide marginal lobes; hypothecium hyaline; paraphyses simple, thick (3–4 µm) and distinctly capitate (5–7 µm) ... Lecidoma
On calcareous soils. Thallus squamulose; hypothecium pale brown; paraphyses thinner ... Romjularia
-
24(22) Saxicolous on calcareous rocks. Paraphyses partly moniliform, 2–3 µm wide ... Clauzadea (C. monticola)
Usually bryophilous, rarely on damp siliceous rock. Paraphyses not moniliform, 1·0–1·5 µm wide ... 25
-
25(24) Paraphyses without distinctly swollen apices; conidia bacilliform, 7–8×1 µm; thallus inconspicuous ... Bryobilimbia
Paraphyses with distinctly swollen apices; conidia ellipsoid, 4–6×2–3 µm; thallus granular to minutely squamulose ... Lecidea berengeriana group
-
26(15) Cephalodia usually present; apothecia immersed, with a poorly developed exciple. Not yet reported from the Southern Hemisphere ... Amygdalaria
Cephalodia absent; apothecia usually with a well-developed exciple ... 27
-
27(26) Conidia filiform ... 28
Conidia bacilliform ... 29
-
28(27) Exciple reduced to a thin brownish rim; hypothecium very pale brownish; hymenium 110–145 µm; apothecia large, completely sunken into the thallus. Known only from South America ... Xenolecia
Exciple very well developed, very irregular in shape and segmented in parts that meet at sharp angles, usually conspicuously longitudinally grooved; hypothecium ± confluent with the exciple, blackish brown. Southern Hemisphere genus ... Stephanocyclos
-
29(27) Young apothecia completely sunken into the cretaceous thallus with a flat, greyish (covered with minute thalline remnants), very broad and involucrellum-like margin; disc black and relatively small. In mature apothecia the margin becomes reduced and then resembles that of Porpidia species (ontogeny is figured in Reference HertelHertel 1977a : 242); ascospores large (28–30 µm long); hypothecium much reduced. Restricted to calciferous rock in alpine habitats (European Alps, China) ... Stenhammarella
Margin of young apothecia not flat (circular or semi-circular) in section (sometimes reduced); ascospores usually smaller; hypothecium well developed ... 30
-
30(29) Exciple thick and carbonaceous throughout, clearly separated from the paler hypothecium ... 31
Excipulum not carbonaceous throughout ... 32
-
31(30) Paraphyses moniliform; short bacilliform conidia (±4–6×1·5 µm) are born laterally and apically; epihymenium brown; apothecia with a black disc, becoming brownish when wet ... Clauzadea monticola (extreme forms)
Paraphyses slender, not moniliform; conidia bacilliform, 5–14×0·7–1·2 µm; apothecia sessile with a black or bluish pruinose disc, never becoming brownish when wet. On limestone, dolomite, mortar and other richly calcareous substrata ... Farnoldia
-
32(30) Growing on limestone (and other rock types very rich in CaCO3). Thallus often endolithic ... 33
Growing on acidic rock (exceptionally on calciferous siliceous rock). Paraphyses usually slender, moderately branched and anastomosing, very rarely moniliform; discs of apothecia usually black or pruinose, rarely becoming brownish after moistening; the bacilliform conidia [6–14 (–22)×0·8–1·2 µm] are developed apically ... Porpidia p.p.
-
33(32) Epihymenium intensely blue-green; exciple thick, paler than hypothecium (seen in thin sections!), K+ reddish violet (Atra-red); ascospores often pointed; apothecia black (even when moistened), becoming large (up to 3 mm in diam.). Holarctic ... “Farnoldia” hypocrita
Epihymenium brownish or olivaceous ... 34
-
34(33) Hypothecium concolorous with exciple; brown and or olivaceous pigments present internally; conidia developed apically. High montane to alpine regions ... Porpidia p.p.
Hypothecium paler than exciple; only brown, K− pigments present internally; conidia developed laterally and apically. In lowland to high montane regions ... Clauzadea
We gratefully acknowledge support from the US National Science Foundation, Award No. DBI–9808 735 (Alan Prather, PI) to Michigan State University that facilitated access to Henry Imshaug's collections. We also thank Henry Imshaug and co-workers for collecting most of the material described here, Gintaras Kantvilas, Ronald Lewis Smith, Ryszard Ochyra and Ulrik Søchting for sending us their named collections, and the curators of COLO, M, UCR and W for the loan of comparative material. Andreas Beck (M) is thanked for providing the photograph in Fig. 4A, and Gintaras Kantvilas (HO) for identifying the taxa associated with Poeltiaria tasmanica.












