INTRODUCTION
Tropical montane forests are highly diverse ecosystems (Myers et al. Reference MYERS, MITTERMEIER, MITTERMEIER, FONSECA and KENT2000) and are important providers of ecosystem functions and services (Costanza et al. Reference COSTANZA, D’ARGE, DE GROOT, FARBER, GRASSO, HANNON, LIMBURG, NAEEM, O’NEILL and PARUELO1997). Vast areas of these forests have been deforested (FAO 2011), resulting in landscapes comprising forest fragments in matrices of deforested habitats. Soil seed banks (SSB) are important seed sources for early forest regeneration after disturbance (Castillo & Stevenson Reference CASTILLO and STEVENSON2010, Garwood Reference GARWOOD, Leck, Parker and Simpson1989, Young et al. Reference YOUNG, EWEL and BROWN1987).
Forest recovery at deforested sites is slow compared with recovery after natural disturbances (Aide et al. Reference AIDE, ZIMMERMAN, HERRERA, ROSARIO and SERRANO1995, Kappelle et al. Reference KAPPELLE, GEUZE, LEAL and CLEEF1996), and missing sources of plant seeds are a major barrier for forest regeneration (Myster Reference MYSTER2004, Zimmerman et al. Reference ZIMMERMAN, PASCARELLA and AIDE2000). Seed density and species richness of SSB are reduced by deforestation (Ewel et al. Reference EWEL, BERISH, BROWN, PRICE and RAICH1981, Miller Reference MILLER1999), and seed density and species richness tend to decline in deforested habitats with increasing distance to adjacent forests (Aide & Cavelier Reference AIDE and CAVELIER1994, Cubiña & Aide Reference CUBIÑA and AIDE2001, Zimmerman et al. Reference ZIMMERMAN, PASCARELLA and AIDE2000). Forest fragments are exposed to edge effects from the deforested matrix, leading to altered habitat conditions at forest edges (Saunders et al. Reference SAUNDERS, HOBBS and MARGULES1991, Williams-Linera et al. Reference WILLIAMS-LINERA, DOMÍNGUEZ-GASTELÚ and GARCÍA-ZURITA1998). Modified habitat conditions often cause a proliferation of small-seeded pioneer species (Laurance et al. Reference LAURANCE, NASCIMENTO, LAURANCE, ANDRADE, FEARNSIDE, RIBEIRO and CAPRETZ2006, Oliveira et al. Reference OLIVEIRA, GRILLO and TABARELLI2004) and the extirpation of large-seeded climax species at forest edges (Melo et al. Reference MELO, LEMIRE and TABARELLI2007). These changes in vegetation at forest edges may consequently alter the characteristics of SSB at forest edges (Lin & Cao Reference LIN and CAO2009, Lindner Reference LINDNER2009).
Tropical montane forests are characterized by steep gradients in altitude that strongly modify habitat conditions (Beck et al. Reference BECK, BENDIX, KOTTKE, MAKESCHIN and MOSANDL2008). Due to changes in habitat conditions, seed input from the standing vegetation (Espinosa et al. Reference ESPINOSA, LUZURIAGA, CRUZ, MONTERO and ESCUDERO2013, Ortega et al. Reference ORTEGA, LEVASSOR and PECO1997) and the persistence of seeds in the soil (Cavieres & Arroyo Reference CAVIERES and ARROYO2001, Wagner & Mitschunas Reference WAGNER and MITSCHUNAS2008) have been reported to change along altitudinal gradients. However, responses of SSB to differences in altitude are intricate, and some studies have reported an increase in seed density and species richness of SSB with increasing altitude (Espinosa et al. Reference ESPINOSA, LUZURIAGA, CRUZ, MONTERO and ESCUDERO2013, Funes et al. Reference FUNES, BASCONCELO, DÍAZ and CABIDO2003), while others have reported decreasing density and species richness (Ortega et al. Reference ORTEGA, LEVASSOR and PECO1997, Thompson Reference THOMPSON1978).
Knowledge of the effects of disturbance on tropical SSB is largely restricted to lowland forests. To address this gap of knowledge, we sampled SSB in a fragmented montane landscape in the tropical Andes of Bolivia in three different habitat types that were associated with different degrees of disturbance, i.e. in forest interior, at forest edges and in deforested habitats. Sampling was replicated at six sites ranging in altitude from 1950 to 2450 m asl. Previous studies have shown that altitude influenced the composition of vegetation both in remnant forests and in deforested habitats along this gradient (Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013; in press). We extracted seeds by sieving and classified seeds into morphospecies and size classes. We tested effects of disturbance (i.e. habitat type) and altitude on density and richness of different seed sizes and on species composition. We predicted that (1) seed density, species richness and species composition of SSB differ among habitat types, with depauperate SSB in deforested habitats, and that (2) seed density and species richness increase and species composition changes with increasing altitude (cf. Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013; in press).
METHODS
Study area
Our study was conducted in the eastern Cordillera of Bolivia, near the village of Chulumani (16°24′36″S, 67°31′32″W). The mean annual temperature is about 21°C and the mean annual precipitation about 1459 mm (Molina-Carpio Reference MOLINA-CARPIO2005). Temperature decreases with increasing altitude by approximately 0.5°C per 100 m of altitude. The tropical montane forests in this area have been deforested by frequent anthropogenic burning (Killeen et al. Reference KILLEEN, SILES, SORIA and CORREA2005), resulting in a landscape comprising few forest remnants in a matrix of deforested habitats. Only a small fraction of the deforested areas is used for agriculture, mainly for coca (Erythroxylum coca Lam.) cultivation, while vast deforested areas are burned repeatedly by uncontrolled fires, mostly originating from slash-and-burn practices. Vegetation in the deforested areas is arrested in an early stage of secondary succession and is dominated by bracken fern (Pteridium arachnoideum (Kaulf.) Maxon) and predominantly wind-dispersed small-seeded shrubs, mostly ruderal Asteraceae and Melastomataceae species (Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013). In contrast, vegetation of forest remnants comprises species from Lauraceae (genera: Nectandra, Ocotea), Myrtaceae (Eugenia, Calyptranthes), Melastomataceae (Miconia) and Rubiaceae (Faramea, Palicourea) (Lippok et al. in press).
Sampling of soil seed bank
Sampling comprised six sites covering an altitudinal gradient of 500 m, ranging from 1950 to 2450 m asl. At each site, we sampled SSB in three different habitat types that are associated with different degrees of disturbance, i.e. in forest interior, at forest edges and in deforested habitats. The interior of forests remnants represents a rather low intensity of disturbance by occasional logging and charcoal production. Environmental conditions and vegetation structure at forest edges are altered significantly by edge effects of the deforested matrix (Lippok et al. in press). Deforested habitats adjacent to the forests are regularly burned and covered by a successional vegetation (Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013). The forest interior was sampled at 160 m from forest margins inside forests and forest edges at 20 m inside forests. Deforested habitats were sampled at three different distances from the forest margin, at 5 m, 20 m and 80 m. We lacked detailed information about the date of edge creation and fire history of deforested sites. Based on information of locals, we estimated that deforested sites have been burned at least every 5 y.
SSB was sampled to a depth of 5 cm without the litter layer; thus, we may have missed seeds trapped in the litter layer with our sampling method. At each sampling point (i.e. forest interior, forest edge and 5 m, 20 m and 80 m at deforested sites), we set up a 50 × 2-m plot parallel to the forest margin. Each SSB sample comprised 20 subsamples, sampled at randomly chosen intercepts from a 2 × 2-m grid inside each plot and collected with a circular tube of 3.6 cm diameter and 5 cm depth, resulting in a total area of c. 200 cm2 per sample. The compound SSB sample (volume about 1000 cm3) was homogenized and air-dried for several days to standardize sample volumes among sites. A randomly chosen volume of 500 cm3 of each dried sample (Lindner Reference LINDNER2009) was sieved with three different mesh sizes (i.e. 2 mm, 1 mm and 0.63 mm). We discarded the remainder (<0.63 mm) and analysed the resulting three fractions separately (i.e. 0.63–1 mm, 1–2 mm and >2 mm). The applied extraction by the sieving method has the advantage of a quick and cheap survey of SSB (Lindner Reference LINDNER2009). In contrast to the seedling emergence method, very small seeds are discarded and viable seed densities may be overestimated because viable and non-viable seeds cannot be distinguished (Warr et al. Reference WARR, THOMPSON and KENT1993).
We used a standard binocular microscope to count the seed density and to classify seeds into morphospecies on the basis of seed size, shape, colour, surface and appendage. We categorized seed sizes based on the three sieving fractions as small (0.63–1 mm), medium-sized (1–2 mm) and large (>2 mm). We calculated seed density (number of seeds per 500 cm3 dry soil) and species richness (number of morphospecies per 500 cm3 dry soil) for each of the three seed size classes (i.e. small, medium-sized and large). Although we are missing information about species identities and corresponding life-history traits, seed sizes of plant species are associated with their ecological class (small-seeded pioneer species vs. large-seeded climax species; Foster & Janson Reference FOSTER and JANSON1985), dispersal syndrome (small-seeded wind-dispersed vs. large-seeded animal-dispersed; Leishman et al. Reference LEISHMAN, WESTOBY and JURADO1995) and the ability to persist in the soil (small-seeded long-lived vs. large-seeded short-lived; Thompson et al. Reference THOMPSON, BAND and HODGSON1993).
Data analysis
We tested the effects of disturbance (i.e. habitat type) on seed density and species richness in two steps. First, we fitted linear mixed-effects models with habitat (three levels: forest interior, forest edge and deforested habitat) and altitude as fixed factors and sampling site as random factor, to test the overall effects of habitat and altitude on seed density and species richness. Second, we contrasted seed density and species richness of forest edges and of deforested sites at 5 m, 20 m and 80 m distance to the forest margin with seed density and species richness in the forest interior, i.e. the habitat type with the lowest disturbance intensity. All analyses were conducted for the entire seed pool and separately for each class of seed sizes. To achieve normally distributed model residuals, seed density was log10- and species richness was square root-transformed.
We tested the effects of disturbance (i.e. habitat type) on species composition in two steps. First, we tested effects of habitat and altitude on species composition with a distance-based multivariate analysis of variance (db-MANOVA). The analysis was based on the Bray–Curtis dissimilarities between samples derived from square root-transformed morphospecies abundances excluding all singletons (i.e. morphospecies with only one recorded seed). Significance of effects in the db-MANOVA was determined with Monte Carlo permutations (n = 999), stratified at the level of sampling sites for testing differences among habitats. Second, we calculated pairwise Bray–Curtis dissimilarities between all sampling points (i.e. forest interior, forest edge and 5 m, 20 m and 80m at deforested sites). All statistical analyses were carried out with R and dedicated packages ‘nlme’ and ‘vegan’.
RESULTS
SSB characteristics
We extracted a total of 2731 seeds and classified them into 152 morphospecies whereof 68 morphospecies were only sampled with a single seed (i.e. singletons). Only 21 morphospecies (14%) occurred in all habitats, i.e. in forest interior, at forest edges and in deforested habitats. Small seeds (0.63–1 mm) were most abundant, accounting for 81% of all extracted seeds (from 59 morphospecies). Medium-sized seeds (1–2 mm) accounted for 14% of the extracted seeds (54 spp.) and large seeds (>2 mm) for 5% (39 spp.).
Seed density and species richness
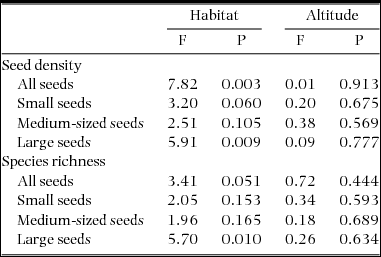
As expected, seed density was positively related to species richness (Pearson's r = 0.50, P = 0.005). Density and species richness of all seeds were affected by habitat type, but not by altitude (Table 1). Effects of habitat type differed among seed sizes and were only significant in large seeds, while density and richness of small and medium-sized seeds were not significantly affected by habitat type (Table 1).
Table 1. Effects of habitat type (i.e. forest interior, forest edge and deforested habitat) and altitude (i.e. a gradient of 500 m in altitude) on seed density and species richness of soil seed banks (SSB) in fragmented tropical montane forests in the Bolivian Andes. Models were computed separately for all seeds, small seeds (0.63–1 mm), medium-sized seeds (1–2 mm) and large seeds (>2 mm). F-statistics are based on analyses of variance with Type II errors.
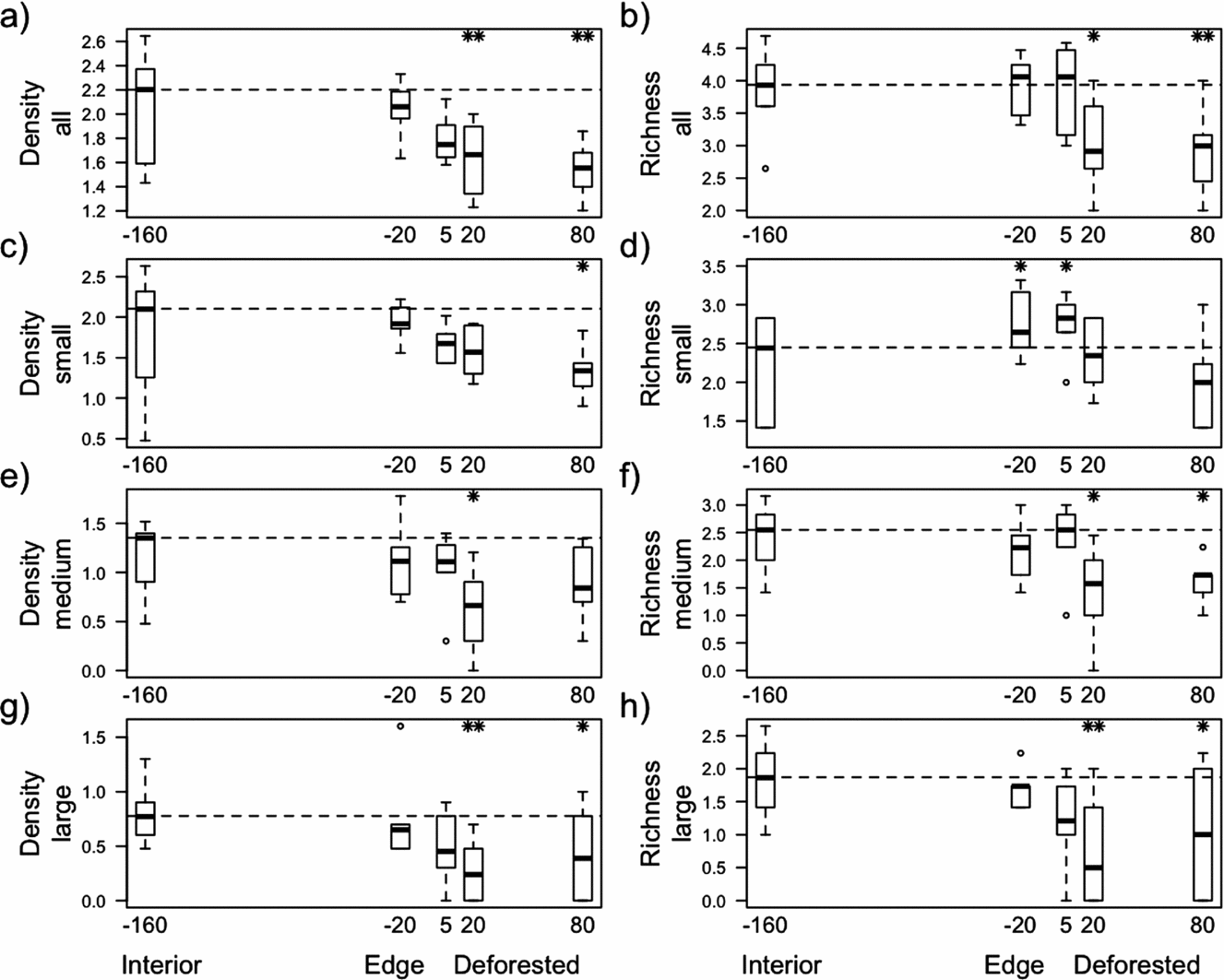
Seed density (Figure 1a) and species richness (Figure 1b) of all seeds decreased in deforested habitats with increasing distance from the forest interior and were significantly reduced at 20 m and 80 m distance from the forest margin. The decrease in seed density with distance was most evident in large seeds (Figure 1g), while the decrease in species richness was significant in large and medium-sized seeds (Figure 1f & h). Despite the overall decrease in species richness with increasing distance from the forest interior, species richness of small seeds was enhanced at forest edges and at 5 m distance from the forest margin at deforested sites (Figure 1d).

Figure 1. Characteristics of soil seed banks in three different habitat types, i.e. in forest interior, at forest edges and in deforested habitats in fragmented tropical montane forests in the Bolivian Andes. Given are the distances (m) from the forest margin, where negative values indicate sampling points inside the forest and the positive values sampling points outside the forest. Seed density (seeds per 500 cm3 dry soil, log10-transformed) and species richness (species per 500 cm3 dry soil, square root-transformed) of all seeds (a, b), small seeds (0.63–1 mm; c, d), medium-sized seeds (1–2 mm; e, f) and large seeds (>2 mm, g, h) are shown. Horizontal lines across boxes are medians, boxes indicate 25th and 75th percentiles, whiskers indicate the data range, and circles are outliers. Dashed lines indicate the median of forest interior values and asterisks indicate significant differences compared with the forest interior. * P ≤ 0.05, ** P ≤ 0.01.
Species composition
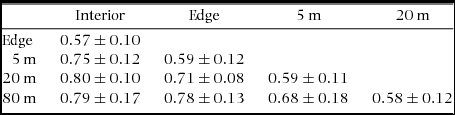
Species composition of SSB was affected by habitat type and altitude. Habitat type explained 13% of variance in species composition (db-MANOVA: F = 1.82, P = 0.001) and altitude explained 8% of variation (db-MANOVA: F = 0.08, P = 0.005). Overall species turnover between sampling points was high (Table 2). The species turnover from forest interior to deforested habitats was almost complete (Bray–Curtis dissimilarity ≈ 0.8) and higher than the species turnover between forest interior and forest edges.
Table 2. Species turnover of soil seed banks (SSB) in three different habitats, i.e. in forest interior, at forest edges and in deforested habitats. Given are mean pairwise Bray–Curtis dissimilarities ± SD of SSB of forest interior, forest edge and of deforested sites at 5 m, 20 m and 80 m distance from forest margin. Sampling comprised six sites along an altitudinal gradient of 500 m in fragmented tropical montane forests in the Bolivian Andes. Dissimilarities were computed from square root-transformed abundances of 84 morphospecies (excluding species with a single observation).
DISCUSSION
SSB characteristics
SSB were dominated by small seeds characteristic of pioneer species (Foster & Janson Reference FOSTER and JANSON1985), which is consistent with other SSB studies of tropical rain forests (Baider et al. Reference BAIDER, TABARELLI and MANTOVANI2001, Dalling & Hubbell Reference DALLING and HUBBELL2002, Quintana-Ascencio et al. Reference QUINTANA-ASCENCIO, GONZALEZ-ESPINOSA, RAMIREZ-MARCIAL, DOMINGUEZ-VAZQUEZ and MARTINEZ-ICO1996). The high seed output of pioneer species (Whitmore Reference WHITMORE1989) contributes to the high density of small seeds in the SSB. In addition to high seed production, small seeds tend to persist over a long time in SSB (Funes et al. Reference FUNES, BASCONCELO, DÍAZ and CABIDO1999) and dormancy is common (Leishman et al. Reference LEISHMAN, WRIGHT, MOLES, WESTOBY and Fenner2000). In contrast, large seeds are characteristic for most climax tree species (Foster & Janson Reference FOSTER and JANSON1985), which usually produce low numbers of seeds (Whitmore Reference WHITMORE1989) and do not persist for a long time in SSB (Hopkins & Graham Reference HOPKINS and GRAHAM1983).
Effects of habitat type
As predicted, seed density, species richness and species composition of SSB differed among habitat types. Seed density and species richness of SSB was lowest in deforested habitats, especially in large-seeded species and distant from adjacent forests. Small-seeded species accumulated near forest margins.
Deforestation caused a decrease in seed density and species richness of SSB (Garwood Reference GARWOOD, Leck, Parker and Simpson1989, Miller Reference MILLER1999), probably due to the reduction in vegetation in deforested habitats. Secondary vegetation in deforested habitats was dominated by pioneer species from Asteraceae and Melastomataceae and had a species composition distinct from near-natural forests (Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013). Thus, seed input from secondary vegetation in deforested habitats might be shifted towards higher amounts of small-seeded species while large-seeded species were lacking. The observed decrease in seed density and species richness of SSB in deforested habitats with increasing distance to adjacent forests is in accordance with other SSB studies (Aide & Cavelier Reference AIDE and CAVELIER1994, Cubiña & Aide Reference CUBIÑA and AIDE2001, Zimmerman et al. Reference ZIMMERMAN, PASCARELLA and AIDE2000) and is often caused by a decrease in seed rain from forest remnants with increasing distance (Cubiña & Aide Reference CUBIÑA and AIDE2001, Holl Reference HOLL1998). This may be explained by the fact that many animal dispersers, which are crucial dispersal vectors especially for large seeds (Markl et al. Reference MARKL, SCHLEUNING, FORGET, JORDANO, LAMBERT, TRAVESET, WRIGHT and BÖHNING-GAESE2012), avoid deforested habitats, especially distant from forests (Ingle Reference INGLE2003, Wunderle Reference WUNDERLE1997). Additionally, the litter of the dominant bracken fern in the deforested habitats acts as a barrier to seed input and may reduce seed density and richness of SSB (Ghorbani et al. Reference GHORBANI, DUC, MCALLISTER, PAKEMAN and MARRS2006). Our findings suggest that deforestation depletes SSB of tropical montane forests, especially in large-seeded species and distant from adjacent forests.
SSB at forest edges were similar to those in the forest interior. However, we observed a higher richness of small-seeded species near forest margins. One potential explanation for this pattern could be a shift in the species composition of forest vegetation at edges towards higher ratios of small-seeded pioneer species (Santos et al. Reference SANTOS, PERES, OLIVEIRA, GRILLO, ALVES-COSTA and TABARELLI2008, Lippok et al. in press). Consistent with this explanation, communities of seed-dispersing animals are shifted towards small-bodied species at forest edges that are more likely to consume and disperse small-seeded plant species (Menke et al. Reference MENKE, BÖHNING-GAESE and SCHLEUNING2012). It is a likely scenario that small-seeded species from deforested habitats could penetrate marginal habitats and eventually invade the vegetation at forest edges (Lin & Cao Reference LIN and CAO2009, López-Toledo & Martínez-Ramos Reference LÓPEZ-TOLEDO and MARTÍNEZ-RAMOS2011).
Effects of altitude
Along the altitudinal gradient, only species composition of SSB changed, while seed density and species richness were unaffected. While previous studies of changes in SSB characteristics with elevation reported either an increase (Espinosa et al. Reference ESPINOSA, LUZURIAGA, CRUZ, MONTERO and ESCUDERO2013, Funes et al. Reference FUNES, BASCONCELO, DÍAZ and CABIDO2003) or a decrease (Ortega et al. Reference ORTEGA, LEVASSOR and PECO1997, Thompson Reference THOMPSON1978) in seed density and species richness, we did not find directed changes along the altitudinal gradient. This suggests that effects of the here investigated altitudinal gradient of 500 m on seed density and richness were negligibly small. The observed altitudinal turnover in species composition of SSB is likely driven by changes in species composition of vegetation, i.e. a high species turnover in forest (Lippok et al. in press) and secondary vegetation (Lippok et al. Reference LIPPOK, BECK, RENISON, GALLEGOS, SAAVEDRA, HENSEN and SCHLEUNING2013) with altitude. Relative to the changes in SSB among habitat types, altitudinal effects on SSB were weak, suggesting that SSB were influenced by similar processes along the entire altitudinal gradient.
Conclusions
In tropical montane forests of the Bolivian Andes, SSB changed strongly among habitat types, but only weakly along the altitudinal gradient of 500 m. In deforested habitats, density and richness of SSB decreased with increasing distance from adjacent forests, especially in large-seeded species, indicating a low potential for natural forest regeneration distant from forest remnants. At forest edges, small-seeded species accumulated in the SSB, which might imply a gradual invasion of small-seeded species from adjacent deforested habitats to forest edges.
ACKNOWLEDGEMENTS
We thank the German Science Foundation (DFG) for funding and the ENEDAS e.V. for financial support of this study by a JCW scholarship. M.S. was also supported by the research funding programme Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz (LOEWE) of Hesse's Ministry of Higher Education, Research, and the Arts. We thank the staff from the Herbario Nacional de Bolivia (LPB) for their support and the staff of the soil labour of the UMSA for help in the laboratory.