Introduction
Profound bradycardia in the newborn is defined as a heart rate of less than 60 beats per minute. Reference Kugler, Gillette and Garson1,Reference Richards, Alexander, Shinebourne, de Swiet, Wilson and Southall2 In infants with complete AV block or structural heart disease, profound bradycardia can result in poor feeding, decreased perfusion, and decreased level of activity. Reference Rein, Simcha, Ludomirsky, Appelbaum, Uretzky and Tamir3 The most common causes of bradycardia in the newborn period are sinus bradycardia, reactive bradycardia secondary to an underlying illness (sepsis, hypothermia, congenital hypothyroidism, hypoxia, and prematurity). Reference Henderson-Smart, Butcher-Puech and Edwards4,Reference Simonsen, Anderson-Berry, Delair and Davies5 Nevertheless, congenital heart disease (CHD) such as congenital heart block, long QT syndrome, sinus node dysfunction, and structural heart disease (L-Transposition of the Great Arteries and Heterotaxy syndrome) should all be explored.
Case
We present the case of a 37-week gestation newborn male born to a mother with well-controlled gestational diabetes. Maternal family history was positive for multiple family members with systemic lupus erythematosus. Prior to delivery, the baby was noted to have an irregular heart rhythm with a normal rate of 120 beats per minute on antenatal ultrasound. After birth, the baby was transferred to the well-baby nursery where an electrocardiogram was done and read as normal.
On day of life 1, the baby began having episodes of sustained bradycardia with rates of 60 beats per minute with poor distal perfusion. He was transferred to the NICU for continuous monitoring, sepsis evaluation, and cardiology consultation. Electrocardiogram appeared to demonstrate complete AV block with atrial rate of 120 beats per minute and a ventricular rate of 72 beats per minute with fixed QRS cycle length (Fig 1). An echocardiogram was performed that revealed no structural abnormalities and normal cardiac function. Systemic perfusion was decreased with decreased urine output and rising lactic acidosis as high as 4 mmol/L. Decision was made to initiate an Isoproterenol drip at 0.05 mcg/kg/minute and titrated to effect to increase ventricular rate and thereby improve systemic perfusion. After titration to a dose of 0.3 mcg/kg/minute, normal sinus conduction was observed.
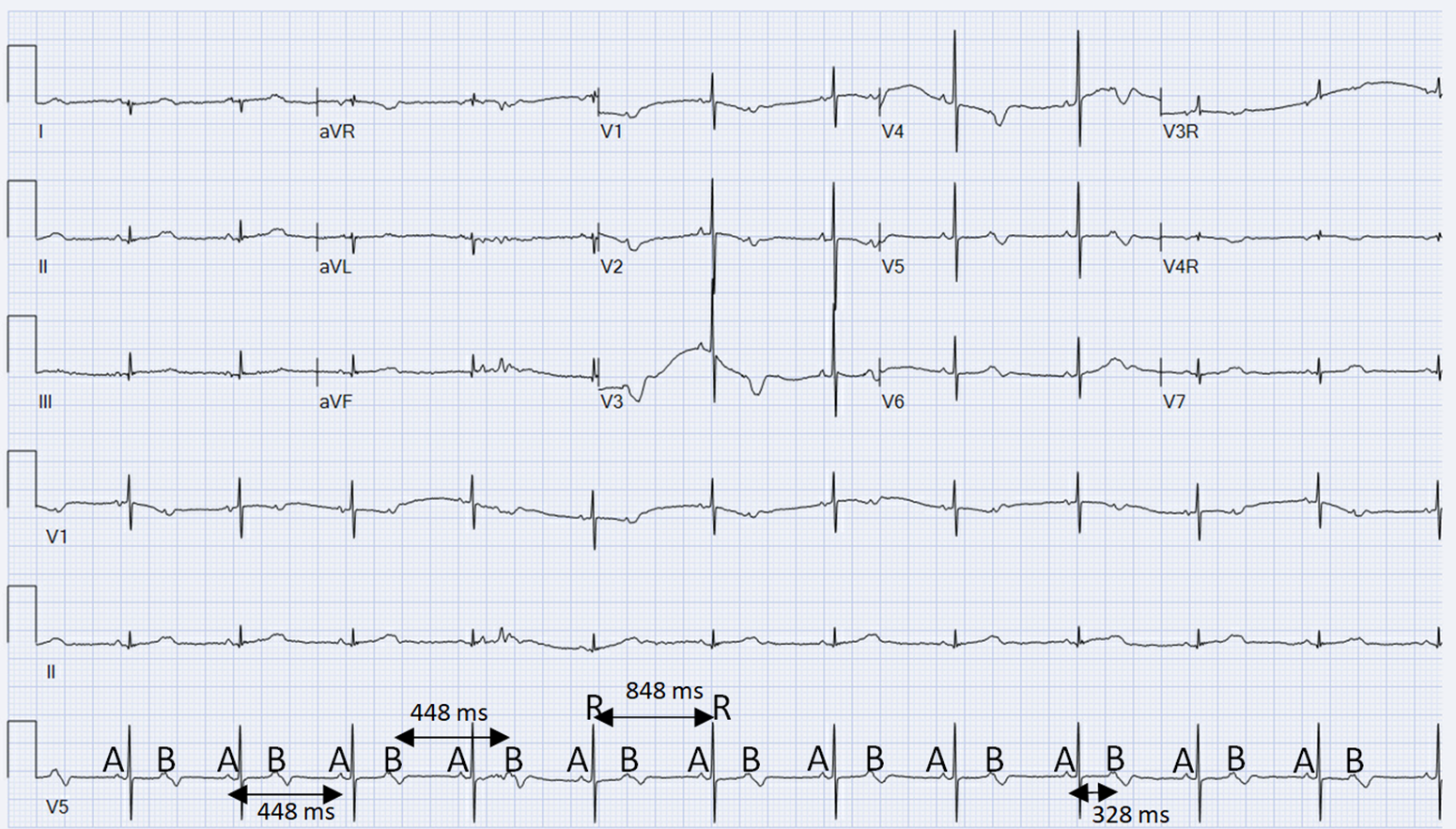
Figure 1. ECG is concerning for complete AV block vs high grade 2:1 AV block. The QRS cycle length (R−R interval) remained stable and unchanged at 848 ms. Sinus P waves (A−A) show a consistent P−P interval of 448 ms, and premature atrial contractions (B−B) show a consistent interval of 448 ms throughout. The Sinus and premature atrial contraction P waves (A−B) show a consistent interval of 328 msc. The QTc is 446 msc throughout.
The patient remained on the Isoproterenol drip to maintain the ventricular heart rate at 150 beats per minute, in normal sinus rhythm, with improved distal perfusion after which lactic acidosis returned to normal and hemodynamic stabilisation was achieved. Mother underwent rheumatologic work-up which was negative for systemic lupus erythematosus and Sjogrens Syndrome-related antibodies. While on Isoproterenol drip, the patient’s heart rate increased to 160 beats per minute, with inability to wean due to resultant bradycardia with poor distal perfusion and the patient was scheduled for pacemaker implantation.
Serial electrocardiograms showed P waves noted to have different morphologies with variable cycle length of P−P intervals, which raised the concern for blocked premature atrial contractions in a pattern of bigeminy. This was confirmed after a complete review of the 24 h Holter monitor (Fig 2), which showed frequent premature atrial contractions; some of which were blocked and others conducted aberrantly confirming the diagnosis of blocked atrial bigeminy as the cause of the symptomatic bradycardia. Flecainide was initiated at 105 mg/m2/day, which resulted in decreased premature atrial contraction’s, consistent atrioventricular conduction, and normalised ventricular rates. After five doses of Flecainide, the Isoproterenol drip was weaned off patient remained in normal sinus rhythm throughout. The pacemaker implantation was cancelled. He was discharged home on hospital day 10 with close follow-up after which the outpatient Holter showed normal sinus rhythm throughout.
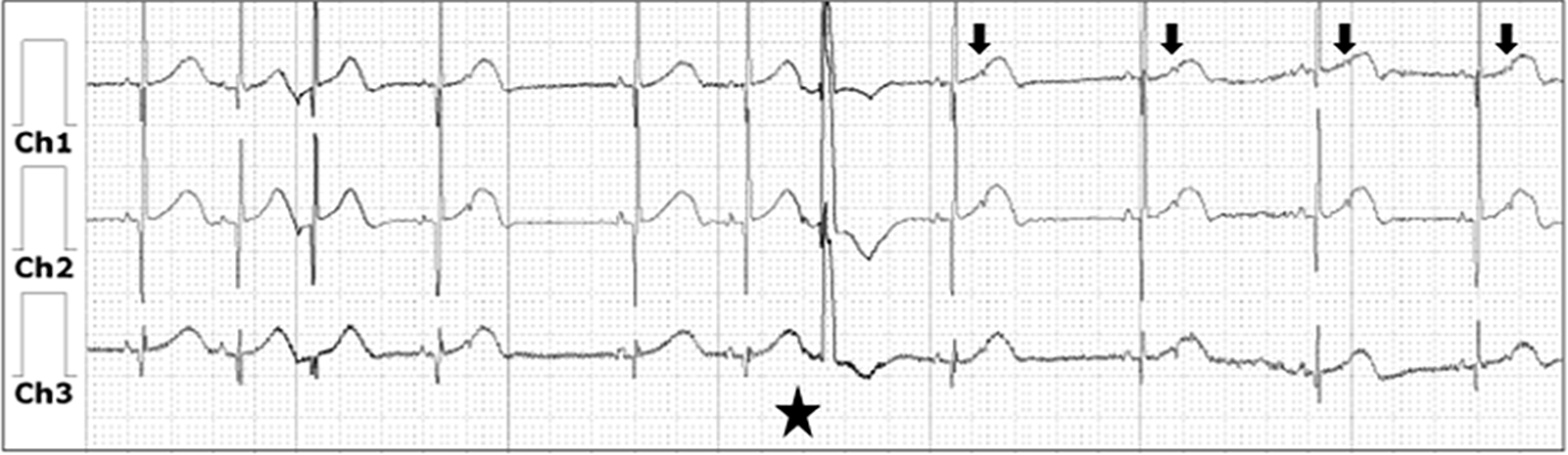
Figure 2. Holter monitor during Isoproterenol infusion with increased conduction from atrium to ventricle as noted by normal sinus rhythm, with persistent premature atrial contractions that conduct aberrantly noted by the widened QRS (star) and blocked premature atrial contractions (arrow).
Discussion
Congenital AV block occurs in approximately 1 out of every ∼20,000–30,000 live births.6 A leading cause of congenital AV block is associated with maternal connective tissue disorders, such as systemic lupus erythematosus and Sjogrens syndrome. Other causes include L-transposition of the great arteries or left atrial isomerism, which is the main role of echocardiogram as part of the diagnostic process. Reference Friedman, Duncanson, Glickstein and Buyon6-Reference Jayaprasad, Johnson and Venugopal8 When complete AV block is diagnosed postnatally, one-third of cases will have associated CHD. Reference Kertesz, Fenrich and Friedman7 Infants born with complete congenital AV block are at increased risk of morbidity and mortality including sudden cardiac death, though mortality is higher in cases with associated CHD with rates ranging from 25 to 50%. Reference Kertesz, Fenrich and Friedman7
Other causes of profound bradycardia in the newborn may masquerade as complete heart block. High-grade AV block and long QT syndrome are two of the more common etiologies. In high-grade AV block, there is variable synchronous conduction from the atria to the ventricles. This is a separate entity from complete AV block where there is total atrio-ventricular dis-synchrony. Reference Kertesz, Fenrich and Friedman7 In some studies, high-grade AV block has been associated with significant mortality as high as 20% despite use of corticosteroids early in the neonatal period. Reference Friedman, Duncanson, Glickstein and Buyon6
Premature atrial contraction’s with blocked conduction is another common and generally benign entity, which may be misdiagnosed as complete AV block. In this setting, there are variable P−P intervals with varying P wave morphologies indicating distinct areas of conduction from the atria. Although PACs are very common, it is extremely unusual that they would result in clinically significant bradycardia. In this case, watchful waiting may be suitable if the patient is asymptomatic, and these findings tend to self-resolve spontaneously soon after birth. However, in the setting of symptomatic atrial bigeminy with blocked conduction, antiarrhythmic therapy used in atrial tachycardias, such as Flecainide, provide a suitable and effective treatment option as noted in our case. Though some literature described atrial bigeminy causing symptomatic bradycardia in adults, to our knowledge, this is the first case in the literature reporting symptomatic blocked atrial bigeminy resulting in hemodynamically significant bradycardia or mimicking as complete AV block in a newborn. Reference Alper, Gungor, Turkkan and Tekkesin9,Reference Gaudio, Di Michele, Ferri, Mirabelli, Franchitto and Alessandri10 The ability to accurately define and diagnose each entity in neonatal bradycardia is essential to the patients’ management, especially when there is concern for complete AV block. Misdiagnosis and failure to recognise medically manageable conditions may lead to unnecessary pacemaker implantation and increased stress and burden on families of these newborn infants.
Conclusion
This case report highlights that blocked atrial bigeminy should be considered in the differential diagnoses of symptomatic bradycardia in neonates, and that this can be managed medically, preventing unnecessary interventions such as premature atrial pacemaker implantation. Our case demonstrates the successful use of Flecainide in such a case resulting in the suppression of atrial ectopy with avoidance of premature atrial pacemaker implantation.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.





