Industrial processing of textiles generates a number of waste streams, including wastewater, air emissions and solid waste. The nature of waste streams depends on installations, processes and technologies, and the types of fibres and chemicals used. Most chemicals are discharged through wastewater into the environment. Effluents from the textile-colouring process cause environmental problems, especially those containing coloured, water-soluble, reactive and acid dyes, including those which tend to pass through conventional wastewater treatment plants (Forss & Welander, Reference Forss and Welander2011). Several techniques have been developed recently for effluent treatments which are both economic and efficient (Khatri et al., Reference Khatri, Peerzada, Mohsin and White2015). One of the processes that may satisfy these criteria is the optimized heterogeneous Fenton process. Modified clays are often used as catalysts in the application of heterogeneous Fenton process for removing dyes from coloured effluents as well as for other various organic transformations (Ramirez et al., Reference Ramirez, Costa, Madeira, Mata, Vicente, Rojas-Cervantes, López-Peinado and Martín-Aranda2007; Dandia et al., Reference Dandia, Bhati, Jain and Sharma2011; Pereira, Reference Pereira, Oliveira and Murad2012). The use of clays as inorganic solid catalysts is considered to be a suitable, practical option because of their abundance, low cost and possible reusability (Hajjaji et al., Reference Hajjaji, Ganiyu, Tobaldi, Andrejkovičová, Pullar, Rocha and Labrincha2013, Reference Hajjaji, Andrejkovičová, Pullar, Tobaldi, Lopez-Galindo, Jammousi, Rocha and Labrincha2016; Xue et al., Reference Xue, Guo, Liu and Chen2015). Impregnation with iron salts is the simplest technique for preparation of clays for this application. This method, like many others used in the preparation of such catalysts, requires a certain period of catalyst aging, which is a drawback for application at an industrial scale (Olaya et al., Reference Olaya, Moreno and Molina2009). An improved method of catalyst preparation, which has a positive effect on the properties of solid catalysts, involves ultrasound waves (Pérez et al., Reference Pérez, Centeno, Odriozola, Molina and Moreno2008; Sanabria et al., Reference Sanabria, Molina and Moreno2012; Dhahri et al., Reference Dhahri, Muñoz, Yeste, Cauqui and Frini-Srasra2016). Ultrasonic techniques are time efficient and use small amounts of water during synthesis of solid catalysts while preserving the physical and chemical properties of materials and also improve catalytic activity.
To the authors’ knowledge, although the synergistic effect of ultrasound and Fenton reaction in dye removal has been investigated (Zhang et al., Reference Zhang, Fu and Zhang2009; Weng et al., Reference Weng, Lin and Yuan2013; Basturk & Karatas, Reference Basturk and Karatas2014; Siddique et al., Reference Siddique, Farooq and Price2014; Acisli et al., Reference Acisli, Khataee, Darvishi, Soltani and Karaca2017), there is insufficient information about the use of ultrasound in the preparation of the catalysts used in the Fenton decolourization process. In the present study, a comparison of the structural characteristics of Fe-loaded bentonite prepared using a conventional method of impregnation and impregnation improved by ultrasound was conducted. Furthermore, investigation of the effect of preparation methods of catalysts on their stability and efficiency in a heterogeneous Fenton process was also carried out. The model pollutant used was Reactive Blue 4 (RB4) dye, an extensively used reactive dye with anthraquinone chromophore and dichlorotriazil group. The disadvantages of its use have been explained and presented in previous studies (Monteagudo et al., Reference Monteagudo, Durán, Aguirre and San Martín2010; Verma et al., Reference Verma, Raghukumar, Parvatkar and Naik2012; Silva & Andrade, Reference Silva and Andrade2016).
MATERIALS AND METHODS
Materials
All the chemicals used, namely H2O2 (30%, w/w), 95–97% H2SO4, Na2CO3, Fe(NO3)3×9H2O and Reactive Blue 4 dye (RB4) were supplied by Sigma-Aldrich Company (Germany). The structure and main chemical properties of RB4 dye are given in Fig. 1 and Table 1, respectively. A Na-bentonite (BNa) was used, which was supplied by the ‘Bentoproduct’ company (Šipovo, Bosnia and Herzegovina), containing 88–92%, montmorillonite with a cation exchange capacity (CEC) of 90–120 meq/100 g, according to the product specification file.
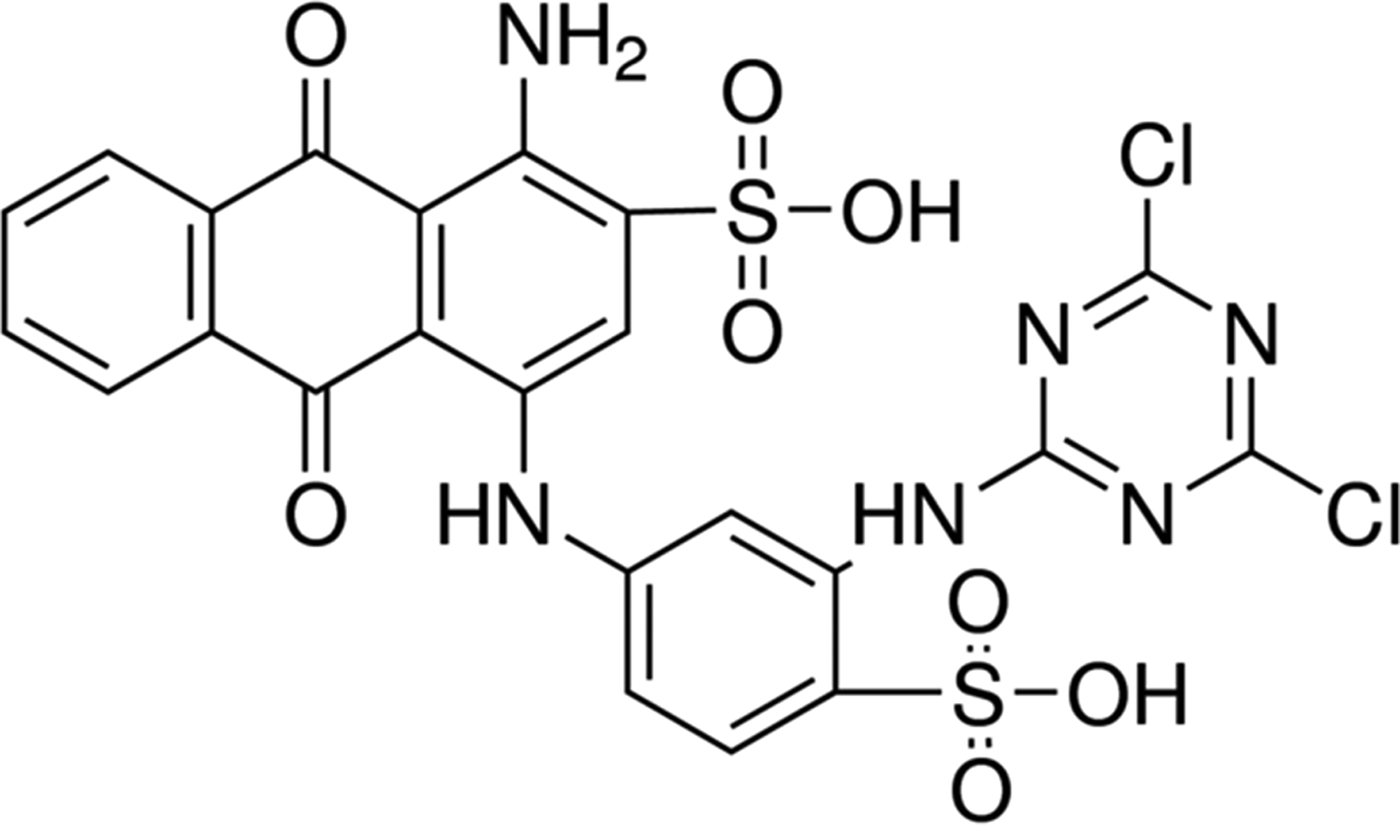
Fig. 1. RB4 dye structure.
Table 1. Main chemical properties of RB4 dye.

Catalyst-preparation methods
The influence of the catalyst-preparation conditions on its stability and efficiency in the heterogeneous Fenton process of decolourization has been studied by means of the influence of calcination temperatures of the impregnated catalysts and the effect of the application of ultrasound on the impregnation process. In all experiments, the preparation procedure for the clay suspension and the solution for impregnation was the same. The suspension of BNa was prepared, under strong stirring of 1 g of clay in 50 mL of distilled water for 30 min. For the preparation of the impregnation solution, sodium carbonate, used in powder form, was added to a 0.2 M solution of Fe(NO3)3×9H2O to obtain a molar ratio of [Na+]/[Fe3+] = 1. Subsequently, seven samples were prepared with different Fe3+/clay ratios (0.6, 1.25, 3, 5, 7, 9 and 11 mmol Fe3+/g) which were subjected to three series of impregnation.
The first batch of samples (marked ‘BFe CMI’) was subjected to the conventional method of impregnation and calcination at two different temperatures. The total preparation time of Fe-polycation and its mixing with the suspension of swollen clay was 4 h, followed by calcination at 350 and 500°C for 2 h. The results of the effect of temperature on the stability of the catalysts were used to prepare the remaining samples.
The other two series of catalysts were prepared by impregnation improved by ultrasound waves (Ultrasonic Homogenizer Sonopuls HD 2200, Bandelin) as follows: the Fe-polycation prepared (5 min) was added to a clay suspension for (1) 5 min (sample BFe UMI5) and (2) 10 min (sample BFe UMI10).
The samples prepared by the impregnation methods were dried at 100°C for 24 h, washed with distilled water several times and centrifuged; subsequently they were dried again for 24 h at 100°C, and were calcined at the specific temperature before being used as catalysts in the Fenton process. The effectiveness of decolourization of synthetic RB4 dye solution was monitored by measuring the absorbance (A) at a wavelength of 594.78 nm with a UV-Vis spectrophotometer (PG Instruments Ltd T80+ UV/VIS, model: Shimadzu UV-1800, Japan). The concentration of leached iron was examined using an atomic absorption spectrophotometer (Perkin Elmer Analyst 700). Experimental conditions of the Fenton process were selected from a previous work (Kulić et al., Reference Kulić, Kerkez, Bečelić-Tomin, Dalmacija and Pucar2016), in which Fenton-process optimization and adsorption were studied using the 3 mmol of Fe3+/g ratio only. The levels of adsorption of RB4 dye were 7.4% and 23.6% for BNa and BFe CMI, respectively. The reaction time was 3 h, [RB4] = 50 mg/L, [H2O2] = 20 mM, [catalyst] = 1 g/L and the pH was adjusted to 3. The efficacy of reaction was calculated by the following formula:
where A is the absorbance after Fenton process and A 0 is the initial absorbance.
Catalyst characterization
The specific surface area, pore size, pore volume and pore-size distribution of the catalysts were measured by the multi-point BET (Brunauer–Emmett–Teller) method with an Autosorb iQ Surface Area Analyzer (Quantachrome Instruments, USA), using the ASiQwin software. The morphology of the catalysts was examined by scanning electron microscopy (SEM) (TM3030, Hitachi High-Technologies, Japan) coupled with energy dispersive spectrometry (EDS) (Bruker Quantax 70 X-ray detector system, Bruker Nano, GmbH Germany). Fourier Transform infrared (FTIR) spectra were collected using a Thermo-Nicolet Nexus 670 (USA) FTIR spectrometer, in the 4000–400 cm–1 range and in a diffuse reflection mode at a resolution of 4 cm–1.
RESULTS AND DISCUSSION
Effect of impregnation methods on the catalyst characteristics in the Fenton process
The efficacy of RB4 removal and the stability of the catalysts were considered during decolourization with the Fenton process. As the production of hydroxyl radicals is affected by Fe concentration, samples with all-Fe3+/clay ratios were subjected to a specific test.
Calcination is an important thermal treatment used to obtain suitable catalysts because it facilitates dispersion of the active phase, an important feature in the implementation of the Fenton process, and prevents development of undesirable properties in the clay minerals (Vimonses et al., Reference Vimonses, Jin, Chow and Saint2009). Calcination of the impregnated materials using the conventional method was carried out at 350 and 500°C. The effects of heat treatment on the reactivity and stability of BFe CMI are illustrated in Fig. 2. The decolourization efficiency of the synthetic dye solution tends to increase when using catalysts prepared at lower temperatures; even at lower molar ratios (0.6 and 1.25 mmol of Fe3+/g) the efficiency was ~90%.
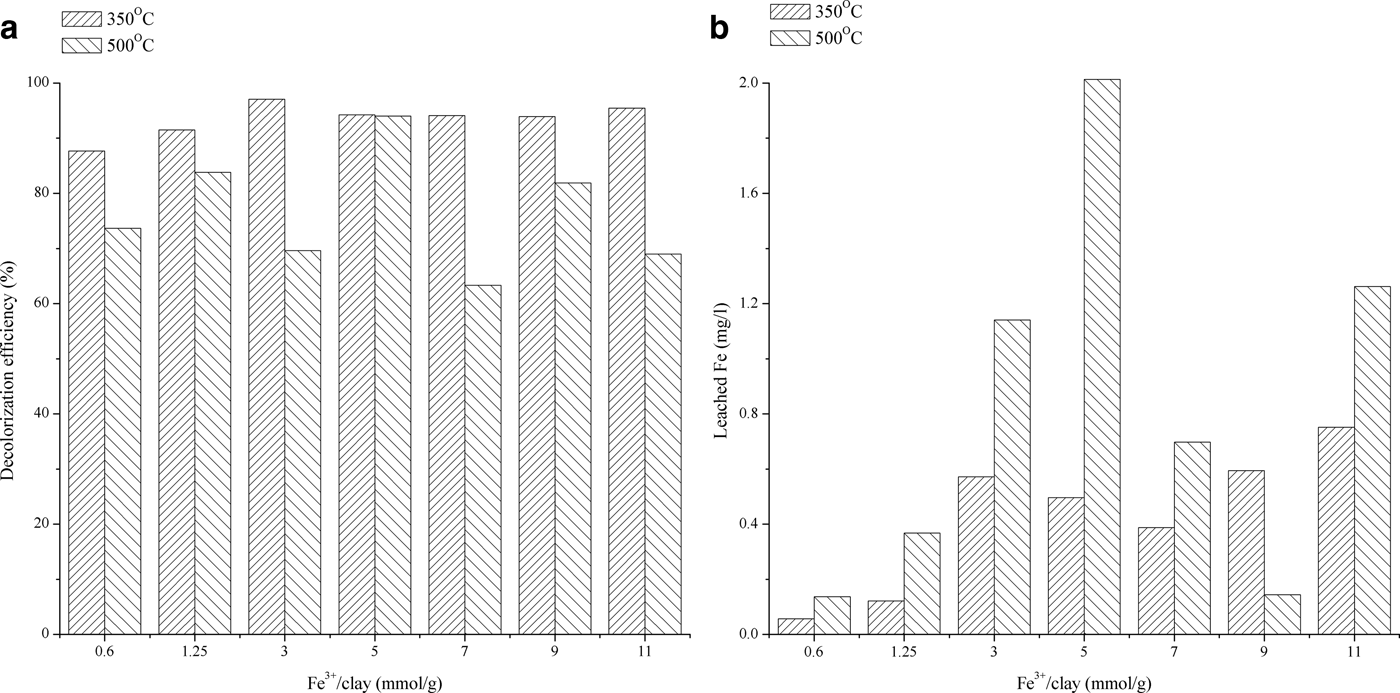
Fig. 2. Catalytic activity and stability of the catalyst (conventional impregnation): (a) Fenton process efficiency; and (b) leaching of Fe.
The amount of Fe leached varied significantly following calcination at various temperatures; less leaching occurred for BFe CMI calcined at 350°C. Fixation of Fe ions might be achieved by thermal treatment whereby the large Fe-polycations are transformed into the Fe oxides, with a considerably smaller diameter (Iurascu et al., Reference Iurascu, Siminiceanu, Vione, Vicente and Gil2009), which allows them to intercalate easily into the clay interlayer space and to affect the final porosity of the modified bentonite. At higher calcination temperatures, however, the clay interlayer may collapse and degrade due to the loss of the adsorbed water (Iurascu et al., Reference Iurascu, Siminiceanu, Vione, Vicente and Gil2009; Yilmaz, Reference Yilmaz2011). Thus, the leaching of active ions may be significant and the effectiveness of these catalysts varies after oxidation. The highest achieved decolourization efficiency was 97.1% at 3 mmol Fe3+/g of bentonite, after heating at 350°C. The other series of catalysts were calcined at 350°C in the final step of the clay-impregnation process.
The removal of RB4 and iron leaching during the Fenton process using catalysts synthesized by ultrasound are shown in Fig. 3. BFe UMI5 samples showed high activity for all molar Fe3+/clay ratios. The decolourization efficiency ranged from 89.5 to 97.1%, with the highest RB4 removal achieved at 9 mmol Fe3+/g. There was a loss of active species into the solution as a result of an increase in the molar ratio Fe3+/clay, however (Fig. 3b). Likewise, the homogeneous Fenton process has a significant impact on the efficacy attained. A possible cause of weak stability might be insufficient preparation time.

Fig. 3. Catalytic activity and stability of the catalysts (impregnation with ultrasound): (a) Fenton process efficiency; and (b) leaching of Fe.
The catalysts prepared with prolonged applied ultrasound (BFe UMI10) showed lower decolourization efficacy (~72%) at 0.6 and 1.25 mmol Fe3+/clay. By increasing the Fe content in the materials, the efficiency of the Fenton process was increased by almost 20% and did not change with further increase of the Fe3+/clay ratio, while catalyst stability did not change.
This behaviour might be explained by better incorporation of Fe ions into the interlayers of BNa by using ultrasound in the synthesis of the catalyst (Darvishi & Morsali, Reference Darvishi and Morsali2011), and in the present case, for a longer period of exposure time (10 min). The increasing efficiency of the decolourization process with increasing Fe content in the solution may indicate the simultaneous occurrence of homogeneous and heterogeneous Fenton processes.
It is suggested that the catalysts prepared by both the conventional and the improved ultrasound method had high catalytic efficacy, but their relative stabilities varied in the acidic conditions of the Fenton process.
Structural characteristics of the catalysts
The BFe CMI catalysts calcined at 500°C and BFe UMI5 were not characterized because of their unsatisfactory stability. The molar ratios of the catalysts tested were 0.6, 3 and 11 mmol Fe3+/g. The 3 mmol Fe3+/g was chosen as an optimum molar ratio for both decolourization and stability in terms of the Fenton process, while 0.6 and 11 mmol Fe3+/g ratios were used to identify possible differences in the structure due to different Fe ratios.
BET method
The N2 isotherms (Fig. 4) were used to study the porosity of the three materials (Table 2). All the isotherms display type IV features with H2 hysteresis loops (according to IUPAC classification), indicating mesoporous structure with disordered pore-size distribution (Alothman, Reference Alothman2012; Caglar et al., Reference Caglar, Cubuk, Demir, Coldur, Catir, Topcu and Tabak2015; Bounab et al., Reference Bounab, Draoui, Ahrouch, Hadri, Bouchta and Barhoun2017). The isotherms are quite similar, with the largest adsorbed N2-gas volume observed in materials with 3 mmol Fe3+/g.

Fig. 4. N2 adsorption-desorption isotherms for: (a) BNa, BFe CMI; (b) BFe UMI10.
Table 2. Specific surface area and porosity of the raw and modified bentonite.

ND – not detected
The porosity data for BNa and BFe CMI (Table 2) were taken from Kerkez et al. (Reference Kerkez, Bečelić-Tomin, Dalmacija, Agbaba, Tomašević-Pilipović, Slijepčević and Kulić2015). The impregnation of BNa led to an increase in specific surface area, from 97 to 122 m2/g for BFe CMI and to 197 m2/g for BFe UMI10 for an optimal molar ratio, due to the presence of small Fe-oxide particles after calcination at 350°C. This feature is also reflected in the isotherms (Fig. 4) (Iurascu et al., Reference Iurascu, Siminiceanu, Vione, Vicente and Gil2009; Hou et al., Reference Hou, Ma, Zhang, Tang, Fan and Wan2011; Nogueira et al., Reference Nogueira, Lopes, Silva, Lago, Fabris and Oliveira2011; Ayodele & Togunwa, Reference Ayodele and Togunwa2014; Nidheesh, Reference Nidheesh2015). The modified samples have slightly higher values for total pore and mesopore volume (BJH method), while the average pore diameter decreased. Furthermore, the influence of molar ratios (0.6, 3 and 11 mmol Fe3+/g) on textural properties of BFe UMI10 is reflected in the change of surface area and total pore volume (Table 2), thus confirming the importance of the optimal Fe ratio for the improved impregnation method.
SEM results
The surface morphology of the original and modified clay samples is shown in Fig. 5. The surface of the BNa is different from that of the impregnated samples, and its layered structure has a fluffy appearance. BNa also has macropores (Idrissi et al., Reference Idrissi, Miyah, Benjelloun and Chaouch2016). After impregnation and calcination, the number of pores with smaller diameters increases and hence the pore volume is larger (Table 2). The use of ultrasound in the synthesis of the impregnated clay yielded more uniform particle-size distribution in comparison to BNa (Nogueira et al., Reference Nogueira, Lopes, Silva, Lago, Fabris and Oliveira2011). Thus, although the influence of molar ratios characterized is similar to the N2 adsorption-desorption isotherms (Fig. 4), sample BFe UMI10, with 3 mmol Fe3+/g, has the greatest surface area with small pores, in accordance with the results obtained from BET analysis.

Fig. 5. SEM images of: (a) BNa, (b) BFe CMI 0.6, (c) BFe CMI 3, (d) BFe CMI 0.6, (e) BFe UMI10, (f) BFe UMI10 3 and (g) BFe UMI10 11.
EDS results
The results obtained from EDS analysis are presented in Table 3. Impregnation of BNa with Fe ions resulted in replacement of the Na, Ca and Mg interlayer cations. The difference between BFe CMI and BFe UMI samples is reflected in the increase of Fe content after conventional and ultrasound modification, followed by a decrease in the amount of exchangeable Ca (Iurascu et al., Reference Iurascu, Siminiceanu, Vione, Vicente and Gil2009; Nogueira et al., Reference Nogueira, Lopes, Silva, Lago, Fabris and Oliveira2011). The amounts of Si and Al in all samples decrease slightly with different synthesis procedures at higher molar ratios, suggesting that the clay layers are mostly preserved during impregnation and calcination with lower ratios. In addition, higher Fe3+/clay molar ratios tested during both impregnation methods increase the Fe content detected, indicating its incorporation onto the surface and into the interlayer space of BNa. With increasing amount of Fe after impregnation, the relative amounts of remaining elements were reduced.
Table 3. Elemental composition of the raw and modified bentonite.

ND – not detected
FTIR results
The FTIR spectra for BNa and the impregnated samples (BFe CMI and BFe UMI10) are shown in Fig. 6. The BNa spectrum has characteristic bands at 3626 cm–1 and 917 cm–1, attributed to Al-OH-Al stretching and bending vibrations, respectively (Hou et al., Reference Hou, Ma, Zhang, Tang, Fan and Wan2011). The band at 3444 cm–1 in BNa that shifted slightly to 3426 cm–1 after impregnation assigned to the H-O-H stretching, and the H-O-H bending band at 1635 cm–1, are attributed to the interlayer water (Hou et al., Reference Hou, Ma, Zhang, Tang, Fan and Wan2011; Idrissi et al., Reference Idrissi, Miyah, Benjelloun and Chaouch2016). The intense band of the in-plane Si–O stretching at 1038 cm–1, shifted to ~1041 cm–1 after modification in all samples. Additionally, the band at 1431 cm–1 which is attributed to calcite in the BNa sample is absent from the impregnated samples. The bands at 530 and 470 cm–1 are associated with Al–O stretching and Si-O bending vibrations (Yuan et al., Reference Yuan, Annabi-Bergaya, Tao, Fan, Liu, Zhu, He and Chen2008). Nevertheless, in both the BFe CMI and BFe UMI10 (for all presented molar ratios), bands assigned to Fe oxides were not detected.
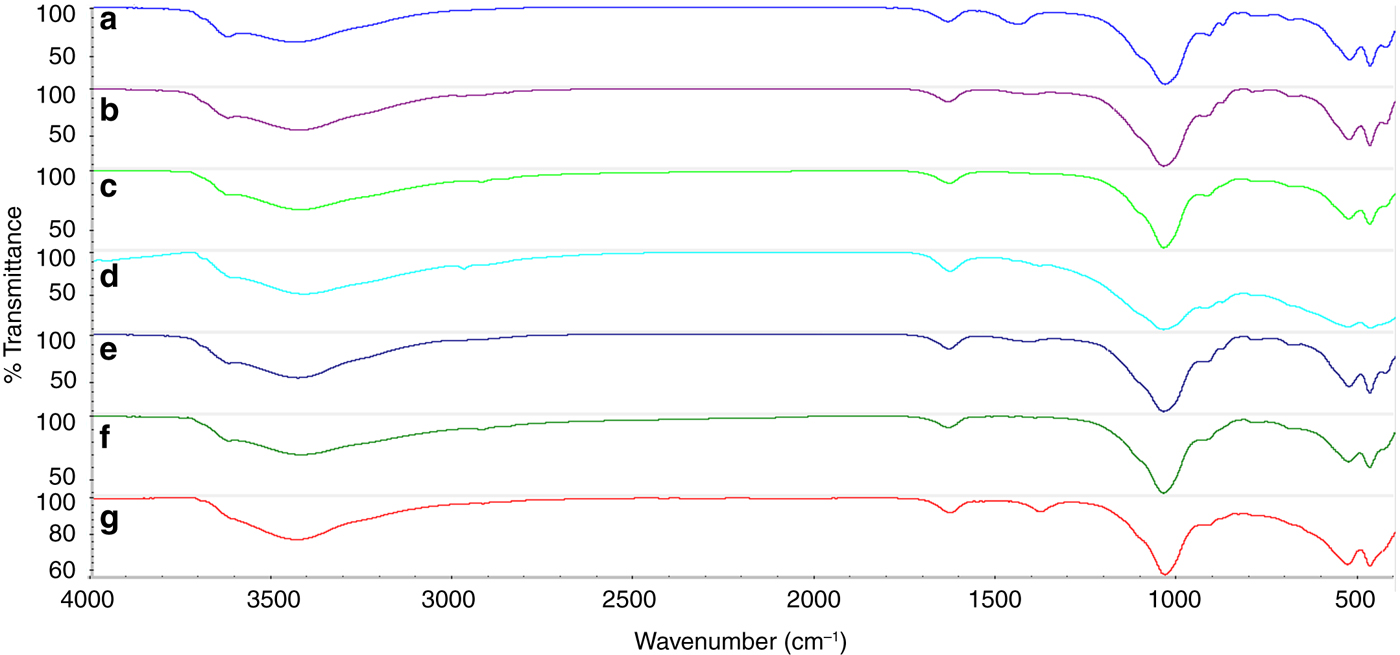
Fig. 6. FTIR spectra of: (a) BNa, (b) BFe CMI 0.6, (c) BFe CMI 3, (d) BFe CMI 11, (e) BFe UMI .6, (f) BFe UMI10 3 and (g) BFe UMI10 11.
SUMMARY AND CONCLUSION
The impregnation method of BNa was improved by ultrasound allowing application in the Fenton process of decolourization of the synthetic dye RB4. This improvement was compared with the conventional method which was tested at two calcination temperatures (350 and 500°C). Better catalyst properties were obtained at lower temperature during the thermal treatment. Examination of the impact of Fe loading on decolourization efficiency indicated that acceptable performance was achieved with 3 mmol Fe3+/g. The stability of this sample in terms of Fe leaching, depends on the exposure time to ultrasound waves at the time of its preparation.
The specific surface area and mesoporosity increased with application of ultrasound for 10 min. Elemental analysis confirmed the increase of Fe content after conventional and ultrasound modification, and also with various molar ratios. On the other hand, the corresponding decrease in Ca content after impregnation, and the slight change in the Si and Al contents indicate the stability of the clay layers during the modification processes.
This work confirmed previous research that it might be possible to reduce the time required for aging of materials in the traditional catalyst-synthesis method and therefore the entire procedure of preparation, while retaining the catalytic activity required.
ACKNOWLEDGMENTS
The research was funded by the Ministry of Education, Science and Technological Development, Serbia (Project III43005 and TR37004). The authors are grateful to the ‘Bentoproduct’ company for provision of the raw bentonite sample. The authors also acknowledge Goran Kitić and Jovana Stanojev of BioSense Institute (Novi Sad, Serbia), for the SEM/EDS analysis.











