INTRODUCTION
The protozoan parasite Trypanosoma cruzi, the causative agent of Chagas' disease, infects nearly 18 million people in Latin America. Haem-compounds are necessary as a growth factor for the parasite. This nutritional requirement would be due to the inability of T. cruzi to synthesize haeme (Lombardo et al. Reference Lombardo, Araujo and Batlle2003). Because high levels of haemin and related porphyrins have an important cytotoxic action through the generation of reactive oxygen species (ROS) such as superoxide anion (O2−), hydrogen peroxide (H2O2) and the highly reactive hydroxyl radical (OH.) (Afonso et al. Reference Afonso, Polo, Enriquez de Salamanca and Batlle1996; Kumar and Bandyopadhyay, Reference Kumar and Bandyopadhyay2005), it was of interest to investigate the effect of haemin on the growth of T. cruzi. The potentially deleterious reactions involved are controlled by an antioxidant defence system that eliminates pro-oxidants and scavenges free radicals. Trypanosomatids do not possess catalase or glutathione peroxidase, the antioxidant defence system of these parasites includes the enzymes superoxide dismutase (SOD), ascorbate peroxidase (APx), tryparedoxin peroxidase (TpxPx) and low molecular weight antioxidants, mainly trypanothione (N1, N8-bisglutathionylespermidina; Try) (Turrens, Reference Turrens2004). In recent years a non-Se-dependent glutathione peroxidase form that reacts with organic peroxides but not with hydrogen peroxide has been detected in both African and American trypanosomes (Wilkinson et al. Reference Wilkinson, Obado, Mauricio and Kelly2002, Reference Wilkinson, Hom, Prathalingam and Kelly2003). Another enzyme involved is trypanothione reductase (TryR), a NADPH-dependent enzyme, which regenerates reduced trypanothione (Tryred). The combined action of APx, TpxPx and TryR would be central to the maintenance of a low steady-state concentration of H2O2.
Independent of its toxic effects, haem plays an essential role in various biological reactions, such as regulation of protein synthesis. Requirement of haem for expression of numerous proteins in mammalian reticulocytes, and also in a number of non-erythroid cells, is well established (Chen and London, Reference Chen and London1995). The effect of haemin on protein synthesis and cell proliferation in Leishmania donovani has also been reported (Pal and Joshi-Purandare, Reference Pal and Joshi-Purandare2001).
Finally, several detoxification-defence mechanisms exist in the cell to counteract the oxidative damage induced by free haem (Kumar and Bandyopadhyay, Reference Kumar and Bandyopadhyay2005). Among them, the most important involves the activity of haeme oxygenase (HaemOx). HaemOx is the rate-limiting enzyme in the degradation of haem. It breaks down the pro-oxidant haem to yield equimolar amounts of carbon monoxide, free iron and the antioxidant biliverdin (Maines, Reference Maines1997; Tomaro and Batlle, Reference Tomaro and Batlle2002). HaemOx is expressed in virtually all forms of life (bacteria, fungi, plants, mammals), to date at least 2 isoforms have been identified: HaemOx-1 and HaemOx-2. It was proposed that the inducible isoform HaemOx-1 could play an effective role to counteract the damage caused by oxidative and nitrosative stress (Maines, Reference Maines1997; Naughton et al. Reference Naughton, Foresti, Bains, Hoque, Green and Motterlini2002). HaemOx-1 is induced by host oxidative stress stimuli (H2O2, haem-compounds, metal ions, GSH depletion and others) and activation of HaemOx-1 gene expression is considered to be an adaptive cellular response to different types of chemicals and mediators that change the redox status of the cell (Motterlini et al. Reference Motterlini, Green and Foresti2002).
Taking into account the different effects of haemin discussed above, our aims have been to investigate the role of haemin on the rate of growth, cellular morphology, total content of proteins, the antioxidant defence system stage and HaemOx activity and expression in T. cruzi. The results obtained could potentially be used for the treatment of Chagas' disease.
MATERIALS AND METHODS
Chemicals
Haemin, NADPH, 5,5′ dithio-bis(2-nitrobenzoic acid) (DTNB), EDTA, NADH, hydrogen peroxide and ascorbic acid were from Sigma Chem (St Louis, MO, USA). Yeast extract, tryptose, powdered beef liver and brain heart infusion were from Difco. Trypanothione was purchased from Bachem Bioscience Inc. (USA). All other chemicals were of the highest purity commercially available. The following primary polyclonal antibodies, rabbit anti-HaemOx-1 (Stresgen Biotech, Victoria, BC Canada) and goat anti-actin (I-10) (Santa Cruz Biotech, Santa Cruz, CA, USA) were used. The secondary antibodies used for HaemOx-1 Western blot analysis were anti-goat IgG-HRP or anti-rabbit IgG-HRP (Santa Cruz Biotech).
Organism and growth conditions
T. cruzi cells, Tulahuén strain, Tul 2 stock, were grown at 28°C with constant shaking in a liquid medium containing 0·3% yeast extract, 0·9% tryptose, 0·4% dextrose, 1% disodium phosphate 2-hydrate, 0·36% sodium chloride, 0·04% potassium chloride, 0·15% powered beef liver, 0·5% brain heart infusion and 0·5 mg/100 ml haemin.
Preparation of extracts from T. cruzi
All manipulation steps were performed at 2–4°C. The cells were harvested by centrifugation at 12 000 g for 10 min and then washed once with either 0·05 m Tris-HCl buffer, pH 7·4, 0·05 m sodium phosphate buffer, pH 7·4, or 0·05 m potassium phosphate buffer, pH 7·2, depending on the measurement under study. The cells from 200 ml of culture were resuspended in 5 ml of (a) 0·05 M Tris-HCl buffer, pH 7·4, for APx activity, (b) 0·05 M sodium phosphate buffer, pH 7·4, for SOD activity and (c) 0·05 M potassium phosphate buffer, pH 7·2, for TryR activity and HaemOx activities and total thiol content. Cells in suspension were disrupted by sonication in a Soniprep 150, MSE Ultrasonic Power for 45 sec. The resulting homogenate was centrifuged at 5000 g for 15 min, the precipitate was discarded and the supernatant was employed as source of enzyme.
SOD activity
Superoxide dismutase activity has been assayed by a spectrophotometric method based on the inhibition of a superoxide-driven NADH oxidation (Paoletti, Reference Paoletti, Aldinucci, Mocali and Caparrini1986). One enzyme unit (EU) is defined as the amount of protein required to inhibit 50% NADH oxidation.
APx activity
APx activity was measured at 25°C following the change in absorbance at 265 nm due to ascorbate oxidation. The method described by Boveris et al. (Reference Boveris, Sies, Martino, Docampo, Turrens and Stoppani1980) was employed. Usually corrections for oxidation of ascorbate in the absence of hydrogen peroxide were not significant, instead non-enzymatic oxidations of ascorbate by hydrogen peroxide had to be considered. Enzyme activity was calculated by using an ε value of 10·36 mm−1. The EU is defined as the amount of enzyme forming 1 nmol of product per sec under the standard incubation conditions.
TryR activity
TryR activity was determined at 25°C following NADPH oxidation at 340 nm (Ariyanayagan and Fairlamb, Reference Ariyanayagam and Fairlamb2001). The S supernatant dialysed against 5 mm potassium phosphate buffer, pH 7·2 (5 changes of 160 volumes during 45 min) was employed as enzyme preparation. The activity was calculated using an extinction coefficient of 6·22×103 m−1 cm−1. One EU is defined as the amount of enzyme forming 1 nmol of product per min under the standard incubation conditions.
HaemOx activity
HaemOx activity was determined by measuring bilirubin formation, which was calculated as the difference in absorbance measured at 455 and 520 nm. From the supernatant of T. cruzi, both a microsomal HaemOx fraction and a biliverdin reductase preparation were obtained according to the method of Erario et al. (Reference Erario, Gonzales, Noriega and Tomaro2002). The standard assay mixture and incubation conditions were those reported by Erario et al. (Reference Erario, Gonzales, Noriega and Tomaro2002). Enzyme activity was determined using an ε value of 50 mm−1 cm−1. The EU is defined as the amount of enzyme forming 1 nmol of product per hour under the standard incubation conditions.
Western-blot analysis for HaemOx-1 expression
T. cruzi supernatants prepared in 0·05 m sodium phosphate buffer, pH 7·4, were subjected to 12% SDS-PAGE, transferred to nitrocellulose membranes, and then incubated with specific antibodies, according to the protocol of Foresti et al. (Reference Foresti, Clark, Green and Motterlini1997). Rainbow-coloured protein molecular mass standards obtained from Amersham were used for the estimation of molecular size. For quantification of immunoblots, relative intensities of bands were quantified by densitometry using IMAGE MASTER image analysis software (Amersham Pharmacia Biotech). Control for loading and transfer was obtained by probing with anti-β-actin.
Assay of total thiol groups
The content of thiol groups was determined by measuring the change in light absorbance (410 nm) occurring when SH-groups reduce DTNB. Quantification was carried out in both supernatant and pellet after precipitation of S supernatant with TCA to a final concentration of 5% (Rossi et al. Reference Rossi, Cardaioli, Scaloni, Amiconi and Di Simplicio1995).
Protein determination
Protein concentration was determined by a modification of Lowry's method for insoluble proteins. Cells from 10 ml of culture were harvested and washed as indicated above. They were then resuspended in 5 ml of 0·6 m NaOH and disrupted by ultrasonic treatment for 45 sec. The broken cell suspension was employed for protein determination as described by Lowry (Reference Lowry, Roseborough, Farr and Randall1951), except that Na2CO3 was dissolved in H2O instead of 0·1 m NaOH.
Statistical analysis
All data are expressed as means±standard deviations of 3 or 4 separate experiments, running in duplicate. The significance of differences was evaluated using Student' t-test, taking P<0·05 as significant.
RESULTS
Growth under different haemin concentrations
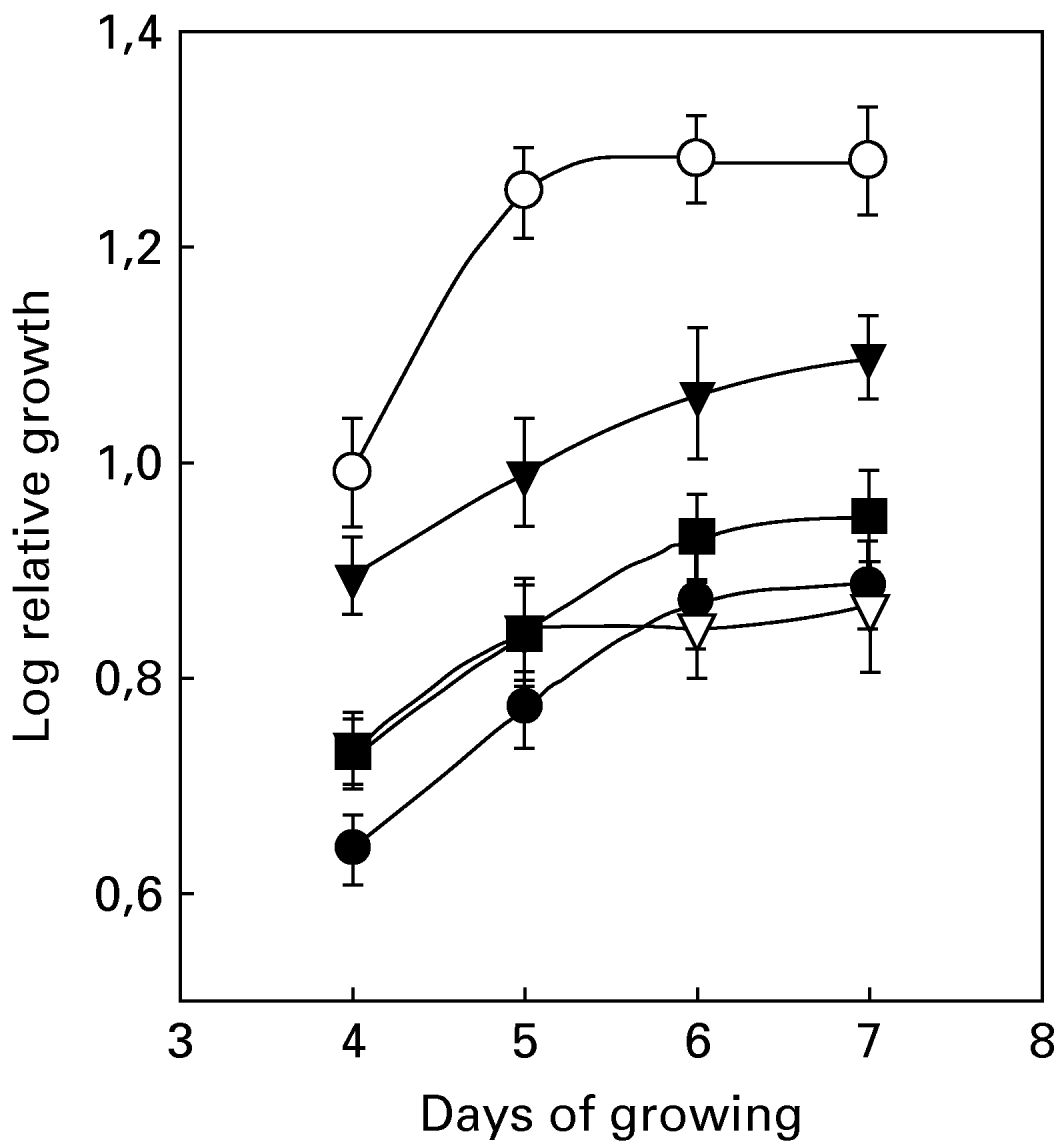
Epimastigotes of T. cruzi were grown in the presence of different haemin concentrations, between 0 and 30 mg/l, at 28°C (Fig. 1). The relative growth of the culture was followed as a measure of parasite proliferation. Cells were counted using a Neubauer chamber at the beginning of the experiment and every 24 h during 4–7 days. The indicated haemin concentration corresponds to the concentration of exogenously added haemin. Measurements before 4 days and after 7 days were not recorded because the cellular mass at intervals shorter than 4 days was insufficient for carrying out determinations of enzymes and metabolites and, at longer times, problems inherent to depletion of nutrients or formation of undesired metabolites could arise.

Fig. 1. Relative growth of Trypanosoma cruzi under different haemin concentrations. (●) Without added haemin, (○) 5 mg haemin per l of culture, (▾) 15 mg haemin per l of culture, (▿, ■) 30 mg haemin per l of culture. The two lines for 30 mg/l haemin correspond to considerer (■) all living cells independent of the morphology or (▿) only living cells showing an extracellular flagellum. Relative growth is defined as the number of cells per ml of culture at the end of the growth period with respect to the number of cells per ml of culture at the beginning of growth. All other experimental conditions were as indicated in the Materials and Methods section.
Curves for haemin concentrations of 10 mg/l and 15 mg/l were practically superimposed (data not shown). After 5 days of growing, 2 different curves were observed for 30 mg/l haemin. The upper curve corresponds to all living cells (independent of the morphology). The lower curve only takes into account the living cells showing an extracellular flagellum. Haemin concentrations higher than 15 mg/l clearly decreased growth rate and induced morphological changes. We have therefore found that 6 days of growth and 5 mg/l haemin were the conditions yielding optimum growth and proliferation.
Effect of haemin on behaviour and morphology of the cells
Optical microscopy observations are presented in Fig. 2. When T. cruzi cells were grown in a medium containing a haemin concentration of 30 mg/l, they appeared less elongated (stumpy), with short flagella and therefore with diminished mobility (Fig. 2B). These morphological changes could correspond to the transformation from epimastigote to amastigote. Such differentiation was corroborated employing the methodology of serial cultivation described by Zaidenberg et al. (Reference Zaidenberg, Tournier, Schinella and Buschiazzo2000). Three consecutive cultures of 5 days each at the 30 mg/l haemin concentration employing transformed cells (like-amastigote) were carried out. Maintenance of the cellular morphology evaluated by optical microscopy would indicate that transformation from epimastigote to amastigote was induced by high haemin concentrations.

Fig. 2. Morphology of Trypanosoma cruzi epimastigotes grown in the absence (A) and presence of haemin (B). Cells were grown without haemin (A) and in the presence of 30 mg/l haemin (B) during 6 days. Then they were examined under light microscopy (ZEISS West Germany AXIOSKOP). Original magnification, ×400.
For haemin concentrations between 0 and 10 mg/l in the culture medium, the proportion of trypomastigotes increased with time, reaching 25–30% after 7 days.
Effect of haemin on total soluble protein content
We have analysed, the effect of haemin (0–30 mg/l) on total protein content dissolved in 0·6 m NaOH (see Materials and Methods section) in cultures growing between 3 and 7 days (Fig. 3). Considering the results shown in Fig. 3A and B, Fig. 3C can be drawn, which shows total protein content per cell with time. In the absence of haemin, the protein concentration progressively increased from 6·41±0·18 to 8·73±0·25 μg/106cells. In the presence of haemin 10 mg/l values were 0–15% higher, showing a maximum level after 5 days of growth, without further changes. For a haemin concentration of 30 mg/l a different response was obtained, protein concentration increased within 3–5 days (from 9·26±0·10 to 10·12±0·08 μg/106cells) and then rapidly decreased to 7·24±0·18 μg/106cells.

Fig. 3. Effect of different haemin concentrations on cellular density (A) and protein content expressed per ml of culture (B) as well as per cell number (C). (●) Without added haemin, (○) 10 mg haemin per l of culture, (▾) 30 mg haemin per l of culture.
We have seen that between 3 and 6 days of growth, significant differences (P<0·05) among the 3 different haemin concentrations were found.
Effect of haemin on content of thiol groups
Levels of reduced low molecular mass thiols are indicative of the redox state of the cell. Therefore we could expect that if haemin induces the formation of oxidant species it will affect thiol group levels.
In Fig. 4 we can see that SH-content remains constant for haemin concentrations between 0 and 5 mg/l, diminishing between 22% and 37% for haemin 15 and 30 mg/l respectively.

Fig. 4. Effect of haemin on thiol group content. The cells were grown under different haemin concentrations during 6 days. Thiol groups were measured as described in the Materials and Methods section.
Effect of haemin on antioxidant enzymes
As seen in Table 1, the activities of antioxidant enzymes SOD, TryR and APx showed a maximum value at 5 mg/l of haemin. For higher concentrations of haemin (15 and 30 mg/l) all activities diminished, attaining values very similar to the controls for TRyR and APx or slightly lower for SOD.
Table 1. Effect of haemin on antioxidant enzyme activities
(T. cruzi cells were grown for 6 days in the presence of different haemin concentrations. Enzymatic activities were assayed and expressed as described in the Materials and Methods section. Activity corresponding to haemin 0 mg/l was considered as the control value.)

a No significant differences (P>0·05) when compared to the control (0 mg/l haemin) as assessed by Student's t-test.
Diminished activities of these enzymes cannot be attributed to an inhibitory effect of the haemin present in S supernatant (which varies between 0·23±0·08 and 42·46±7·84 μg/ml for haemin concentrations in the culture medium of 0 to 30 mg/l, respectively) on the enzyme activity evaluated in vitro.
Haem oxygenase activity and expression
T. cruzi cells were grown during 6 days in the absence and in the presence of 5, 15 and 30 mg haemin per 1 of culture. The values of HaemOx activity (data not shown) corresponding to 0 and 5 mg/l haemin did not show significant differences (average value: 1·22±0·15 EU/mg), for 15 mg/l haemin the activity slightly increased (up to 2·01±0·23 EU/mg) whilst for 30 mg/l haemin an activity approximately 3 times higher (3·81±0·12 EU/mg) was observed. Western blot analysis for HaemOx-1 (Fig. 5) showed only a single band with a molecular mass of 32–34 kDa. The maximum expression of the enzyme (9·5 to 10·2-fold) was observed for 30 mg/l haemin, whilst for 5 and 15 mg/haemin the increase was lower, 1·4 to 1·9-fold and 4·5 to 4·9-fold respectively (0 mg/l haemin was considered as the control value).

Fig. 5. Effect of haemin on HaemOx-1 expression in Trypanosoma cruzi. Western blotting was performed by standard procedures with polyclonal antibody to HaemOx-1. β-actin was used as a loading and transfer control. Other experimental conditions were as described in the Materials and Methods section.
DISCUSSION
T. cruzi is the aetiological agent of the Chagas' disease, affecting a significant proportion of the American population. In this parasitic protozoa the effects of haemin on proliferation, differentiation and the redox state of the cells have been investigated. A haemin concentration of 5 mg/l and 6 days of growth at 28°C with constant shaking, were the optimum conditions to obtain epimastigotes in culture. Haemin concentrations higher than 10 mg/l produced a marked decrease in the rate of growth, together with significant morphological changes, such as shortening of extracellular flagellum, reduction in size and rounding up. Taking into account the changes in cellular morphology and the behaviour observed employing the methodology described by Zaidenberg et al. (Reference Zaidenberg, Tournier, Schinella and Buschiazzo2000), these changes correspond to cell differentiation towards the amastigote state. These results are in agreement with those obtained in Leishmania donovani culture in vitro in medium RPMI 1640, in which a haemin concentration of 50 μm (equivalent to 32·6 mg/l) triggered promastigote-amastigote transformation (Pal and Joshi-Purandare, Reference Pal and Joshi-Purandare2001). Haem regulation of differentiation and proliferation in other cell types, such as a human leukaemic cell line, mouse 3T3 cells and embryonic cells in culture, have also been reported (Kumar and Bandyopadhyay, Reference Kumar and Bandyopadhyay2005).
Because high haemin levels stimulate protein translation, we have investigated the effect of haemin on total protein content in cells grown for between 3 and 7 days. We have found that up to day 5 of growth our results are in agreement with the described behaviour, i.e. the higher the haemin concentration, the greater the intracellular total protein content. However, from day 5 onwards this response changes, and a 30% reduction in protein content was found in cells grown in the presence of 30 mg/l haemin. This concentration of haemin not only down regulated protein synthesis but also led to its degradation. Similar results were obtained in other trypanosomatids where high concentrations of haemin (50 μm) reduced the level of protein synthesis, in general and beta-tubulin in particular (Pal and Joshi-Purandare, Reference Pal and Joshi-Purandare2001). In mammals, the haemin-catalysed degradation of protein to small peptide fragments has also been reported (Kumar and Bandyopadhyay, Reference Kumar and Bandyopadhyay2005). In our T. cruzi cultures, high haemin concentrations, induced non-synchronized morphological changes, leading to a heterogeneous culture, whereby the process of cellular replication corresponding to the amastigote stage is favored over the synthesis of proteins.
The well-known capacity of haemin to generate ROS, could well support the process of haemin-mediated oxidative degradation of proteins. The increased expression of heat shock protein hsp90 in trypanosomatids and mammalian cells cultured with high haemin concentrations (50 μm) would be further evidence that haemin does act as a stress-inducing compound (Pal and Joshi-Purandare, Reference Pal and Joshi-Purandare2001). As far as we know this is the first report in T. cruzi where a correlation between haemin levels in the culture medium and the state of the antioxidant defence system into the cells has been established. We have shown that the 3 enzymes examined, SOD, APx and TryR showed their maximum activity with haemin at a concentration of 5 mg/l. However, at higher haemin concentrations enzyme activities were lower, in the case of SOD to values even lower than controls. Reduced thiols show the same behaviour profile as the antioxidant enzymes. It is important to consider that evaluation of the antioxidant defence system has been carried out after 6 days of growth, when the deleterious action of higher haemin concentrations is evident. If measurements had been made at earlier time-points we may have detected a stimulation of the antioxidant defence system to prevent or overcome the oxidative deleterious actions (experiments in progress).
HaemOx is a haem-degrading enzyme that also plays a significant role in protecting cells from haeme-induced oxidative stress. HaemOx activity and expression have also been investigated. So far, HaemOx activity in T. cruzi cells had not been reported. According to our results, a positive correlation between enzyme activity and expression was observed. If we compare results obtained for HaemOx and those for the antioxidant enzymes defence system we observe a different response. For low concentrations of haemin (5 mg/l), the antioxidant enzymes activity and the reduced thiol content show their maximum levels, instead the activity of HaemOx was not modified and its expression slightly increased. For concentrations of haemin higher than 15 mg/l, the antioxidant defence system was reduced while HaemOx-1 was markedly induced, increasing both its activity and expression. These findings indicate that the mode of action of both antioxidant systems is different.
In conclusion, to obtain T. cruzi epimastigotes in culture, the haemin concentration in the medium should not exceed 5–10 mg/l and the time of growth should be 6 days. Higher haemin concentrations produced oxidative stress which induced transformation of the epimastigote to the amastigote form, along with an injury of the enzymatic machinery of the parasite, leading to diminished protein synthesis as well as lower activity of the antioxidant defence system. Under these conditions HaemOx-1 acts as a stress-responsive antioxidant enzyme.
Our results suggest the possibility that HaemOx participates in the catabolism of haeme in T. cruzi. At present we are purifying the protein recognized by the anti-HaemOx-1 polyclonal antibody.
Finally, better knowledge of the biochemical systems affording the antioxidant defences of the parasite, would provide a valuable target to be exploited for the development of an effective chemotherapy.
María Elisa Lombardo and Alcira Batlle hold the post of Scientific Researchers at the Argentine National Research Council (CONICET). Alejandra Beatriz Ciccarelli is a Research Fellow of CONICET. Lidia Susana Araujo is a Research Fellow of the University of Buenos Aires (UBA). This work was supported by grants from CONICET, UBA and the Agencia de Promoción Científica y Tecnológica.