A woman was referred as an emergency to the Regional Fetal Medicine Centre at 29+2 weeks gestation in her first pregnancy, with an ultrasound diagnosis of hydrops and bradycardia, following presentation at her local unit with abdominal pain and reduced foetal movements. Complete congenital heart block with a ventricular rate of 39 beats per minute (bpm) and gross foetal hydrops were diagnosed. The heart was structurally normal. After consultation with the on-call paediatric cardiologist and neonatologist, the couple were counselled regarding the high risk for foetal demise in utero, as well as the poor prognosis post-natally in a preterm hydropic baby. It was felt that the best option was delivery.
A female infant weighing 1825 g was delivered by emergency caesarean section that day. Following delivery, the baby was ventilated and started on isoprenaline and dobutamine infusions (Fig 1). The heart rate remained 40–50 bpm and the baby was urgently transferred to the tertiary cardiac centre for emergency epicardial pacing. Echocardiography revealed a structurally normal heart. Initial stabilisation was performed by drainage of ascites and pleural effusions and insertion of temporary epicardial pacing wires (Fig 2). On day 13, a St. Jude Microny 2525T™ single-chamber epicardial pacemaker was inserted with a Medtronic CAPSURE® EPI 4968–25 cm ventricular lead (Fig 3). The initial lead capture threshold was 1.2 V at 0.5 ms with lead impedance 940 ohms, R wave amplitude sensing 26.9 mV. The pacing mode was VVI at 130 bpm. The baby was extubated on day 16, weighing just 1.2 kg with her hydrops completely resolved. The mother was found to be positive for anti-Ro, SSA, antibodies, despite being asymptomatic from rheumatological disease. At 8 months of age, the post-implant threshold remains low at 0.9 V at 0.58 ms with lead impedance of 400 ohms. The child is asymptomatic, thriving, and developing appropriately (Figs 1, 2 and 3).

Figure 1 Initial X-ray showing evidence of severe hydrops: ascites, skin oedema, an enlarged heart, and small pleural effusions.
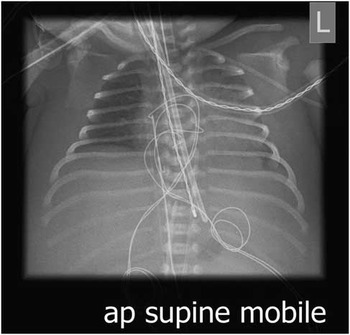
Figure 2 Chest X-ray post-emergency temporary epicardial leads inserted.

Figure 3 Post-operative X-ray showing the pacemaker extending from behind the rectus muscle in the abdomen with the epicardial leads in situ.
Premature hydropic babies with complete congenital heart block have a very poor prognosis with a high mortality.Reference Shepard, Kochilas and Vinocur 1 The most common cause of complete congenital heart block is cardiac structural anomalies followed by maternal autoimmune disease, often undiagnosed, which was the case here.Reference Shepard, Kochilas and Vinocur 1 There are only a few reported cases in the literature with good outcomes of infants with severe hydrops of this gestation and weight surviving and having successful pacemaker insertion. This case highlights the success of initial medical stabilisation in a tertiary neonatal unit with a close link to tertiary cardiac care.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.





