Introduction
Winter legume cover crops furnish essential plant nutrients, particularly nitrogen (N), to subsequent summer crops (Ranells, Reference Ranells1992; Wagger et al., Reference Wagger, Cabrera and Ranells1998), but estimates of this N contribution have traditionally been based solely on the decomposition and N release dynamics of shoot residues (Wilson and Hargrove, Reference Wilson and Hargrove1986; Utomo et al., Reference Utomo, Frye and Blevins1990; Stute and Posner, Reference Stute and Posner1995), while N contributions of roots have largely been ignored. Legume cover-crop shoots decompose and release N rapidly following spring termination, in large part because their biochemical composition (low C/N ratio and percent lignin) is conducive to fast rates of decomposition and N release (Buchanan and King, Reference Buchanan and King1993; Sainju et al., Reference Sainju, Whitehead and Singh2005). In contrast, legume cover-crop roots have higher C/N ratios, elevated lignin content, and are often physically protected from microbial decomposition within soil aggregates (Buchanan and King, Reference Buchanan and King1993; Puget and Drinkwater, Reference Puget and Drinkwater2001; Schmidt et al., Reference Schmidt, Torn, Abiven, Dittmar, Guggenberger, Janssens, Kleber, Kögel-Knabner, Lehmann, Manning, Nannipieri, Rasse, Weiner and Trumbore2011; Dungait et al., Reference Dungait, Hopkins, Gregory and Whitmore2012), all of which lead to slower rates of legume cover-crop root decomposition and N release relative to shoots. Although legume cover-crop root decomposition and N release is relatively slow, roots can account for nearly 30% of total legume cover-crop biomass (Sainju et al., Reference Sainju, Whitehead and Singh2005), and it is, therefore, important to include root-derived N in estimates of legume cover-crop contributions to soil inorganic N pools.
Several authors have found that in legume pasture systems where shoots are removed from cover crops such as alfalfa (Medicago sativa L.) and subterranean clover (Trifolium subterraneum L.), roots play an important role in providing N for subsequent crops (Rasse et al., Reference Rasse, Smucker and Schabenberger1999; Bolger et al., Reference Bolger, Angus and Peoples2003). However, the quantity of N released from decomposing roots of spring-terminated winter legume cover crops during subsequent summer crop growth, as well as the temporal dynamics of this release, have not been thoroughly investigated. The effect that a spring termination approach, which varies in its level of soil disturbance and placement of N-rich winter legume cover-crop shoot residues, may have on root decomposition and N release is of interest to growers utilizing cover crops for nutrient management, and is also poorly understood.
In the Southeastern USA, winter legume cover crops are terminated in the spring using both traditional and novel approaches. Termination with a tractor-mounted mechanical disk that incorporates legume cover-crop shoots into the soil is a traditional spring termination approach. Disking results in rapid shoot decomposition and nutrient release, compared to termination approaches that leave surface mulch, due to greater shoot–soil contact and elevated levels of soil oxygen in disked systems, both of which stimulate microbial decomposition (Buchanan and King, Reference Buchanan and King1993; Coppens et al., Reference Coppens, Garnier, Findeling, Merckx and Recous2007; Paul and Clark, Reference Paul and Clark2007). These faster rates of shoot decomposition and nutrient release under disking result in higher soil inorganic N levels at an earlier date compared with traditional herbicide-based no-till termination approaches (Wilson and Hargrove, Reference Wilson and Hargrove1986; Sarrantonio and Scott, Reference Sarrantonio and Scott1988). Additions of soil inorganic N are known to have a positive effect on shoot decomposition (Fog, Reference Fog1988; Mary et al., Reference Mary, Recous, Darwis and Robin1996), but the effect of added N on legume cover-crop root decomposition is poorly understood and may be positively impacted by the addition of N-rich disked legume cover-crop shoot biomass.
Terminating cover crops with a roller-crimper is a novel approach that has been gaining popularity in the Southeastern USA due to its proven effectiveness in weed suppression and soil moisture conservation (Ashford and Reeves, Reference Ashford and Reeves2003; Kornecki et al., Reference Kornecki, Price, Raper and Arriaga2009; Mirsky et al., Reference Mirsky, Curran, Mortensen, Ryan and Shumway2011). This tractor-mounted cylindrical drum implement with chevron-shaped blades rolls over and crimps cover-crop stems when the crop is in full bloom, leaving plants flattened on the soil surface as protective mulch (Davis, Reference Davis2010; Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011). When cover-crop biomass production is sufficiently high, resulting in a thick surface layer of mulch, roller-crimping can reduce or eliminate herbicide usage (Mirsky et al., Reference Mirsky, Curran, Mortensen, Ryan and Shumway2009, Reference Mirsky, Curran, Mortensen, Ryan and Shumway2011) offering the possibility of no-till organic farming systems. Roller-crimping also leaves cover-crop root systems intact and soil undisturbed, which is in stark contrast to disking where soil disturbance can lead to relocation of cover-crop biomass and enhanced decomposition through increased soil respiration (Paul and Clark, Reference Paul and Clark2007; Yaduvanshi and Sharma, Reference Yaduvanshi and Sharma2008; Soriano et al., Reference Soriano, Àlvarez, Landa and Gómez2012). Research in North Carolina (NC) indicates that roller-crimped legume cover crops can furnish 10–217 kg N ha−1 depending on species and termination date (Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011), and that peak soil inorganic N occurs 4–6 weeks after roller-crimping (Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2014). However, comparisons of legume cover-crop N release dynamics under disking and roller-crimping termination at the same field site and during the same season are lacking.
In the present study, we investigated the effect of a spring termination approach on decomposition and N release dynamics of roots from pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth). These species were selected because they are well-adapted to the moderately acid soils of the Southeastern USA, are among the most common winter legume cover crops grown by farmers in the region, and are compatible with roller-crimper termination (Wilson and Hargrove, Reference Wilson and Hargrove1986; Ranells, Reference Ranells1992; Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011). Field studies at separate sites in the NC Coastal Plain were conducted in the summer of 2012. Our main objectives were to: (i) quantify decomposition and N release dynamics of pea, clover and vetch roots; (ii) determine if roots decompose and release N faster when terminated by disking than roller-crimping; and (iii) determine if roots decompose and release N faster under higher soil inorganic N levels. We hypothesized that the most rapid decomposition and N release would occur in roots from species with favorable biochemical composition, and in disked plots over rolled plots.
Materials and Methods
Field sites and experimental setup
Legume cover-crop root decomposition and N release were investigated in Goldsboro (35.39°N, 78.03°W) and Kinston (35°26′N, 77°65′W), NC, during the summer of 2012. In Goldsboro, the study was conducted at the Center for Environmental Farming Systems on Wickham loamy sand (fine loamy, mixed, thermic, Typic Hapludult), while in Kinston the study took place at the NC Department of Agriculture & Consumer Services Caswell Research Farm on Portsmouth loam (fine loamy fluviomarine deposits over sandy skeletal fluviomarine, mixed, thermic Typic Umbraquult). These sites are located in the NC Coastal Plain, an important agricultural production area of the state characterized by mild winters and hot, humid summers. Long-term state weather data (1971–2011) indicates that these sites have similar air temperatures (~23°C) during the growing season (April–September), but Kinston has higher average rainfall during this period (750 versus 650 mm) (State Climate Office of North Carolina, NC State University, 2014). These sites allowed us to test our hypothesis on different soil types and in locations likely to experience some climatic variability over the course of the experiment. Pea, clover and vetch were planted at both sites in September 2011 (Table 1) in a randomized complete block split-plot design. Main plots (termination method) were 324 m2 in Goldsboro and 549 m2 in Kinston, while subplots (legume cover-crop species) measured 84 m2 in Goldsboro and 90 m2 in Kinston. Each treatment plot was replicated four times. Within each main plot, one (Goldsboro) and two (Kinston) subplots were not planted to legume cover crops and were left bare over the winter and early spring. Cover crops were terminated by disking and roller-crimping on April 19 and May 10, 2012 in Kinston and Goldsboro, respectively (Table 1). At spring termination, plots not planted to cover crops were also subject to disking and roller-crimping, which terminated the small amount of natural vegetation that had grown in these cover crop-free bare plots during winter and early spring. In Goldsboro, cover crop-free bare plots received no N addition (N00), while in Kinston in addition to the N00 plots there were also cover-crop-free bare plots that received 150 kg N ha−1 (N150) as urea and ammonium nitrate solution approximately 2 weeks after termination (Table 1). There were no N150 plots established in Goldsboro because the land is certified-organic, which prohibits use of synthetic fertilizers. These N00 and N150 plots served the purpose of allowing us to follow root decomposition and N release under natural and elevated soil N conditions without the history of a legume cover crop which would add large amounts of N-rich biomass and likely increase soil microbial activity (Paul and Clark, Reference Paul and Clark2007). Pea, clover, vetch, N00 and N150 plots are collectively referred to as ‘cover-crop treatment plots’. All cover-crop treatment plots were planted to unirrigated corn (cv. AgVenture AV8262) approximately 2 weeks after cover-crop termination at both sites.
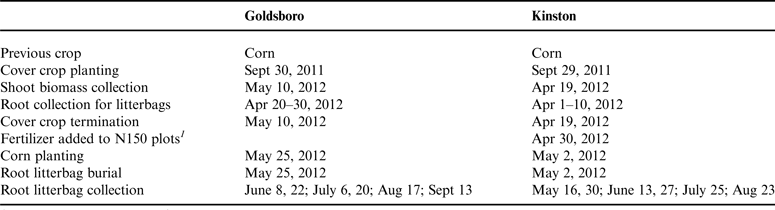
Table 1. Field activities schedule at Goldsboro and Kinston, NC study sites.

1 In Kinston, 150 kg N ha−1 as urea and ammonium nitrate solution was added to half of plots where cover crops were not grown.
Cover-crop biomass determination
A 0.5 m2 quadrat was used to collect legume shoot biomass from pea, clover and vetch plots just prior to termination (Table 1). One sample was taken from a representative location within each plot. Biomass samples were transported to North Carolina State University (NCSU), dried at 65°C until constant weight was achieved, and final dry weights were recorded. Root biomass of pea, clover and vetch remaining in the field was estimated using root:shoot ratios for these species previously determined in a greenhouse study (unpublished data). Briefly, in this greenhouse study pea, vetch, and clover were grown in replicates of six in a completely randomized design for 16 weeks in transparent plastic sleeves placed in 75 cm tall, 10 cm diameter polyvinyl chloride cylinders. The greenhouse temperature range was 10–30°C. One plant was grown per cylinder in a sand (90%) and top soil (10%) medium. Plants were irrigated and fertilized daily using 200 ml N-free Hoagland solution per cylinder (Hoagland and Arnon, Reference Hoagland and Arnon1950). At harvest, sleeves were removed from cylinders, cut open along their length, and the medium was washed off with a gentle hose spray. Plants were cut with scissors at the soil line to separate shoots and roots, which were then oven-dried at 65°C until constant weight was achieved. Oven-dried root and shoot weights were used to determine root:shoot ratios. The range of root:shoot ratios determined in the greenhouse (0.27–0.3) was slightly higher than the values reported for these species in field studies (0.21–0.28) (Puget and Drinkwater, Reference Puget and Drinkwater2001; Williams et al., Reference Williams, Myrold and Bottomley2006; Kong and Six, Reference Kong and Six2010; Vasileva, Reference Vasileva2015). These small differences likely relate to greater root production in the greenhouse compared with the field due to optimal greenhouse growing conditions, our ability to more easily capture fine roots (<2 mm diameter) using this greenhouse method compared with field methods (Puget and Drinkwater, Reference Puget and Drinkwater2001; Kong and Six, Reference Kong and Six2010), and the deeper root excavation depth (75 cm) we used compared with field studies (15–40 cm) (Puget and Drinkwater, Reference Puget and Drinkwater2001; Williams et al., Reference Williams, Myrold and Bottomley2006; Kong and Six, Reference Kong and Six2010; Vasileva, Reference Vasileva2015). Since these greenhouse-derived root:shoot ratios were relatively close in value to those reported for these species under field conditions, we used these ratios to estimate standing pea, clover and vetch root biomass at termination.
Cover-crop root litter collection and burial
The litterbag method (Bocock and Gilbert, Reference Bocock and Gilbert1957) was used to determine the effect of termination approach on root decomposition and N release. In April 2012, prior to legume cover-crop termination in Goldsboro and Kinston, root litter from flowering pea, clover and vetch was collected to 15 cm soil depth at both sites (Table 1) and processed separately. To collect root litter, we identified the base of legume cover-crop plants and used a shovel to dig up plants carefully removing weed roots manually. Roots were soaked and washed thoroughly using tap water, air-dried at room temperature on greenhouse benches until constant weight was achieved, and cut into 3–5 cm pieces (Berg et al., Reference Berg, Muller and Wessen1987). Root litter was placed in nylon bags (9 × 9 cm2, 1 mm mesh) in the following quantities: 0.8 g bag−1 for pea and clover, 0.7 g bag−1 for vetch. Root litterbags were buried approximately 2 weeks after cover-crop termination on the same day as corn planting, and were placed between corn rows to 15 cm depth in all plots. Treatment combinations are summarized as follows: cover-crop treatment (pea, clover, vetch, N00 and N150) × termination (disking and roller-crimping) × time (0, 2, 4, 6, 8, 12 and 16 weeks after root litterbag burial) whereas pea, clover and vetch roots were buried in pea, clover and vetch plots, respectively, and roots from all species were buried in N00 and N150 plots. When inserting litterbags into soil precautions were taken to minimize soil disturbance, which was especially important for roller-crimped treatments since undisturbed soil is a defining characteristic of this termination approach. A high level of soil disturbance in roller-crimped plots at litterbag burial would have made them indistinguishable from disked plots thereby weakening the termination component of this study. To bury litterbags, a spade (20 cm width) was pressed vertically into moist soil to 15 cm depth creating approximately a 20 cm × 2 cm slot just wide enough for vertical placement of litterbags. Slots were gently closed in by pressing soil down and slightly inward. This method of litterbag placement minimized the area and level of soil disturbance compared to digging with a shovel. The presence of surface mulch, another defining characteristic of roller-crimping termination that differentiates it from disking, was not affected by this insertion method. Litterbags were retrieved 2, 4, 6, 8, 12 and 16 weeks after burial and were oven-dried at 70°C for 48 h. Oven-dried roots were removed from litterbags and a brush and forceps were used to gently remove adhering soil (Wang et al., Reference Wang, Liu and Mo2010). Oven-dried root weight was recorded.
Soil collection and N analysis
Soil samples were collected at litterbag burial and at each litterbag retrieval date from all plots to 15 cm depth using a 2.54 cm diameter soil probe. Ten subsamples were taken per plot in a ‘W’ pattern, homogenized manually, and bulked. Soils were dried at 40°C to constant mass, then ground to pass 2 mm mesh screen. Samples were extracted with 1 mol l−1 KCl with shaking for 1 h, and allowed them to settle for 20 min before filtering. Extracts were refrigerated at 4°C until analysis for NH4 + and NO3 − on a QuikChem 2000 injection auto analyzer (Lachat Instruments, Loveland, CO). Separate samples were also taken to determine percent total soil N using a Perkin Elmer 2400 CHNS/O Elemental Analyzer (Norwalk, CT) in the Environmental and Analytical Testing Service facility at NCSU (Raleigh, NC).
Root litter chemical analysis, root mass loss and root N release
Root litter subsamples were collected prior to placement in litterbags, ground to pass a 1 mm mesh screen, and analyzed for initial C, N and lignin content. Carbon and N were determined using the Perkin Elmer 2400 CHNS/O Elemental Analyzer described previously. Lignin was measured by Dairy One (Ithaca, NY) using 72% w/w sulfuric acid digestion followed by near infrared reflectance spectroscopy. Due to insufficient root biomass at both locations, only single composite samples from each species were analyzed for lignin, and multiple comparisons to determine differences in lignin content between species were not possible. At each litterbag retrieval date, litterbag content oven-dried weight was determined, and was followed by taking subsamples of litterbag content to determine C, N and ash concentration. For ash determination, litterbag contents were combusted in a muffle furnace at 550°C for 16–18 h. Remaining ash represented the soil mineral contaminant in litterbags. To determine root mass loss at each collection date, subsample weight used for ash determination was recorded before and after combustion. We assumed that differences in weight resulting from combustion were due to root C loss as CO2. The proportion root in subsamples is equal to the root subsample weight before combustion minus root subsample weight after combustion divided by the root subsample weight before combustion. The proportion of originally buried root mass remaining in retrieved litterbags at time (t) is equal to the collected oven-dried litterbag content weight multiplied by the percent root mass of the subsample and divided by the original weight of the buried root mass. Litterbag root N mass remaining at each collection was determined by subtracting litterbag soil-derived N mass, which was calculated by multiplying litterbag ash content by percent total soil N, from litterbag content N mass. Percent original root N mass remaining in litterbags was determined by dividing litterbag root N mass remaining at each collection by original litterbag root N mass at burial multiplied by 100.
Statistical analyses
Analysis of variance with the PROC MIXED procedure (SAS Institute Inc., Cary, NC) was used to analyze the following variables: shoot and root biomass, percent root mass remaining, percent root C and N, root N mass remaining, percent total soil N and soil inorganic N content. Termination approach, cover-crop treatment plots and time were fixed effects, while block was treated as a random effect. Multiple comparisons between treatment means were made with Tukey–Kramer's HSD (P < 0.05) on least-squared means.
Plant litter decomposition and nutrient release are best described by first-order decomposition kinetics characterized by initial rapid mass loss or nutrient release and followed by a slower rate of mass loss or nutrient release (Wilson and Hargrove, Reference Wilson and Hargrove1986; Buchanan and King, Reference Buchanan and King1993; Luna-Orea et al., Reference Luna-Orea, Wagger and Gumpertz1996; Paul and Clark, Reference Paul and Clark2007). Percent root mass and root N mass remaining for each litterbag retrieval date were regressed with time from all plots using nonlinear regression procedures of SigmaPlot 12.5 (Systat Software, Inc., San Jose, CA) to find the most suitable exponential model to describe root decomposition and N release. The following exponential models were tested:
where f is the percent root or N mass remaining at time x (week), a and b represent the percent root or N mass at week 0, k is the decomposition or N release rate constant, and ε is the random error term.
The single exponential, two-parameter model (1) assumes an equal rate of residue decomposition throughout the experiment, while the single exponential, three-parameter model (2) assumes a portion of litter does not decompose during the experiment. The double exponential, four-parameter model (3) assumes that litter decomposes at two different rates during the experiment (Lopp and Guillard, Reference Lopp and Guillard2004; Soto et al., Reference Soto, Luna-Orea, Wagger, Smyth and Alvarado2005). Model selection to best describe decomposition and N release was based on adjusted coefficient of determination values produced using nonlinear regression.
Results and Discussion
Soil inorganic N dynamics
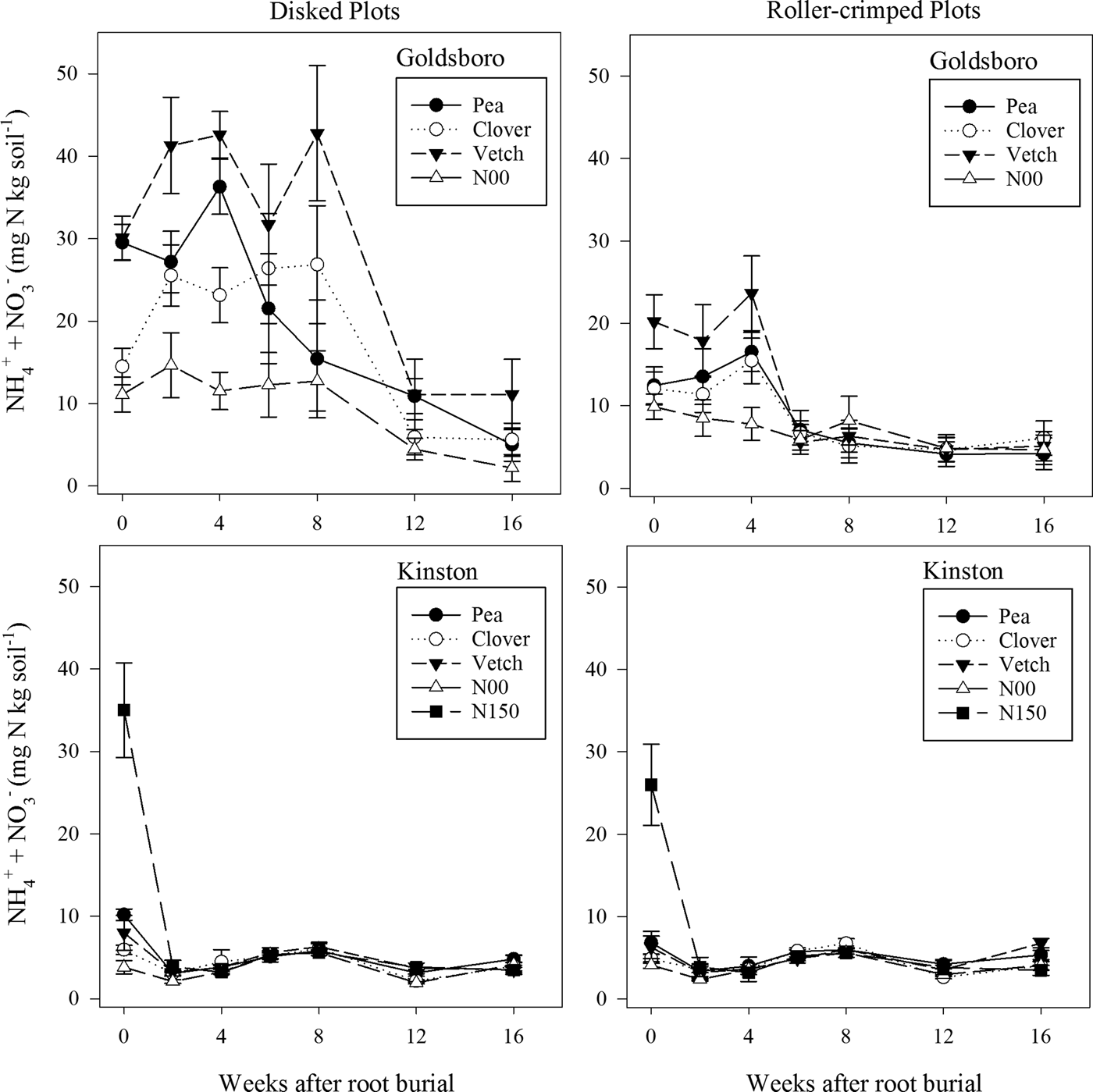
In Goldsboro, soil inorganic N content in plots was significantly affected by the higher order interaction of cover-crop treatment × termination × time (P = 0.0001). Pea, clover and vetch plots terminated by disking had higher soil inorganic N levels than their corresponding plots terminated by roller-crimping until week 12 for pea and clover, and through week 16 for vetch (Fig. 1). In Goldsboro, disked vetch plots had higher soil inorganic N levels than disked pea or clover plots for several sampling dates through week 8 of the study, while roller-crimped vetch plots also had higher soil inorganic N levels than roller-crimped pea and clover, but for a much shorter duration (Fig. 1). Parr et al. (Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2014) similarly reported higher soil inorganic N levels in roller-crimped vetch plots than in roller-crimped clover plots at sites in the NC Coastal Plain. Not surprisingly, N00 plots had lower soil inorganic N levels than pea, clover and vetch plots under both termination approaches for several sampling dates (Fig. 1). In Goldsboro, peak soil inorganic N occurred in disked pea and vetch plots 4 weeks after litterbag burial (~6 weeks after cover-crop termination), which is in agreement with earlier findings by Varco et al. (Reference Varco, Frye, Smith and MacKown1989). However, disked clover plots peaked in soil inorganic N 8 weeks after litterbag burial (~10 weeks after cover-crop termination). In roller-crimped plots, peak soil inorganic N was reached for all species 4 weeks after litterbag burial (Fig. 1), which falls within the general time frame of peak soil inorganic N following legume cover-crop spring termination by roller-crimping in the NC Coastal Plain (Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2014). At its peak in Goldsboro, soil inorganic N in disked plots was 73–117% higher than in roller-crimped plots, depending on cover-crop species (Fig. 1).

Figure 1. Soil inorganic N (mg N kg soil−1) fluctuation in disked and roller-crimped pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) plots and in cover crop-free bare plots with no N addition (N00) or with 150 kg N ha−1 (N150) added as urea and ammonium nitrate solution at Goldsboro and Kinston, NC study sites.
In Kinston plots, the higher order interaction of cover-crop treatment × termination × time also had a significant effect (P < 0.0001) on soil inorganic N levels (Fig. 1). However, soil inorganic N fluctuation over the course of the study was drastically different in Kinston compared with Goldsboro as differences in soil inorganic N levels between plots were only observed at litterbag burial (~2 weeks after termination). Not surprisingly, N150 plots had the highest levels of soil inorganic N under both disking and roller-crimping at litterbag burial, while N00 plots had the lowest soil inorganic N levels (Fig. 1). Disked pea plots also had approximately 49% higher soil inorganic N content than roller-crimped pea plots (Fig. 1) at litterbag burial. Unlike in Goldsboro, roller-crimped pea, clover and vetch plots had similar soil inorganic N levels throughout the study in Kinston. The highest recorded measurements of soil inorganic N in Kinston occurred at litterbag burial in both disked and roller-crimped plots, 4 weeks earlier than in Goldsboro.
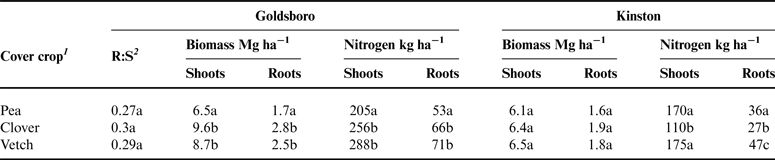
There were substantial differences in soil inorganic N levels in pea, clover and vetch plots between sites, with soil inorganic N levels generally being higher in Goldsboro than in Kinston (Fig. 1). Clover and vetch biomass production was considerably higher in Goldsboro than in Kinston (Table 2), and there was a larger quantity of standing cover-crop N (shoots plus roots) for all species in Goldsboro (258–359 kg N ha−1) compared with Kinston (137–222 kg N ha−1) at termination. However, our data also indicate that N00 plots in Goldsboro had a higher range of soil inorganic N (2.2–14.7 mg N kg soil−1) than N00 plots in Kinston (1.9–5.6 mg N kg soil−1), which may relate to differences in rainfall distribution between sites. Rainfall was approximately three times higher in Kinston than in Goldsboro during the month following cover-crop termination (Table 3). We speculate that this higher rainfall may have accelerated cover-crop N release and subsequent N losses in Kinston plots. High rates of NO3 − leaching from legume cover-crop residues have been observed in both disked and surface mulched systems where soil moisture content exceeds soil water-holding capacity (Moller and Reents, Reference Moller and Reents2009; Campiglia et al., Reference Campiglia, Mancinelli, Radicetti and Marinari2011). The very sharp decline in soil inorganic N in Kinston N150 plots within 2 weeks of litterbag burial and fertilizer application provides additional evidence in support of leaching-related N losses (Table 1; Fig. 1). The Portsmouth loam soil at the Kinston site also does not drain as rapidly as the Wickham loamy sand of Goldsboro, and excessive rainfall in Kinston may have generated soil moisture conditions conducive to denitrification (Mosier et al., Reference Mosier, Doran and Freney2002), which may have also contributed to soil inorganic N losses in Kinston. In Goldsboro, total rainfall was relatively low compared with Kinston, and N losses through leaching or denitrification likely were not major events. These results suggest that in high rainfall environments N released from decomposing legume cover-crop residues may have a relatively low soil residence time.
Table 2. Shoot and root biomass and N yields for pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) at Goldsboro and Kinston, NC study sites.

1 Values within a column followed by the same letter are not significantly different (Tukey–Kramer's HSD; P < 0.05).
2 Root:Shoot ratios (R:S) were determined from 16-week-old greenhouse-grown plants in replicates of six for each species.
Table 3. Monthly means of daily maximum air temperature (°C) and total precipitation (mm) at Goldsboro and Kinston, NC study sites.

1 Data collected until the last litterbag collection date at Goldsboro (September 13) and Kinston (August 23) sites.
Cover-crop root decomposition dynamics
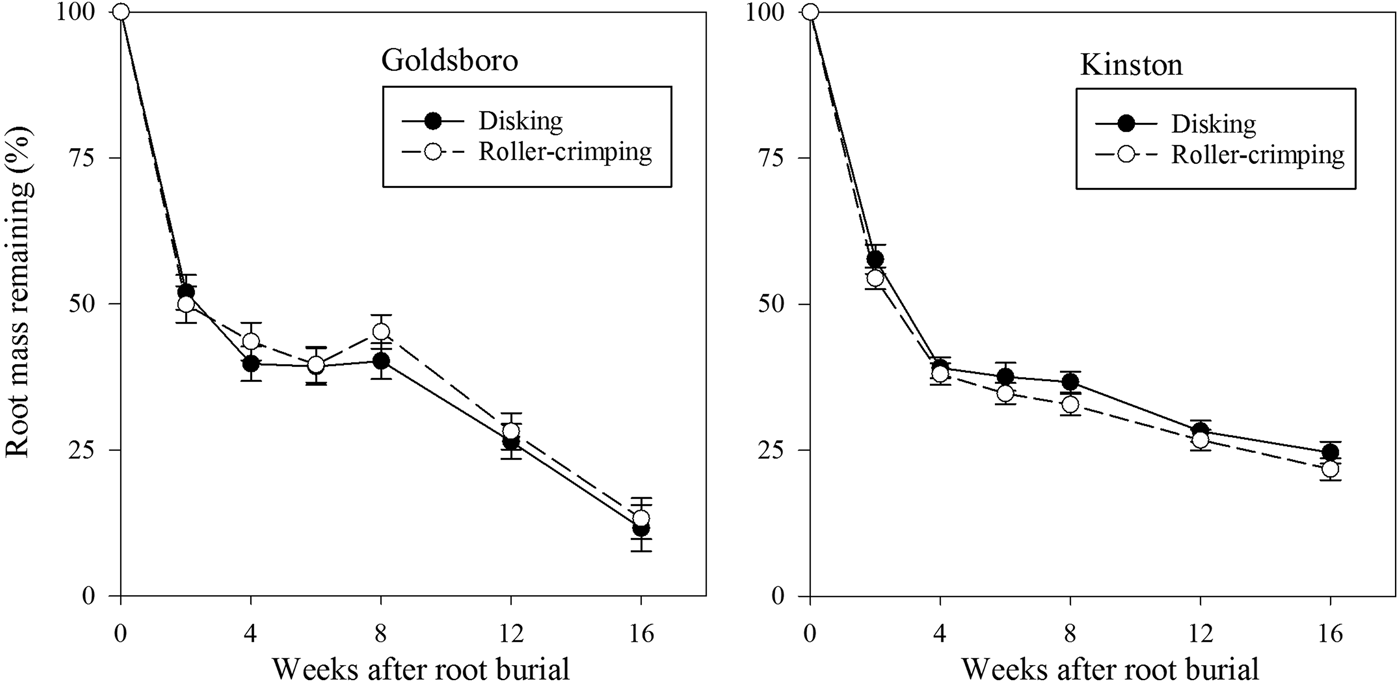
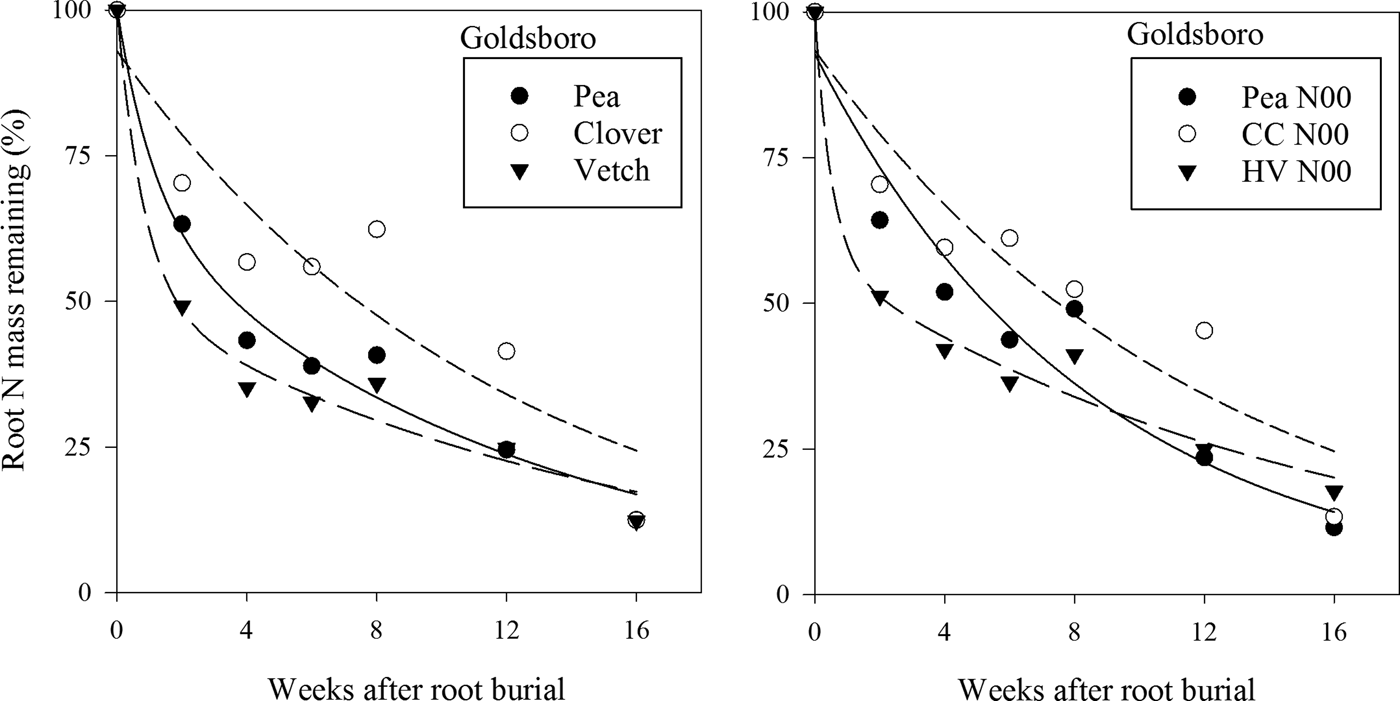
Although disking and roller-crimping generated different levels of soil inorganic N over a prolonged period in Goldsboro, root decomposition rate was not affected (P = 0.79) by termination approach at this site (Fig. 2). There also was not a higher-order interaction among termination, cover-crop treatment and time (P = 0.07). Only the cover-crop treatment × time interaction was significant (P = 0.04) in Goldsboro. When averaged across termination approaches, pea and vetch roots buried in respective pea and vetch plots decomposed faster than when buried in N00 plots at week 8 (Fig 3). In Kinston, termination approach also did not affect (P = 0.82) root decomposition (Fig. 2). The cover-crop treatment × termination × time interaction also was not significant (P = 0.31), but like in Goldsboro, there was a significant cover-crop treatment × time interaction (P < 0.001). When averaged across termination approaches, clover roots buried in clover plots decomposed faster than all pea and vetch treatments at weeks 8 and 12 (Fig. 4). At week 16, clover roots buried in clover plots decomposed faster than clover roots buried in N00 and N150 plots, pea roots buried in N150 plots and vetch roots buried in vetch plots (Fig. 4).
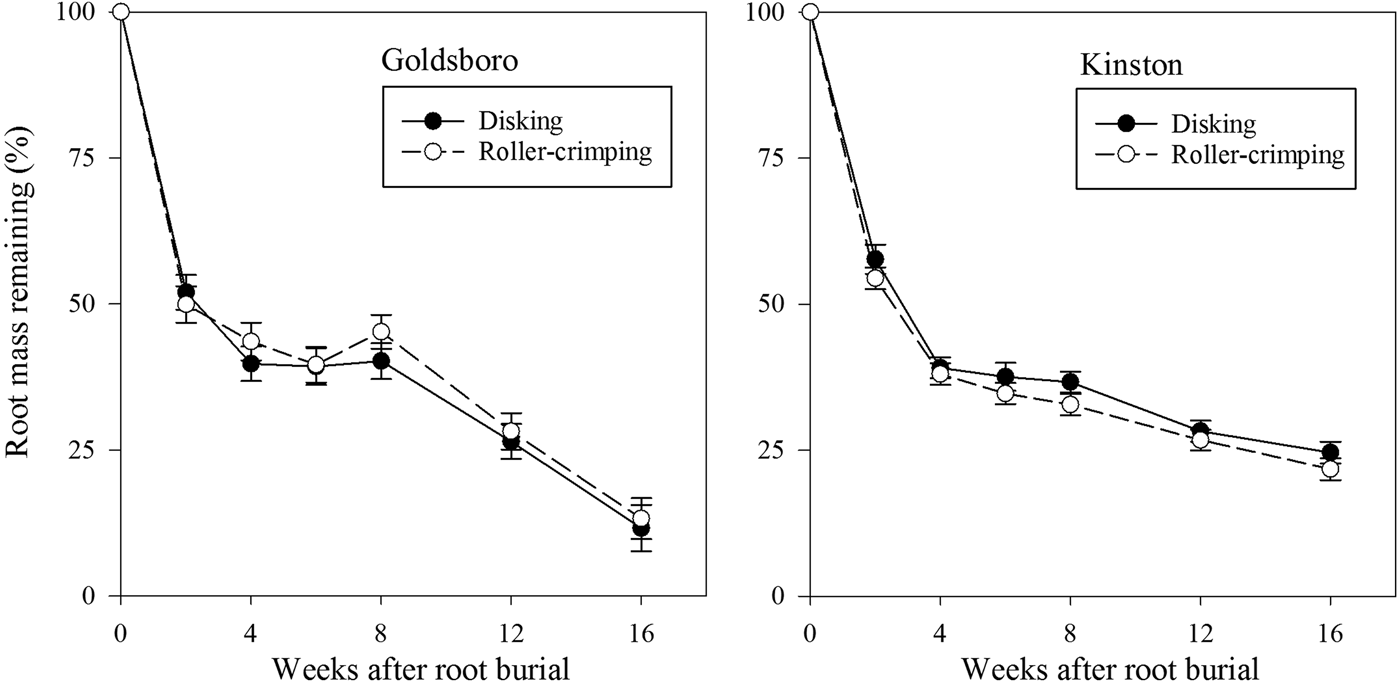
Figure 2. Percentage of original root mass remaining over time averaged across cover crop treatments as a function of termination approach at Goldsboro and Kinston, NC study sites.

Figure 3. Percentage of original pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) root mass remaining over time in cover crop treatment plots averaged across termination approaches at the Goldsboro, NC study site. Equations for these curves are given in Table 4.

Figure 4. Percentage of original pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) root mass remaining over time in cover crop treatment plots averaged across termination approaches at the Kinston, NC study site. Equations for these curves are given in Table 4.
In Goldsboro, there was large variability in model selection to best describe root decomposition, while in Kinston model 3, and in one case model 2, best described root decomposition, suggesting that in all but one case, root litter decomposed at two different rates in Kinston (Table 4). The decomposition rate constants (k) produced by models 1 and 3 reflect the faster rates of decomposition for clover roots buried in clover plots compared with other treatments during the latter portion of the study in Kinston (Table 4). The exponential models used to describe root decomposition at both sites have also been selected by other authors to best describe legume cover-crop decomposition using litterbag methods (Buchanan and King, Reference Buchanan and King1993; Luna-Orea et al., Reference Luna-Orea, Wagger and Gumpertz1996).
Table 4. Coefficients for the single exponential two-parameter model (1), single exponential three-parameter model (2) and double exponential four-parameter model (3) used to describe pea, clover and vetch root decomposition averaged over termination in pea, clover, vetch, N00 and N150 plots. Model selection was based on the highest adjusted R 2 values.

1 Values within a column (separated by site) followed by the same letter are not significantly different (Tukey–Kramer's HSD; P < 0.05).
2 Model 1: f = ae−kx + ε. Model 2: f = a + be−kx + ε. Model 3: f = ae−k1x + be −k2x + ε. In models, f is the percent of original root mass remaining at time x (week); a and b represent the percent root mass at week 0; k is the decomposition rate constant, and ε is the random error term.
3 ‘Not valid’ indicates data exceeded maximum number of iterations and did not converge. Thus, model coefficients could not be validated.
Averaged over termination approach, pea, clover and vetch roots buried in plots of their respective species lost 56–65% of their initial root mass within 4 weeks of burial in Goldsboro (Fig. 3). Taking into account our estimates of standing root biomass for each of these species immediately before termination (Table 2), this rate of decomposition indicates that approximately 1037, 1568 and 1625 kg ha−1 of pea, clover and vetch root biomass, respectively, was lost within 1 month of root burial. After 16 weeks, it is estimated that these species lost 87–90% of their original root biomass, which is equivalent to 1479, 2520 and 2200 kg ha−1 for pea, vetch and clover, respectively. Root decomposition was also rapid immediately following termination in Kinston, but slowed considerably during the second half of the study in comparison with Goldsboro. Averaged over termination approach, pea, clover and vetch roots buried in plots of their respective species lost 60–65% of their original mass within 4 weeks of burial in Kinston (Fig. 4), which is equivalent to 960, 1235 and 1100 kg ha−1 of pea, clover and vetch root biomass, respectively. After 16 weeks, 73–85% of roots had decomposed, and 1232, 1615 and 1314 kg ha−1 of pea, clover and vetch root biomass, respectively, was lost.
The rates of clover and vetch root decomposition observed in both Goldsboro and Kinston resembled rates of shoot decomposition reported for these species in the Southeastern USA. For example, clover shoots lost 70% of their biomass within 4 weeks under conventional tillage in a study in the nearby Piedmont region of NC (Buchanan and King, Reference Buchanan and King1993), while clover roots in our study, averaged across sites and termination approaches, lost 61% of their initial biomass during this same time period (Figs. 3 and 4). After 16 weeks, approximately 90% of original clover shoot biomass was lost in the Piedmont region study (Buchanan and King, Reference Buchanan and King1993), which closely mirrors the 87% of clover roots lost during this time frame averaged across sites and termination approaches in our study. In a Mississippi field study, Varco et al. (Reference Varco, Frye, Smith and MacKown1993) reported that 44 and 76% of vetch shoot biomass was lost from no-tillage and conventional tillage plots, respectively, within 4 weeks, while approximately 58 and 78% of shoot biomass was lost from no-tillage and conventional tillage plots, respectively, after 16 weeks. In our study, averaged across sites and termination approaches, 63% of vetch roots decomposed within 4 weeks, while 80% of vetch roots decomposed by week 16 (Figs. 3 and 4). The rate of root decomposition observed in our study was substantially faster than rates of legume cover-crop root decomposition observed in field studies in the Northern and Western USA (Puget and Drinkwater, Reference Puget and Drinkwater2001; Kong and Six, Reference Kong and Six2010). However, Buchanan and King (Reference Buchanan and King1993) also found that clover roots decomposed more slowly than shoots in a NC field study, but differences between root and shoot decomposition rates were smaller compared with differences under more temperate conditions (Puget and Drinkwater, Reference Puget and Drinkwater2001). Climatic factors, particularly temperature and soil moisture, are a major determinant of plant residue decomposition (Gijsman et al., Reference Gijsman, Alarcon and Thomas1997; Berg and McClaugherty, Reference Berg and McClaugherty2008), and may help explain these regional variations in legume cover-crop root decomposition.
The initial rapid rates of root decomposition observed at both sites in this study have also been observed for clover root decomposition in the Southeastern USA (Buchanan and King, Reference Buchanan and King1993). These initial faster rates of plant litter decomposition are most often attributed to microbial consumption of easily decomposable materials such as simple carbohydrates, hemicelluloses and proteins (Berg et al., Reference Berg, Muller and Wessen1987; Gunnarsson and Marstorp, Reference Gunnarsson and Marstorp2002). After this initial rapid rate of root mass loss, decomposition slowed considerably for the remainder of the study in Kinston, while in Goldsboro root decomposition unexpectedly increased during the final 8 weeks of the study after having slowed during weeks 4–8 (Fig. 2). From weeks 8–16 of the study, 21–27% of initial pea, clover and vetch roots buried in plots of their corresponding species were lost in Goldsboro (Fig. 3) compared with only 7–15% of root mass loss for these species in Kinston (Fig. 4) averaged across termination methods. Our findings are unusual in investigations of plant litter decomposition as initial rapid rates of decomposition are normally followed by slower rates as microbial decomposers consume less palatable materials (Paul and Clark, Reference Paul and Clark2007; Berg and McClaugherty, Reference Berg and McClaugherty2008).
The faster rate of root decomposition in Goldsboro observed during the latter part of this study may relate to greater soil microbial activity in Goldsboro compared with Kinston during this period. Liang et al. (Reference Liang, Grossman and Shi2014) measured several soil microbial properties in disked plots over the course of this study, and reported higher soil enzyme activity (β-glucosidase and β-glucosaminidase) in disked Goldsboro plots than in disked Kinston plots during the latter portion of this study. The increase in rainfall in Goldsboro (Table 3) 1 month after litterbag burial, while still lower than in Kinston, likely played an important role in sustaining soil microbial activity during the later stages of the study in Goldsboro. Although Liang et al. (Reference Liang, Grossman and Shi2014) did not measure soil microbial properties in roller-crimped plots, it is conceivable that soil enzyme activity may have also been greater in Goldsboro roller-crimped plots later in the study leading to faster root decomposition in Goldsboro compared with Kinston.
One of our main objectives in this study was to determine if root litter would decompose faster under elevated soil inorganic N levels. While disking cover crops generated higher levels of soil inorganic N than roller-crimping for varying lengths of time at both sites, the lack of termination effect at either site indicates that factors other than soil inorganic N played a more pivotal role in driving root decomposition in disked and roller-crimped plots. Soil inorganic N levels were also higher in pea, vetch and clover plots than in N00 plots for multiple sampling dates in Goldsboro and at litterbag burial in Kinston disked plots (Fig. 1), but faster root decomposition in these higher N plots was limited to week 8 where vetch roots in vetch plots decomposed faster than vetch roots in N00 plots (Fig. 3). Higher soil microbial activity in vetch plots than in N00 plots is a possible explanation for this finding (Liang et al., Reference Liang, Grossman and Shi2014).
Legume cover-crop root N release dynamics
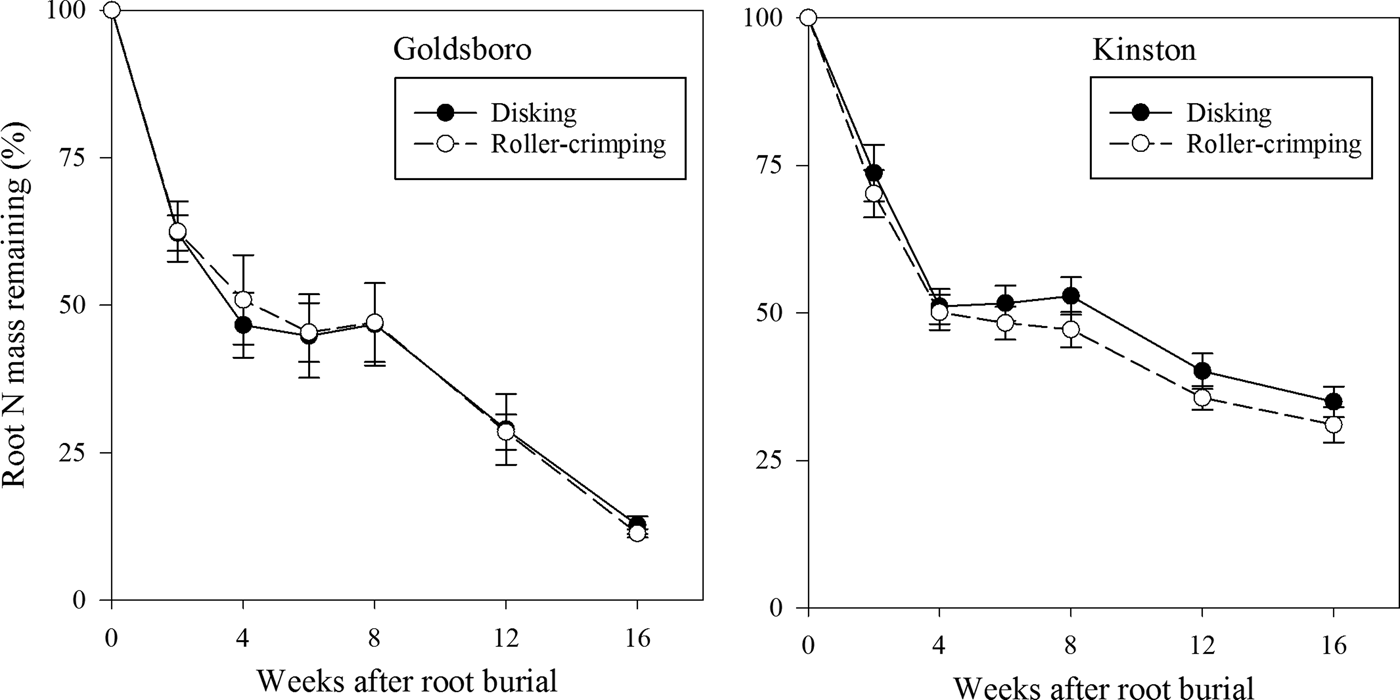
Legume cover-crop termination approach did not affect root N release (P = 0.71) in Goldsboro (Fig. 5). There also was not a significant higher-order interaction among termination, cover-crop treatment and time (P = 0.87) on root N release at this site. However, the cover-crop treatment × time interaction did have a significant effect on root N release (P = 0.008) in Goldsboro. Vetch roots buried in vetch plots released N at a faster rate than clover roots buried in clover plots through week 12 in Goldsboro (Fig. 6), whereas in N00 plots in Goldsboro vetch roots released N at a faster rate than clover roots at all but two sampling dates (weeks 8 and 16).

Figure 5. Percentage of original root N mass remaining over time averaged across cover crop treatments as a function of termination approach at Goldsboro and Kinston, NC study sites.

Figure 6. Percentage of original pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) root N mass remaining over time in cover crop treatment plots averaged across termination approaches at the Goldsboro, NC study site. Equations for these curves are given in Table 6.
Termination approach also did not affect root N release (P = 0.56) in Kinston (Fig. 5). Neither was there a significant higher-order interaction among termination, cover-crop treatment and time (P = 0.97). However, like in Goldsboro, the cover-crop treatment × time interaction was significant (P = 0.005) with vetch roots buried in vetch plots releasing N faster than clover roots buried in clover plots until week 12 (Fig. 7). In N00 and N150 plots, vetch roots released N faster than clover roots through the entire study (Fig. 7). Clover roots never released N at a faster rate than pea or vetch roots at either site, which was likely due to the lower initial N content in clover roots compared with pea and vetch roots (Table 5) (Luna-Orea et al., Reference Luna-Orea, Wagger and Gumpertz1996; Lawson et al., Reference Lawson, Fortuna, Cogger, Bary and Stubbs2012). In Goldsboro, pea and vetch roots buried in their respective pea and vetch plots as well as in N00 plots released N at similar rates (Fig. 6), while vetch roots buried in vetch plots released N faster than pea roots buried in pea plots during the 2nd and 8th week of the study in Kinston (Fig. 7).

Figure 7. Percentage of original pea (Pisum sativum), clover (Trifolium incarnatum) and vetch (Vicia villosa Roth) root N mass remaining over time in cover crop treatment plots averaged across termination approaches at the Kinston, NC study site. Equations for these curves are given in Table 6.
Table 5. Initial root chemical characteristics and root N mass (mg) at the beginning (N0) and end (N16) of the study for pea, clover and vetch at Goldsboro and Kinston, NC study sites.

1 Values within a column (separated by site) followed by the same letter are not significantly different (Tukey–Kramer's HSD; P < 0.05).
2 Only single composite samples from pea, clover and vetch were analyzed for lignin and multiple comparisons were not possible.
Root N release was best described in most cases by model 3 in Goldsboro, while in Kinston there was greater variability in model selection (Table 6). Only N released from vetch roots in cover-crop treatment, N00 and N150 plots was best described using the same model at both sites. The faster rate of vetch root N release from vetch, N00 and N150 plots compared with several other treatments in the study is in some, but not all, instances reflected by the higher N release rate constants associated with vetch treatments that were produced by these models (Table 6).
Table 6. Coefficients for the single exponential two-parameter model (1), single exponential three-parameter model (2), and double exponential four-parameter model (3) used to describe pea, clover and vetch root N release averaged over termination in pea, clover, vetch, N00 and N150 (Kinston only) plots at Goldsboro and Kinston, NC study sites. Model selection was based on the highest adjusted R 2 values.

1 Values within a column (separated by site) followed by the same letter are not significantly different (Tukey–Kramer's HSD; P < 0.05).
2 Model 1: f = ae −kx + ε. Model 2: f = a + be −kx + ε. Model 3: f = ae −k1x + be −k2x + ε. In models, f is the percent of original root N mass remaining at time x (week); a and b represent the percent root N mass at week 0; k is the N release rate constant, and ε is the random error term.
3 ‘Not valid’ indicates data exceeded maximum number of iterations and did not converge. Thus, model coefficients could not be validated.
In legume cover-crop-based production systems in the Southeastern USA, corn can be planted at cover-crop termination in roller-crimped systems (Parr et al., Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011, Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2014), or immediately after seed bed establishment in disked systems. From a nutrient management perspective, it is most critical that the legume cover crop releases a large enough portion of its N to meet corn demands during its period of rapid N consumption, beginning at the six-leaf stage (Magdoff, Reference Magdoff1991) and continuing until tasseling and silking (Jones, Reference Jones1985), which begins approximately 70 days after planting for the corn cultivar planted in this study. Clover and vetch shoots have been reported to release approximately 60% of their N within 1 month of being disked, and N released at this rate has been found to satisfactorily meet the demands of corn during its period of peak N demand (Wilson and Hargrove, Reference Wilson and Hargrove1986; Stute and Posner, Reference Stute and Posner1995). In this study, clover and vetch root N release rates closely mirrored the rates of clover and vetch shoot N release described in the previous studies, which was unexpected since legume cover-crop shoots generally release nutrients faster than roots (Buchanan and King, Reference Buchanan and King1993; Puget and Drinkwater, Reference Puget and Drinkwater2001). Within 2 weeks after root burial, vetch roots buried in vetch plots released 45–50% of their N at both sites, while pea and clover roots buried in plots of their respective species released 19–37 and 24–29.5% of their N, respectively (Figs. 6 and 7). After 4 weeks, these figures rose to 62–65, 49–57 and 39% for vetch, pea and clover, respectively. While it is clearly evident that roots released N rapidly, it is important that root-derived N be quantified to determine if its reservoir is large enough to play an important role in furnishing N for subsequent summer crops.
Our estimates of root biomass production for pea, clover and vetch indicated that just prior to termination, these species contained 53–71 and 36–47 kg N ha−1 in Goldsboro and Kinston, respectively (Table 2). Taking into account the rate at which these roots released N, within 1 month after termination, pea, clover and vetch roots would have released approximately 30, 26 and 46 kg N ha−1, respectively, in Goldsboro, and 18, 11 and 29 kg N ha−1, respectively, in Kinston. The availability of this N would coincide with the beginning of the period of rapid N uptake for corn (Magdoff, Reference Magdoff1991). By corn silking, pea, clover and vetch roots in Goldsboro would have released 38, 39 and 52 kg N ha−1, respectively, whereas in Kinston, 21 , 14 and 30 kg N ha−1, respectively, would have been released. In the NC Coastal Plain, Parr et al. (Reference Parr, Grossman, Reberg-Horton, Brinton and Crozier2011) found application of 168 kg N ha−1 to produce the highest corn yields for this cultivar. Our N release data suggest that roots from these species could potentially contribute 28–37 and 14–20% of this N recommendation in Goldsboro and Kinston, respectively.
Conclusions
This study suggests that in the humid Southeastern USA pea, clover and vetch roots decompose and release N rapidly and that termination by disking or roller-crimping does not affect these processes. Root decomposition and N release were driven primarily by favorable root biochemistry rather than available soil inorganic N. This study also highlighted large regional differences in root decomposition for these species, with roots in this study decomposing at a much faster rate than roots reported in studies from more temperate climates. Cover-crop roots released N rapidly in this study supplying subsequent corn with a small, but notable, quantity of N during its phase of peak N demand.
From a production standpoint, it is essential that farmers utilizing legume cover crops have an understanding of their total N contributions (shoots plus roots) that can be used by subsequent crops. This information can allow producers to better gauge yield expectations of summer crops and plan for additional fertilization, if needed. Our results indicate that vetch, in particular, may be an especially appealing option to farmers in the Southeastern USA, due to its large biomass production and relatively large contribution to soil inorganic N pools in the weeks following spring termination.
Acknowledgements
We wish to thank Dr Consuelo Arrellano, Sarah Seehaver, Matthew Brown, Sean Bloszies and North Carolina State University Environmental and Analytical Testing Service staff for their help with this project. The USDA-NIFA Organic Transitions program (grant number 2010-51106-21872) provided the primary support that made this project possible. We also thank Southern Sustainable Agriculture Research and Education (Southern SARE) for providing the graduate student research grant to Arun Jani.