Introduction
Behavioural economics is increasingly being applied to examine pathological decision-making across a range of psychological disorders and unhealthy behaviours (Bickel & Vuchinich, Reference Bickel and Vuchinich2000; Bickel et al. Reference Bickel, Johnson, Koffarnus, MacKillop and Murphy2014). One widely studied behavioural economic construct is delay discounting (DD), which reflects the degree to which delay to an outcome reduces its value. It is commonly measured using choices between small rewards that are available immediately and larger rewards that are available after a delay. Common rewards on DD measures include money (e.g. $40 today or $100 in 1 month) or food (e.g. two pieces of chocolate now or 10 pieces in 5 h). Independent of the reward type assessed, DD measures typically involve varying the size of the immediate reward and length of the delay to estimate the rate at which the delayed rewards lose value over time. As depicted in Fig. 1, a steeper rate of DD reflects greater preference for immediate rewards and is often conceptualized as a form of impulsivity (Ainslie, Reference Ainslie1975; Madden & Bickel, Reference Madden and Bickel2009).
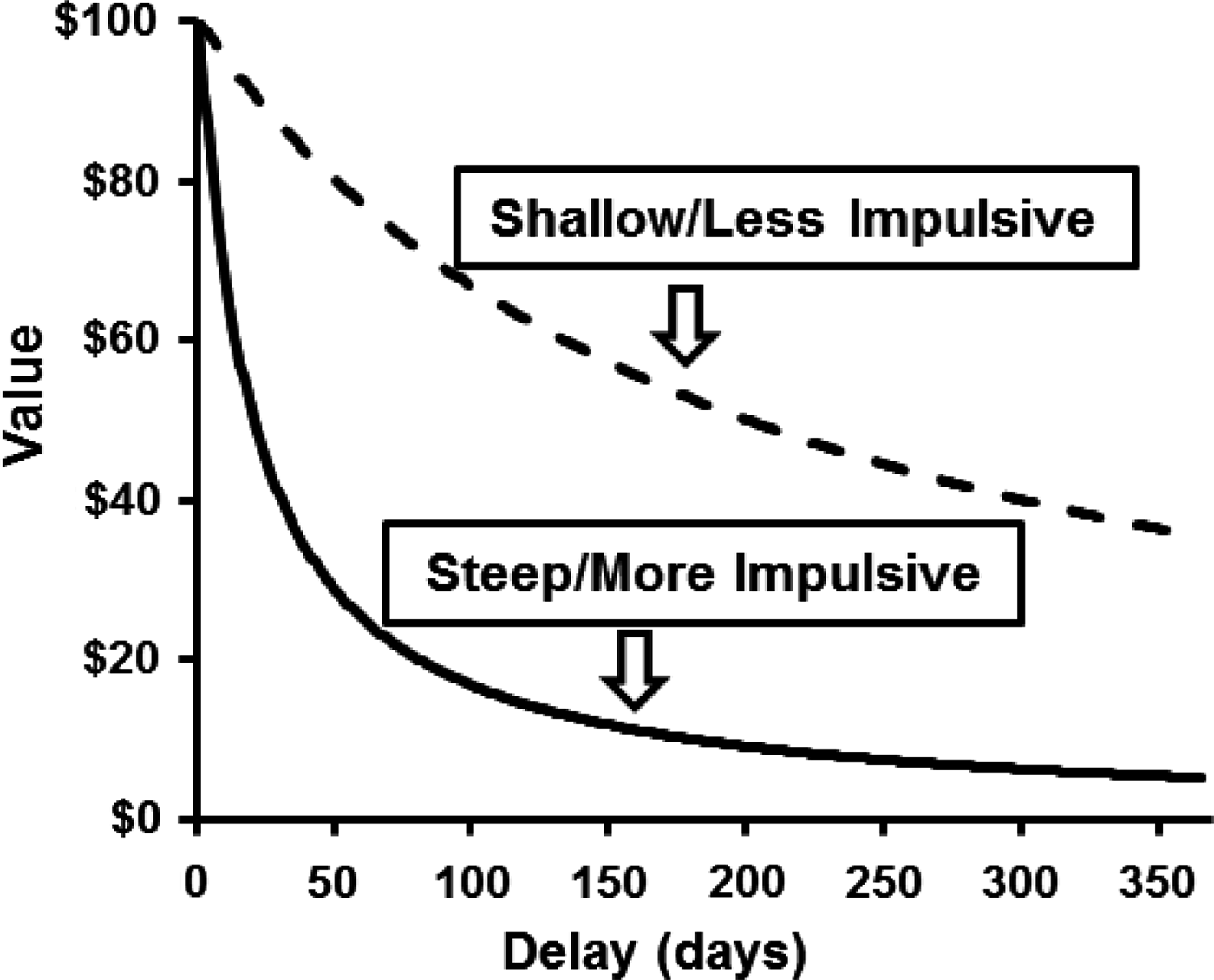
Fig. 1. Prototypical hyperbolic delay discounting curves showing the devaluation of a delayed monetary reward ($100) over a 1-year period. The solid line depicts a steep/more impulsive discounting rate; the dashed line depicts a shallow/less impulsive discounting rate.
Steep DD has been theorized to be a ‘trans-diagnostic’ feature of a number of clinical disorders (Bickel et al. Reference Bickel, Jarmolowicz, Mueller, Koffarnus and Gatchalian2012), including drug addiction (e.g. MacKillop et al. Reference MacKillop, Amlung, Few, Ray, Sweet and Munafo2011), attention-deficit/hyperactivity disorder (e.g. Scheres et al. Reference Scheres, Lee and Sumiya2008; Jackson & MacKillop, Reference Jackson and MacKillop2016) and, more recently, obesity (e.g. Bickel et al. Reference Bickel, Jarmolowicz, Mueller, Koffarnus and Gatchalian2012; Volkow & Baler, Reference Volkow and Baler2015). Obesity represents one of the most serious public health problems and is increasingly investigated using behavioural economics (Epstein & Saelens, Reference Epstein, Saelens, Bickel and Vuchinich2000). In particular, DD has emerged as a novel behavioural phenotype in obesity research, with a growing focus on exploring the clinical applications and neural correlates of DD in individuals who are obese. In the context of weight-loss interventions, individuals must repeatedly choose between immediate rewards from food, or delay/resist food to obtain future greater rewards of health. In this way, DD may play an important role in achieving long-term weight goals (e.g. Best et al. Reference Best, Theim, Gredysa, Stein, Welch, Saelens, Perri, Schechtman, Epstein and Wilfley2012; Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015). In addition, the neural processes underlying DD may also contribute to self-control and successful weight loss. Recent obesity research using functional magnetic resonance imaging (fMRI) has shown that DD decisions are associated with increased blood oxygen level-dependent (BOLD) activation in regions of the prefrontal cortex, parietal cortex and anterior insula (Kishinevsky et al. Reference Kishinevsky, Cox, Murdaugh, Stoeckel, Cook and Weller2012; Stoeckel et al. Reference Stoeckel, Murdaugh, Cox, Cook and Weller2013; Martin et al. Reference Martin, Pollack, McCune, Schulte, Savage and Lundgren2015; Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015). Specifically, the level of activation observed varies with the difficulty of the choices (i.e. greater activation for choices between two similarly valued rewards compared with rewards that differ widely in value). Reduced neural activation in areas of the frontal lobes (i.e. prefrontal cortex) has also been shown to predict weight gain over a 1- to 3-year period (Kishinevsky et al. Reference Kishinevsky, Cox, Murdaugh, Stoeckel, Cook and Weller2012; Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015).
Despite the increased focus on this form of decision-making, the existing literature in obesity is mixed. Prior research has typically used one of two designs, either a case–control design in which individuals who are obese are compared with normal-weight controls, or a dimensional design examining correlations between body size (e.g. body mass index; BMI) and DD. Case–control studies have found that individuals who are obese exhibit more impulsive DD compared with controls (Manwaring et al. Reference Manwaring, Green, Myerson, Strube and Wilfley2011; Lawyer et al. Reference Lawyer, Boomhower and Rasmussen2015; Mole et al. Reference Mole, Irvine, Worbe, Collins, Mitchell, Bolton, Harrison, Robbins and Voon2015; Schiff et al. Reference Schiff, Amodio, Testa, Nardi, Montagnese, Caregaro and Sellitto2015), although with mixed findings (Weller et al. Reference Weller, Cook, Avsar and Cox2008; Rasmussen et al. Reference Rasmussen, Lawyer and Reilly2010; Eisenstein et al. Reference Eisenstein, Gredysa, Antenor-Dorsey, Green, Arbeláez, Koller, Black, Perlmutter, Moerlein and Hershey2015). Similarly, in continuous designs, increased BMI has been shown to correlate with steeper DD (Chabris et al. Reference Chabris, Laibson, Morris, Schuldt and Taubinsky2008; Epstein et al. Reference Epstein, Jankowiak, Fletcher, Carr, Nederkoorn, Raynor and Finkelstein2014; Lu et al. Reference Lu, Tao, Hou, Zhang, Sun, Xu, Xu and Zhao2014; Dassen et al. Reference Dassen, Houben and Jansen2015; Garza et al. Reference Garza, Ding, Owensby and Zizza2016), but again with some inconsistency (Appelhans et al. Reference Appelhans, Woolf, Pagoto, Schneider, Whited and Liebman2011; Stoeckel et al. Reference Stoeckel, Murdaugh, Cox, Cook and Weller2013; Stojek et al. Reference Stojek, Fischer, Murphy and MacKillop2014; Hendrickson et al. Reference Hendrickson, Rasmussen and Lawyer2015).
There are a number of factors that may explain these mixed findings. First, some studies have reported significant sex differences between males and females. Weller et al. (Reference Weller, Cook, Avsar and Cox2008) found significantly greater discounting among women who were obese compared with non-obese women, but no significant differences for men. However, others have not found significant sex differences (e.g. Hendrickson & Rasmussen, Reference Hendrickson and Rasmussen2013; Lawyer et al. Reference Lawyer, Boomhower and Rasmussen2015). Second, the DD assessment method used may play a role, such as the type of reward or whether the outcomes are actually received. The majority of prior studies have used monetary rewards; however, some studies (e.g. Manwaring et al. Reference Manwaring, Green, Myerson, Strube and Wilfley2011; Hendrickson & Rasmussen, Reference Hendrickson and Rasmussen2013) have used food-based tasks that may better approximate real-world decisions. Moreover, while DD rates have been shown to be generally equivalent for real and hypothetical rewards (Johnson & Bickel, Reference Johnson and Bickel2002; Madden et al. Reference Madden, Begotka, Raiff and Kastern2003), this has not been systematically evaluated in obesity. Finally, the literature is heterogeneous with respect to the age of the participants examined, which is a relevant factor given the increased focus on obesity among children (e.g. Wang & Beydoun, Reference Wang and Beydoun2007). A number of studies have focused on child/adolescent samples (e.g. Duckworth et al. Reference Duckworth, Tsukayama and Geier2010; Fields et al. Reference Fields, Sabet and Reynolds2013; Lu et al. Reference Lu, Tao, Hou, Zhang, Sun, Xu, Xu and Zhao2014), but once again the results are mixed.
Given the increasing number of DD studies in obesity, a consolidated and quantitative review is timely. The current study is a meta-analysis of the link between obesity (operationalized via BMI) and DD. The study had three aims. The first was to characterize the relationship between DD and obesity in both case–control comparisons and continuous designs. The second aim was to investigate potential moderators of effects across studies (e.g. study design type, reward type, sample sex distribution, and age of the participants), as these parameters may reveal important nuances of the findings and be relevant for power calculations in future studies. The third aim was to examine the presence of publication bias on the aggregate findings.
Method
Study selection
The initial criterion for inclusion was any published peer-reviewed study reporting either a case–control comparison of DD between an obese/overweight group and controls, or a continuous relationship between BMI and DD. Studies were identified via searches of PubMed, Medline and PsycINFO databases (to 31 December 2015) using the Boolean terms (‘obesity’ OR ‘overweight’ OR ‘body mass index’ OR ‘BMI’) AND (‘discounting’)Footnote † Footnote 1 . Additional studies were identified via scanning relevant reviews (e.g. Vainik et al. Reference Vainik, Dagher, Dubé and Fellows2013; Volkow & Baler, Reference Volkow and Baler2015). Records were irrelevant if they used non-human models, were reviews, used a clinical population other than obesity, or if neither DD nor BMI were measured. Studies were restricted to DD of monetary or food rewards. To avoid inferences based on a small number of associations, a minimum of five effect sizes for any individual category or moderator were required. For studies reporting multiple effects, all effect sizes were included if DD was measured at different magnitudes or reward types; however, effect sizes were also aggregated within study (see below). Records were screened by two raters (M.A. and T.P.), with discrepancies resolved by a third rater (J.M.). The study selection flow diagram is shown in Fig. 2. A total of 39 studies were included, comprising 59 effect sizes (29 case–control; 30 continuous). The study selection procedure followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards (Stewart et al. Reference Stewart, Clarke, Rovers, Riley, Simmonds, Stewart and Tierney2015).

Fig. 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) inclusion flow diagram. BMI, Body mass index; DD, delay discounting.
Sample characteristics
Characteristics of the included studies are provided in Table 1; online Supplementary Table S1 provides a comprehensive listing of associations included. The aggregated sample size was 10 278, with an average sample size of 245 (range 19–2987). The average age was 28.40 (s.d. = 10.26) years. For case–control studies in adults, the criterion group was classified as obese (BMI ⩾ 30 kg/m2) for 75% of studies, and as overweight (BMI = 25–29.99 kg/m2) for 25% of studies. For case–control studies in child/adolescent samples, obesity was defined as BMI >95th percentile in two studies, >85th percentile in one study, and two studies did not report cut-offs.
Table 1. Meta-analytic sample a

DD, Delay discounting; HC, healthy controls; k, hyperbolic discounting rate; MCQ, Monetary Choice Questionnaire; AUC, area under the curve; MICT, multi-item choice task; SS, study-specific discounting index; PBF, percentage body fat; FCQ, adapted MCQ for food rewards.
a See online Supplementary Table S1 for a complete list of all effect sizes included in the meta-analysis.
Measures
The measures used to assess DD were a multi-item choice task (67% of studies), the Monetary Choice Questionnaire (MCQ; Kirby et al. Reference Kirby, Petry and Bickel1999) or a food-related MCQ (28%), or both task types (5%). In all cases, the measures assessed dichotomous choices between smaller-sooner and larger-later rewards, with a mean of 49 items (range 6–160). In terms of DD indices, 46% of studies used the hyperbolic discounting function (k) (e.g. Mazur, Reference Mazur, Mazur, Nevin and Rachlin1987); 46% used area under the curve (AUC) (e.g. Myerson et al. Reference Myerson, Green and Warusawitharana2001) and 8% used alternative indices.
Meta-analytic approach
Analyses were conducted using Comprehensive Meta-analysis 2.2 (Biostat; USA). The effect size of interest was Cohen's d (Cohen, Reference Cohen1988) for case–control studies and Pearson's r for continuous relationships. When these values were not reported or could not be generated based on reported statistics, corresponding authors were contacted to request data (five authors were contacted: three provided data; one indicated data were not accessible; and one did not respond). Effect directions for comparisons using AUC were inverted to be consistent with k values. Due to significant heterogeneity in methods, the primary analytic approach utilized a random-effects model; however, a fixed-effects approach is also reported to be comprehensive. For the fixed-effects approach, Cochran's Q and I 2 are two common indices of effect-size heterogeneity. Cochran's Q reflects the sum of squared differences between each weighted effect size and the overall mean whereas I 2 reflects the percentage variation within effect sizes that is explained by heterogeneity. To examine the influence of individual effect sizes, a ‘jack-knife’ analysis was conducted by systematically omitting each individual association and re-estimating the aggregate effect sizes. To evaluate over-representation by studies contributing multiple effect sizes, the primary analysis was repeated after consolidation of studies with multiple associations into a single effect size.
Moderator analyses examined systematic differences based on study type (case–control v. continuous), reward type (money v. food), sample sex distribution (females only v. mixed samples containing male and female participants), and age [child/adolescent (<18 years) v. adult]. As only one study reported data specific to male participants (Weller et al. Reference Weller, Cook, Avsar and Cox2008), the only viable means of examining sex differences was to compare studies with female-only samples to studies with mixed samples. Moderators were tested using the Q statistic associated with the between-groups difference in a mixed-effects analysis.
Five indices of publication bias were examined. The classic fail-safe N reflects the number of missing studies needed to render the overall effect non-significant. Funnel plots of effect size and standard error were examined via the two-tailed Begg–Mazumdar test (Begg & Mazumdar, Reference Begg and Mazumdar1994), which reports the rank correlation between effect size and standard error, and the one-tailed Egger's test (Egger et al. Reference Egger, Smith, Schneider and Minder1997), which regresses the standardized effect size on the inverse of the standard error. In both cases, significant values indicate an association between effect size and standard error, reflecting potential small study bias. A meta-regression between publication year and effect size was performed to examine change in effect size over time. Finally, adjusted estimates of effect size based on imputed missing studies were generated using a trim-and-fill approach (Duval & Tweedie, Reference Duval and Tweedie2000).
Results
Meta-analysis findings
The random-effects model revealed a medium effect size across studies (d = 0.43) that was highly statistically significant (p < 10−14) (Table 2). The forest plot is presented in Fig. 3. The fixed-effects analysis yielded comparable results (d = 0.48, p < 10−14), but with substantial heterogeneity across studies (Q = 267.79, p < 10−15; I 2 = 78.34). Re-running the primary analysis and systematically excluding each study generated comparable effect sizes and significance levels (d's = 0.40–0.44, p's < 10−14). Finally, after consolidation of effect sizes within studies contributing multiple effect sizes, a similar effect size was found (d = 0.44, p < 10−15).

Fig. 3. Forest plot providing effect sizes (standard difference in means; Std diff in means) and 95% confidence intervals (CI) for case–control and continuous comparisons. Individual data points reflect effect size ±95% CIs, with the size of data point proportional to the study sample size. Effects to the right of zero reflect steeper delay discounting (DD). Study letters refer to multiple comparisons within the same study.
Table 2. Delay discounting in relation to case–control and continuous study design, and reward type

k, No. of effect sizes; n, total number of unique individuals represented in each category; d, Cohen's d effect-size statistic; RE, random effects; p, statistical significance of effect size; OSR, range of effect sizes obtained from one-study-removed ‘jack-knife’ analysis; FE, fixed effects; Q, Cochran's Q test of homogeneity; p q , p value corresponding to Cochran's Q; I 2, proportion of variability due to heterogeneity.
a Heterogeneity statistics from the fixed-effects analysis.
Moderator analyses
Results of the moderator analyses are also presented in Table 2. First, although the effect size was notably larger for case–control studies (d = 0.55, p < 10−9), compared with continuous studies (d = 0.34, p < 10−6), the difference was only marginally significant (p = 0.050). Second, a larger effect size was found for tasks using food rewards (d = 0.74, p < 10−6) compared with monetary rewards (d = 0.41, p < 10−14); however, many fewer studies used food rewards and the difference in effect size was not statistically significant (p = 0.17). Comparable effect sizes were found for studies with female-only samples (d = 0.44, p < 10−9), compared with mixed (d = 0.43, p < 10−9), with no significant difference (p = 0.98). Finally, a larger effect size was present for child/adolescent studies (d = 0.61, p < 10−11), relative to adults (d = 0.40, p < 10−11), and the difference was statistically significant (p = 0.048).
Publication bias
The classic fail-safe N suggested there would need to be 4331 unpublished studies to render the primary meta-analytic outcome as non-significant. The Begg–Mazumdar test was significant (τ = 0.34, p < 0.001), indicating a positive association between the standardized effect size and the variance of the effect. However, the Egger's test intercept was non-significant (intercept = −0.63, p = 0.11). The funnel plot is presented in online Supplementary Fig. S1. Duval and Tweedie's trim-and-fill method did not suggest the presence of unpublished studies. Lastly, a meta-regression of publication year and effect size indicated a small magnitude but significant decrease in effect size over time (slope = −0.03, p < 0.0001).
Discussion
Despite the somewhat mixed existing literature on the link between DD and obesity in terms of individual studies, the present meta-analysis provides relatively strong evidence of a robust cumulative association between steeper discounting of future rewards and obesity. The overall effect size was of medium magnitude, and no single study had a substantial effect on the results. Moreover, moderator analyses revealed a significantly larger effect size in child/adolescent studies compared with adult studies, but did not indicate statistically significant differences between case–control versus continuous study designs, food versus money DD, or female-only versus mixed samples. However, in the case of study design, the differences approached statistical significance (p = 0.050), suggesting that DD may be more sensitive in group-level comparisons between individuals who are obese and controls as compared with continuous associations with body size. Finally, most of the indices did not indicate publication bias, but that was not uniformly the case. The Begg–Mazumdar test suggested possible over-representation of smaller studies with significant effects and the meta-regression suggested that the effect sizes grew smaller over time. With regard to this latter finding, it is possible it is a function of methodological differences in the published literature, with earlier studies being smaller, more deliberately designed examinations of the relationship that would be expected to reveal larger effect sizes, although this is necessarily speculative. In both cases, however, the magnitude of the effects reflected in the test statistics was generally modest, suggesting that the results probably are not substantially affected by publication bias. Collectively, these findings suggest that elevated DD for monetary and food rewards is a robust distinguishing feature of obesity that is relatively consistent across the types of study designs and DD methodologies examined here.
Parallels between obesity and drug addiction have been increasingly drawn (Volkow & Wise, Reference Volkow and Wise2005; Davis et al. Reference Davis, Curtis, Levitan, Carter, Kaplan and Kennedy2011), as both conditions are characterized by overconsumption and self-regulatory impairments. In addition, many contemporary foods have been suggested to have pharmacodynamic profiles that resemble psychoactive drugs (Kenny, Reference Kenny2011). Therefore, it is worthwhile to consider the present results in relation to a previous meta-analysis of DD in addiction studies (MacKillop et al. Reference MacKillop, Amlung, Few, Ray, Sweet and Munafo2011). That study similarly found evidence of a medium effect-size difference between individuals exhibiting addictive behaviours and controls. Thus, the effect size for case–control studies in obesity is generally consistent with findings in addiction. However, the previous meta-analysis did not examine continuous associations in addiction studies, which limits comparisons with continuous associations in the present study. Finally, individuals with addictive disorders have been shown to exhibit steeper DD for drug rewards compared with money rewards (Madden et al. Reference Madden, Petry, Badger and Bickel1997; Bickel et al. Reference Bickel, Odum and Madden1999; Petry, Reference Petry2001; Coffey et al. Reference Coffey, Gudleski, Saladin and Brady2003), which is generally consistent with the larger (albeit non-significant) effect size for food rewards in the present study.
These results offer further support for clinical applications of DD in the context of obesity. Steeper DD has been shown to predict worse addiction treatment outcomes (Yoon et al. Reference Yoon, Higgins, Heil, Sugarbaker, Thomas and Badger2007; Krishnan-Sarin et al. Reference Krishnan-Sarin, Reynolds, Duhig, Smith, Liss, McFetridge, Cavallo, Carroll and Potenza2007; MacKillop & Kahler, Reference MacKillop and Kahler2009), and a limited number of studies have reported similar findings in obesity. Steeper DD predicted decreased weight loss in children who are obese following a 16-week obesity intervention (Best et al. Reference Best, Theim, Gredysa, Stein, Welch, Saelens, Perri, Schechtman, Epstein and Wilfley2012). Less impulsive DD also predicted long-term success following a diet in a sample of obese adults (Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015). Finally, an emerging line of research has begun to explore novel interventions for reducing DD such as episodic future thinking (e.g. Peters & Buchel, Reference Peters and Buchel2010). Two recent studies found that episodic future thinking reduces ad libitum eating in individuals who are obese or overweight (Daniel et al. Reference Daniel, Stanton and Epstein2013) and healthy women (Dassen et al. Reference Dassen, Jansen, Nederkoorn and Houben2016).
Our results may also be relevant in the context of understanding the neural correlates of DD in obesity. Neuroimaging studies in obesity samples found that difficult, similarly valued DD choices were associated with activation in prefrontal, insular and parietal cortices (Kishinevsky et al. Reference Kishinevsky, Cox, Murdaugh, Stoeckel, Cook and Weller2012; Stoeckel et al. Reference Stoeckel, Murdaugh, Cox, Cook and Weller2013; Martin et al. Reference Martin, Pollack, McCune, Schulte, Savage and Lundgren2015; Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015). Lower activation in the prefrontal and parietal cortices also predicted greater weight gain across periods of 1–3 years (Kishinevsky et al. Reference Kishinevsky, Cox, Murdaugh, Stoeckel, Cook and Weller2012; Weygandt et al. Reference Weygandt, Mai, Dommes, Ritter, Leupelt, Spranger and Haynes2015). In general, these findings are consistent with fMRI studies in addiction samples (Boettiger et al. Reference Boettiger, Mitchell, Tavares, Robertson, Joslyn, D'Esposito and Fields2007; Monterosso et al. Reference Monterosso, Ainslie, Xu, Cordova, Domier and London2007; Hoffman et al. Reference Hoffman, Schwartz, Huckans, McFarland, Meiri, Stevens and Mitchell2008; Claus et al. Reference Claus, Kiehl and Hutchison2011; Amlung et al. Reference Amlung, Sweet, Acker, Brown and MacKillop2014) which have been taken as further evidence of common neurobiological substrates of obesity and addiction (Volkow & Wise, Reference Volkow and Wise2005; Volkow & Baler, Reference Volkow and Baler2015). However, an important caveat is that the fMRI studies in obesity have not included comparisons with healthy-weight individuals, an important future direction.
It is important to note a number of limitations and considerations for the current study. First, obesity was operationalized via BMI, which is a relatively coarse measure of body density that may overestimate obesity (World Health Organization, 2000) and fail to capture relevant physical characteristics, such as body fat and anthropometric features (World Health Organization, 2011). Equally, the literature search did not yield a sufficient number of studies on binge eating disorder or ‘food addiction’ (Davis et al. Reference Davis, Curtis, Levitan, Carter, Kaplan and Kennedy2011) to permit a valid meta-analysis. Although the majority of moderators examined were non-significant, these findings should not be considered definitive. Notably larger effect sizes were present for food DD tasks, case–control designs and child/adolescent studies, with the latter two moderators approaching statistical significance. However, these analyses necessarily had less statistical power as they focused on smaller groups of studies. As there were essentially no studies examining differences between males and females (with the exception of Weller et al. Reference Weller, Cook, Avsar and Cox2008), the analysis of sex differences did not provide a thorough analysis of these effects. Finally, there were only 10 effect sizes for discounting of food, and there was a relative absence of studies examining actual outcomes, with none of the food studies using real rewards. This may limit the generalizability of these findings to real-world food and money choices. In sum, examining DD in studies with higher resolution measures of obesity, DD tasks for real and hypothetical rewards, related forms of eating pathology, comparisons between males and females, and in paediatric populations are important future priorities.
In conclusion, this meta-analysis suggests that steep discounting of future food and money rewards is robustly associated with obesity at a medium effect size. This relationship appears to be largely independent of the study designs or DD assessment modalities examined here, and with generally limited influence of publication bias. Although there is a need to continue to refine the understanding of the connection between DD and obesity, these findings provide a strong basis for focusing on DD in aetiological and clinical approaches to obesity. Characterizing these cognitive processes and the underlying brain mechanisms has implications for how obesity is conceptualized and may reveal specific therapeutic targets.
Supplementary material
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0033291716000866
Acknowledgements
The authors are grateful to Drs Bradley Appelhans, Bart Golsteyn and Annette Horstmann for helpful correspondence and providing data to permit calculation of effect sizes. The authors also thank Ms Lana Vedelago for contributions to this project.
J.M.'s roles were partially supported by National Institutes of Health grant P30 DA027827 and the Peter Boris Chair in Addictions Research.
Declaration of Interest
None.







