Introduction
Invasion of ecosystems by exotic species is one of the greatest threats to biodiversity and community structure (Elton, Reference Elton1958). When introduced to new habitats by humans, invasive species interact with indigenous species in a wide range of habitats (Mills et al., Reference Mills, Rader and Belk2004; Rodriguez, Reference Rodriguez2006). Most studies of biological invasions have focused on the negative effects of exotic species on native biota (Human & Gordon, Reference Human and Gordon1996; Callaway & Aschehoug, Reference Callaway and Aschehoug2000). On the other hand, some studies show that indigenous species might either assist or resist invasion of exotic species (Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010; McGlone et al., Reference McGlone, Sieg and Kolb2011). In some cases, when invasions are made by insect species, microorganisms can contribute to the success of the invasion (Jiu et al., Reference Jiu, Zhou, Tong, Xu, Yang, Wan and Liu2007; Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010).
Invasive insects often carry a variety of fungal associates; and, in some cases, fungi can have intimate interactions with its insect vector (Lu et al., Reference Lu, Decock, Zhang and Maraite2008; Lu et al., Reference Lu, Zhou, De Beer, Wingfield and Sun2009a). Relationships between fungi and their insect vectors can be broadly grouped into mutualism (Chapela et al., Reference Chapela, Rehner, Schultz and Mueller1994; Farrell et al., Reference Farrell, Sequeira, O'Meara, Normark, Chung and Jordal2001; Aanen et al., Reference Aanen, Eggleton, Rouland-Lefevre, Guldberg-Froslev, Rosendahl and Boomsma2002) commensalism and antagonism (Madelin, Reference Madelin1966; Hofstetter et al., Reference Hofstetter, Cronin, Klepzig, Moser and Ayres2006). These interactions can have important effects on the behavior, reproductive success, population dynamics and evolution of both invasive insects and their fungal associates. Whether or not fungal associates play a significant role in facilitating or inhibiting the invasion of insect vectors remains to be answered.
The red turpentine beetle (RTB), Dendroctonus valens LeConte (Coleoptera: Curculionidae, Scolytinae), is an invasive pine-killing bark beetle in China that was introduced from North America (Yan et al., Reference Yan, Sun, Don and Zhang2005). Over six million Chinese pines, Pinus tabuliformis Carriére, have been killed by RTB since 1999 in Shanxi Province (Li et al., Reference Li, Chang, Song, Wang and Chang2001). One of the most striking characteristics of RTB is its widespread association with ophiostomatoid fungi (Wingfield, Reference Wingfield1983; Lu et al., Reference Lu, Zhou, De Beer, Wingfield and Sun2009a). Adult RTBs carry fungi on their exoskeleton; pioneer beetles select a suitable pine host, bore into trees and release pheromones to recruit conspecifics; afterwards, the host is killed by mass attack (Zhang & Sun, Reference Zhang and Sun2006; Zhang et al., Reference Zhang, Sun and Clarke2006; Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010). Adult beetles mate, construct egg galleries, lay eggs and inoculate fungi in the phloem layer of the tree (Smith, Reference Smith1971). Fungi hyphae then spread in the phloem adjacent to eggs. When eggs hatch, larvae gregariously feed on the phloem colonized by fungi (Zhang et al., Reference Zhang, Chen and Zhang2002). In North America, Leptographium procerum and L. terebrantis have been isolated from RTB (Harrington & Cobb, Reference Harrington and Cobb1983; Wingfield, Reference Wingfield1983; Owen et al., Reference Owen, Lindahl, Wood and Parmeter1987). In China, Ophiostoma minus, L. sinoprocerum and L. procerum have been isolated from cuticule and galleries of D. valens (Lu et al., Reference Lu, Decock, Zhang and Maraite2008; Lu et al., Reference Lu, Zhou, De Beer, Wingfield and Sun2009a). We have isolated several species of fungi, including O. minus, L. sinoprocerum and L. procerum, from phloem adjacent to RTB larvae at different developmental stages in the field (Wang et al., unpublished data). Fungi benefit from the association by being transported to new plant resources (i.e. pine trees) by adult beetles and also may facilitate invasion of RTB by contributing to overcome host defense (Lu et al., Reference Lu, Decock, Zhang and Maraite2009b; Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010). However, the influence of these fungal associates on RTB larvae has not been studied. Volatile chemicals produced by host pine trees can inhibit fungal growth (Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010), but the effect of volatile chemicals produced by beetles on fungal growth is unknown. Volatile chemicals released by beetles are produced via detoxification of host defensive phytochemicals and often function as aggregation pheromones (Renwick et al., Reference Renwick, Hughes and Ty1973; Shi & Sun, Reference Shi and Sun2009).
The goal of this study was to determine whether RTB fungal associates from native and invaded ranges affect feeding activity, development and survival of larvae, and to explore whether chemicals produced by adult beetles affect fungal growth. We also investigated if chemicals produced by adult beetles were also produced in different larval instars. We tested five RTB fungal isolates, two from North America, L. terebrantis and L. procerum, and three from China, O. minus, L. sinoprocerum and L. procerum. Understanding the interactions between invasive and indigenous species is important because these relationships can have deep ecological consequences, including cascading effects through trophic levels, changes in the community structure and evolutionary changes. Studying these interactions can also open new windows to understand the biology of invasion and to develop new control methods for D. valens and many other notorious pests.
Materials and methods
Beetle collection and rearing
RTB larvae were collected from infested Chinese pine, P. tabuliformis, in the Tunlanchuan Forestry Preserve (N 37°48′, E 111°44′; average elevation 1400 m), Gujiao City, Shanxi Province in September 2008. These larvae were used in the ‘Effects of fungi on RTB larvae’ experiment (below). Approximately 4000 larvae were collected from two infested pine trees. Two thousand 2nd to 3rd instar larvae were selected and reared on artificial medium (water 200 ml, phloem 100 g, agar 7.5 g) at 25°C. Medium composition was adapted after Wallin & Raffa (Reference Wallin and Raffa2000) and Kopper et al. (Reference Kopper, Illman, Kersten, Klepzig and Raffa2005). Phloem was scraped from healthy mature P. tabuliformis, freeze-dried (Christ Alpha 2–4 LD) for 48 h, and ground with a laboratory blender (model 24CB10C, Waring Commercial, Torrington, CT, USA). Larvae were fed on the artificial medium for one week prior to experiments. Lethargic larvae were excluded. Larvae collected in September 2010 at the same location were used in the ‘RTB larvae hindgut volatiles extraction and analysis’ experiment (below); these larvae were collected from 16 pine trees.
Fungal isolates tested
Ophiostoma minus, L. terebrantis and L. procerum isolates came from the culture collection of the Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa (Lu et al., Reference Lu, Zhou, De Beer, Wingfield and Sun2009a). Leptographium sinoprocerum is a newly described species associated with RTB in China (Lu et al., Reference Lu, Decock, Zhang and Maraite2008), and its isolates came from the culture collection of the Research Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry. Information on the fungal isolates used is shown in table 1.
Table 1. Fungal isolates tested in the experiments. All isolates were recovered from Dendroctonus valens adults.
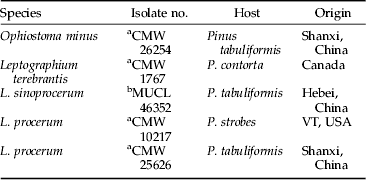
a CMW, Culture Collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa.
b MUCL is a part of the Belgian Coordinated Collections of Microorganisms, BCCMTM.
Effects of fungi on RTB larvae
A different artificial medium (water 400 ml, phloem 15 g, agar 15 g) was used to test the effects of fungi on RTB larvae boring, weight change and mortality. To make the medium, ground phloem and the agar-water mixture were autoclaved separately for 30 min at 126°C and 0.14 mPa of pressure, and then mixed together in a 500 ml conical flask; 15 milliliter of the mixture were poured in Petri dishes (65 mm diameter×15 mm high). After cooling, each agar-phloem disk was removed from the base of the Petri dish and transferred to a larger Petri dish (75 mm diameter×15 mm high), thus providing a gap surrounding the medium to accommodate beetles. Fungal isolates were individually inoculated on the surface of the medium and incubated at 25°C in darkness for 20 days. Although fungal growth rate on the medium was different, all the media discs were fully covered by fungi after 20 days.
Five 2nd to 3rd instar larvae were randomly chosen, weighed together and placed into the gap surrounding the medium in the Petri dish. The Petri dish was sealed with parafilm (Parafilm M, Pechiney Plastic Packaging, Menasha, WI, USA) to maintain moisture. The assays were conducted in a dark growth chamber (model LRH-250, Shanghai Yiheng Biotech Co., Ltd.) at 25°C. At least ten replications were done for each treatment (media with fungus). A group of 14 petri dishes with fungi free media were used as controls.
Effects of fungal isolates on larval feeding activity, larval development and larval mortality were evaluated. Total number of larvae inside the media and larvae outside of the media for each treatment and control at 6, 12, 24, 48 and 96 h were recorded. For data analysis, average number of larvae inside the media (ALI) and the average number of larvae outside the media over all time periods (ALO) for each treatment and control was calculated. Five larvae in each Petri dish were weighed together after six days. Weight change was calculated by subtracting the recorded weight after six days from the recorded weight at the beginning of the experiment. Petri dishes with dead beetles were excluded. Number of dead larvae was recorded 18 days later for each treatment and control.
RTB larval hindgut volatiles extraction and analysis
Due to the difficulty of precisely distinguishing larval instars, larvae were grouped as first/second, second/third, third/fourth, and fourth/fifth instar by comparing head capsule size. Five larvae in each group were randomly chosen, then dissected, and hindguts were immediately immerged into a 250-μl vial insert that was inside a 2-ml glass vial (Agilent, vial insert, 250 μl glass with polymer feet); 20 μl of hexane were used as solvent and 100 ng heptyl acetate (TCI Shanghai Development Co., Ltd. purity 95%) were added as internal standard (Shi & Sun, Reference Shi and Sun2009). Each instar group had five replications (five vials). Vials were stored at −20°C before chemical analyses.
Extracts were identified on a Hewlett-Packard 6890 gas chromatograph-mass spectral detector (GC-MS) equipped with DB-WAX column (60 m×250 μm×0.15 μm). The temperature program was 50°C for 1 min, 5°C min−1 to 95°C, 2°C min−1 to 110°C, 5°C min−1 to 220°C, 10°C min−1 to 230°C. The flow of nitrogen was 1.0 ml min−1. Aliquots of extracts (2 μl) were injected splitless at 250°C. Compounds were identified by comparing retention times and mass spectra of synthetic standards. Chemicals used as standards were (s)-cis-verbenol (Sigma-Aldrich, Inc, purity 95%), (+)-verbenone (Acros Organics, purity 94%), (1R,-)-myrtenol (Acros Organics, purity 95%), and (-)-myrtenal (Acros Organics, purity 98%).
Extracts were quantified on an Agilent 7890 GC-FID equipped with the same DB-WAX column mentioned above. Compound quantification was based on the internal standard (IS 100 ng of heptyl acetate in each sample; assuming similar or identical response factors between the analytes and the IS). Nitrogen was used as the carrier gas at a constant flow of 1.0 ml min−1, and the injector and detector temperatures were 220°C and 300°C, respectively. Column temperature was 50°C for 1 min, 5°C min−1 to 95°C, 2°C min−1 to 110°C, 5°C min−1 to 220°C, 10°C min−1 to 230°C.
Effects of RTB hindgut volatiles on fungal growth
For each fungus, four chemicals (verbenol, verbenone, myrtenol and myrtenal) in three concentrations (0.1, 0.5 and 1 mol l−1), and n-hexane controls were tested. Each treatment and a control had five replications. The method was modified from Hofstetter et al. (Reference Hofstetter, Mahfouz, Klepzig and Ayres2005). A 5-mm diameter disk of 2% malt extract agar (MEA) medium colonized by one of the five test fungal isolates (incubated at 25°C in darkness for three weeks) was placed on the center of a 60×15 mm Petri dish of 2% MEA. The four volatiles tested in the experiment were (s)-cis-verbenol, (+)-verbenone, (1R,-)-myrtenol and (−)-myrtenal. Each compound was dissolved in n-hexane (Beijing Chemicals Works) to get a concentration of 1 mol l−1, 0.5 mol l−1 and 0.1 mol l−1.
For each treatment, 20 μl of test solution were absorbed onto a sterile filter paper (15×15 mm) and the paper was placed on the lid of a Petri dish containing fungal culture. Plates were sealed with parafilm, placed upside down and incubated in darkness at 25°C. Linear fungal growth was measured in four directions (0°, 90°, 180°, 270°) at 24-h intervals. Tests were continued until one of the fungal treatments reached the edge of the Petri dish. Fungal growth in the four directions was averaged for each replicate.
Statistical analysis
In the ‘Effects of fungi on RTB larvae’ experiment, we tested for fungal effects by comparing feeding activity (proportion of ALI and ALO) from all the treatments, including the control, with a Pearson's chi-square test (two-tail, α=0.05) (i.e. null hypothesis fungiA=fungiB=fungiC=fungiD=fungiE=control). Differences in feeding activity between each treatment and the control were also tested with a Pearson's chi-square test (two-tail, α=0.05) (i.e. null hypotheses fungiA=control, fungiB=control, etc.) (Rosner & Sun, Reference Rosner, Sun, Rosner and Sun2004). Average weight change of five larvae in each Petri dish among five fungal isolates and a control was compared using one-way ANOVA followed by a LSD multi-comparison. Larval mortality among fungal isolates was compared by Fisher's exact test (two-tail α=0.05) (Rosner & Sun, Reference Rosner, Sun, Rosner and Sun2004).
In the ‘RTB larval hindgut volatiles extraction and analysis’ experiment, quantity of larval hindgut volatiles was compared by one-way ANOVA (two-tail, α=0.05), followed by LSD multi-comparison for each chemical among larval instars.
In the ‘Effects of RTB hindgut volatiles on fungal growth’ experiment, for each fungal isolate, average fungal linear growth rate (fungal growth divided by number of days cultured) was compared at three specific concentrations separately by one-way ANOVA (chemicals as factor) followed by a Dunnett's multi-comparison with hexane as control. All tests were performed with the statistical software SPSS for windows 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Effects of fungi on RTB larvae
The feeding activity among treatments, including the control, was significantly different (Pearson's χ2=17.92, df=5, N=380, P<0.01). In O. minus colonized-media, larvae had a low feeding activity (52%), significantly different from the control (77%) (Pearson's χ2=9.78, df=1, N=135, P<0.01). Feeding activity of L. sinoprocerum (81%) (Pearson's χ2=0.37, df=1, N=115, P=0.54), L. terebrantis (78%) (Pearson's χ2=0.02, df=1, N=135, P=0.89), L. procerum (North America, 70%) (Pearson's χ2=0.85, df=1, N=125, P=0.36) and L. procerum (China, 69%) (Pearson's χ2=0.80, df=1, N=140, P=0.28) was not significantly different from the control (fig. 1).

Fig. 1. Percent of Dendroctonus valens larval feeding activity on phloem-agar media inoculated with fungi and fungi-free control at 6, 12, 24, 48 and 96 h. ALI, average number of larvae inside the media over all the time periods; ALO, average number of larvae ouside the media over all the time periods. Pearson's chi-square test was used to compare boring percent between each fungal colonized media and fungal-free control; control, fungal-free media; O.min, Ophiostoma minus-colonized media; L.sin, Leptographium sinoprocerum-colonized media; L.te, L. terebrantis-colonized media; L. proNA, L. procerum North American isolate-colonized media; L. proC, L. procerum Chinese isolate-colonized media.
Mean weight change was significantly affected by fungal isolates after six days (F 5,66=6.00, P<0.001). Mean weight change on media discs colonized by O. minus decreased and was significantly different from that of L. terebrantis (P<0.001), L. procerum (Chinese isolate, P<0.001) and control (P<0.01) (fig. 2).

Fig. 2. Average weight change of five Dendroctonus valens larvae over six days of feeding on phloem-agar media inoculated with fungi. Bars indicate standard error. Bars with a different letter are significantly different (α=0.05) (LSD multiple comparisons). Abbreviations for fungal names are the same as those in fig. 1.
Total larval mortality was 12%, and no significant differences were found in mortality among the treatments, including control (Fisher's exact test=10.00, P=0.07).
RTB larval hindgut volatiles extraction and analysis
We identified verbenol, verbenone, myrtenol and myrtenal from the hindgut extracts of RTB larvae. From these volatiles, verbenone and myrtenol were major components and verbenol and myrtenal were minor components in all larval stages. Verbenol and myrtenal were not detected in first/second and fourth/fifth instar larvae (table 2).
Table 2. Mean (±SE) of hindgut volatiles extracted from different stages of Dendroctonus valens larvae.
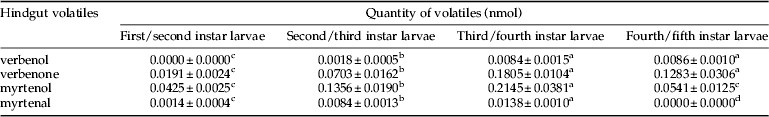
In each row, values with the same letter in superscript are not significantly different (α=0.05; LSD multi-comparison).
There were significant differences among larval stages in volatile contents verbenone (F 3,16=34.07, P<0.001), myrtenol (F 3,16=24.48, P<0.001), verbenol (F 3,16=40.53, P<0.001) and myrtenal (F 3,16=87.92, P<0.001).
Effects of RTB hindgut volatiles on fungal growth
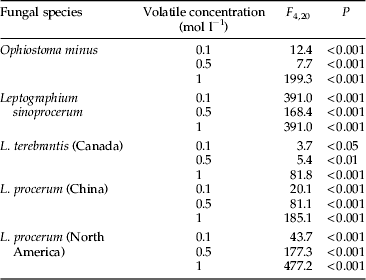
For each fungus, all the three concentrations of volatile chemicals significantly affected linear growth rates (table 3). Multi-comparison with the hexane control showed that O. minus was significantly inhibited by verbenol, myrtenol and myrtenal at 1 mol l−1. Myrtenal caused the lowest linear growth rate, followed by myrtenol and verbenol. However, growth of O. minus was enhanced by verbenol, myrtenol and myrtenal at low concentration (0.1 mol l−1) and was significantly different from control. Leptographium sinoprocerum was significantly inhibited by all four volatiles in the three concentrations compared to the control. Leptographium terebrantis was only significantly inhibited by verbenol, myrtenol and myrtenal at 1 mol l−1. For the Chinese isolate L. procerum, growth rate was inhibited by verbenol, myrtenol and myrtenal at 0.5 mol l−1 and 1 mol l−1; myrtenal significantly inhibited growth rate at 0.1 mol l−1. The North American isolate L. procerum was significantly inhibited by verbenol, myrtenol and myrtenal at 0.5 mol l−1 and 1 mol l−1; myrtenal also significantly inhibited the fungal growth rate at 0.1 mol l−1 (fig. 3).

Fig. 3. Linear growth rate (±SE) for each fungus on 2% malt extract agar in the absence (control) or presence of different concentrations of Dendroctonus valens hindgut volatiles. For each fungal species/isolate, average fungal linear growth rate (fungal growth divided by number of days cultured) was compared at three specific concentrations separately by one-way ANOVA (chemicals as factor) followed by Dunnett's multi-comparison with hexane as control. *P≤0.05; **P≤0.01; ***P≤0.001. Abbreviations for fungal name are the same as those in fig. 1.
Table 3. One-way ANOVA analyzing effects of four volatiles (verbenone, verbenol, myrtenol, and myrtenal) in three concentrations on linear growth rate of five fungal isolates.
Discussion
RTB larvae fed on O. minus had reduced larval boring activity as well as a decrease in body weight. The mechanisms by which O. minus caused this detrimental effect remains to be studied, but one way in which the fungus could achieve this includes a change in the nutritional characteristics of the diet, which may involve reduced protein and caloric content. In North America, O. minus is not reported as an associate of RTB, but in China this fungus is closely related to both larvae (Wang et al., unpublished data) and adults (Lu et al., Reference Lu, Zhou, De Beer, Wingfield and Sun2009a). By affecting larval performance, O. minus may have negative effects at the population level, and this may have constituted an obstacle to its invasion in China. Leptographium (from native ranges and invaded ranges) fungi had no significant effects on RTB larvae. Boring percent and body weight change were not significantly different between larvae feeding on L. sinoprocerum, L. terebrantis and L. procerum (both isolates) and control. Larvae exhibited low mortality, suggesting that these fungi may not lead directly to death. Previous work suggests that the most important role of these fungi is to assist RTB to overcome tree defense (Lu et al., Reference Lu, Wingfield, Gillette, Mori and Sun2010). In China, L. sinoprocerum and L. procerum (Chinese isolate) are most likely fulfilling this role. Further studies should focus on the establishment dynamics of fungal associates in RTB galleries along with the development of larvae.
Fungi growth, in all isolates, was dramatically inhibited by verbenol, myrtenol and myrtenal at high concentration (1 mol l−1). Inhibition by these chemicals decreased at 0.5 mol l−1. At 0.1 mol l−1, these chemicals caused little or no growth inhibition in all isolates, with the exception of O. minus, whose growth was stimulated. Verbenol and myrtenol have also been detected in pine trees (Fernandez et al., Reference Fernandez, Monnier, Ormeno, Baldy, Greff, Pasqualini, Mevy and Bousquet-Melou2009); therefore, the enhanced growth of O. minus may reflect an adaptation of this fungus to host pines and vector beetles. Myrtenol is the dominant compound in the hindgut of RTB in China but is not present in the hindgut of North American beetles (Luxova et al., Reference Luxova, Graves, Gries, Hamud and Seybold2007). Myrtenol is an efficient fungal growth inhibitor and might be an unknown chemical weapon of RTB to restrain growth of detrimental fungi. Although most fungi are only inhibited at high concentrations, we suggest that these chemicals may have a significant ecological function by regulating fungi prevalence in RTB galleries. Verbenol, myrtenol and myrtenal are constantly produced by all larval instars; and, thus, they could have a relatively high concentration in the galleries, which are small spaces. These volatiles may also synergistically affect fungal growth.
Although invasive species can thrive in introduced ranges, the invaded communities may resist invasion through a variety of ecological mechanisms, including predation, competition or disease (Hunt & Yamada, Reference Hunt and Yamada2003; Levine et al., Reference Levine, Adler and Yelenik2004; Parker & Hay, Reference Parker and Hay2005). Our results show a mutually antagonistic relationship between an invasive bark beetle, D. valens, and an indigenous fungal associate, O. minus, in China, which might reflect a natural defense of a native species against an exotic invader. RTB probably adapted to overcome this defense mechanism by suppressing detrimental fungus growth and regulating fungal abundance in the galleries using a chemical arsenal. Detecting these particular interactions is not only crucial to comprehend the complexity of insect multitrophic interactions but also to understand the mechanisms of invasion in organisms as aggressive as D. valens, with over 500,000 ha of invaded forest (Yan et al., Reference Yan, Sun, Don and Zhang2005). From this perspective, our study may also set the base to develop innovative control and management strategies in this and other systems.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (30921063) and the CAS Key Knowledge Innovation Program (KSCX2-EW-N-05). We thank Dr Xingyao Zhang (Chinese Academy of Forestry) for providing the fungal isolate L. sinoprocerum (MUCL 46352). We are especially grateful to Drs Donald R. Owen (California Department of Forestry and Fire Protection, Redding, CA), Zhudong Liu, Zhanghong Shi and Li Li (Institute of Zoology, Chinese Academy of Sciences) for their review of the earlier version of this manuscript. We also thank three anonymous reviewers for their constructive comments, which improved greatly the quality of this paper.








