Introduction
The conservation of plant genetic resources is a global priority (Gepts, Reference Gepts2006). Maize and rice have been extensively collected (Koo et al., Reference Koo, Pardey, Wright, Bramel, Debouck, van Dusen, Jackson, Rao, Skovmand, Taba and Valkoun2004), but further collection is warranted (Henry, Reference Henry2006). Poor farmers often retain high levels of crop diversity (Maxted et al., Reference Maxted, Guarino, Myer and Chiwona2002).
In Panama, rice (Oryza sativa L.) and maize (Zea mays L.) are the primary staple crops (Contraloría, 2001) and swidden agriculture (short cropping periods where fire is used to clear land, followed by long periods of fallow) practised by poor farmers is common (Fischer and Vasseur, Reference Fischer and Vasseur2000). Panama was the bridge over which maize passed from its centre of origin (Mexico) into the maize's largest centre of diversity (South America; Freitas et al., Reference Freitas, Bendel, Allaby and Brown2003). It is also adjacent to the first report of rice cultivation in the New World (c. 1517) and was an early principal port for rice trading (Spijkers, Reference Spijkers1983). There is great natural genetic diversity in Panama due to high levels of environmental heterogeneity (Condit et al., Reference Condit, Pitman, Leigh, Chave, Terborgh, Foster, Núñez, Aguilar, Valencia, Villa, Muller-Landau, Losos and Hubbell2002).
Historically, there have been few collection missions for rice (Lawerence, Reference Lawerence1984) and maize (Kuleshov, Reference Kuleshov1930) in Panama. Modern Panamanian collection missions have been severely limited due to scarce funding, and national germplasm holdings currently total only a few hundred accessions for both maize and rice (CNRFP, 1995). Internationally, the germplasm bank of the International Maize and Wheat Improvement Center (CIMMYT) harbours few Panamanian maize accessions. Specifically, CIMMYT maize germplasm records (Taba et al., Reference Taba, Díaz, Rivas, Rodríguez, Vicarte, Norgaard, Carlos and Brandon2003) indicate that most Panamanian accessions date back to 1958 and only a few are from Panama's Azuero region, which is the heartland of Panamanian agriculture (Jaen-Suarez, Reference Jaen-Suarez1978).
Farmer nomenclature can obscure the relationships between populations (Bellon, Reference Bellon2004) and heterogeneous populations of self-pollinating crops are common (Fukuoka et al., Reference Fukuoka, Suu, Ebana, Trinh, Nagamine and Okuno2006). Notions of ‘modern’ and ‘traditional’ germplasm are complex. One distinguishing attribute is that ‘modern’ varieties/germplasm typically have been improved by formal plant breeding, whereas farmers have managed ‘traditional’ material with or without improvement (Camacho-Villa et al., Reference Camacho-Villa, Maxted, Scholten and Ford-Lloyd2006). Characterization of germplasm after collection is required before utilization can occur (Henry, Reference Henry2006). The objective of this study was to evaluate crop genetic diversity of maize and upland rice farmed by poor farmers in Panama. The study provides baseline information on phenotypic diversity and how this relates to farmers' classifications.
Materials and methods
Study site
The study was carried out in Panama in the tropical forest uplands of Herrera province (Azuero region) where poor farmers continue to practise swidden agriculture. The climate is wet (2000 mm/year) and warm (24°C) and cropping occurs almost exclusively during the rainy season. Steep erosion-prone hills dominate the uplands, and both maize and rice are grown in dry-land conditions on these steep slopes. Tropical forest is the natural vegetation type and fallow fields resemble pioneer or secondary forest growth before being slashed and burned for agricultural production.
Collection strategy
Towns in the upland zone (which were accessible by local transport and not more than 3-h walking distance) were divided into two groups on the basis of agricultural practice (swidden and transition to permanent agriculture). Five towns were randomly selected from each of these two groups. Public meetings were held in each town to recruit farmer participants in December 2004. Farmers attended the meetings voluntarily and those who were interested signed up for the collecting mission. Farmers from nearby towns, who attended the meetings, were allowed to participate as well. Participating farmers were asked to share seed from up to 50 rice panicles and 50 maize ears for each variety they cropped. The study defined a variety as a group of plants or seed managed by a given farmer as a distinctly named unit. Seed from each panicle and ear was packaged separately, treated for insect pests with phosphorous hydride and stored.
A structured interview lasting ~10 min was administered to individual farmers to collect basic data about each variety they cropped. Specifically, each farmer was asked to identify each population by name, indicate whether each population was modern (i.e. the product of a crop improvement program) or traditional (i.e. not the product of a crop improvement program), and specify whether they practised swidden agriculture or were making a transition to permanent plot agriculture. Farmers who provided rice varieties that exhibited heterogeneity during population characterization were visited again and questioned about the origin of the heterogeneity, using an unstructured interview.
Morphological characterization
Varieties for which sufficient seed was collected were evaluated in an agromorphological trial in Panama's lowlands. The trial employed a randomized complete block design with two replicates. The soil at the trial site was a clay loam of light yellow colour, topographically flat, and uniform in appearance, with a pH of 5.6 and an organic matter content of 2%. The area has an average annual rainfall and temperature of 2000 mm and 27°C, respectively (IGNTG, 1988). Mechanical tillage was used to prepare the site. All plots were hand seeded in the second week of July 2005 using a seeding density of 106 seeds/m2 in three rows (30 cm × 30 cm) for rice and a seeding density of 4.7 seeds/m2 in two rows (75 cm × 30 cm) for maize. Trial conditions differed from swidden agriculture in that the soils were richer, the planting density was higher and chemical fertilizer was used instead of ash. Still, the trial site and management mimicked swidden conditions in terms of dry-land planting technique, hand seeding and manual weeding.
One certified variety was planted for both maize (Guararé) and rice (Orisíca) as a check. Commercial farmers grow Guararé throughout Panama and Ministry of Agriculture extensionists encourage upland rice farmers to plant Orisíca. Certified seed of both check varieties was obtained from Panama's Institute for Agricultural Research (IDIAP). At planting 100 kg/ha of 12–24–12 (N–P–K) applied by drilling and 50 kg/ha of 46–0–0 (N–P–K) was applied 3 weeks after emergence by top-dressing. Weeds were controlled manually and pests were controlled with a single spraying of (RS)-ciano-3-fenoxibencil(1RS,3RS;1RS,3SR)-3(2,2-diclorovinil)-2,2-dimetilciclopropanocarboxilato 6.00% at a rate of 9 ml/l. A total of 57 and 49 traits were measured for maize (described in IPGRI, 1991) and rice (described in FAO, 1980), respectively. Traits included nominal (colours, presence of botanical features and type of growth habit), discrete (counts of a botanical features e.g. ears) and continuous (measurements of botanical features e.g. heights, weights) variables (Tables 1 and 2). All weight metrics were adjusted to 12% moisture content for maize and 10% for rice.
Table 1 Means and ranges for selected maize and upland rice traits, and least-squared means for traits that differed between ‘modern’ and ‘traditional’ populations for populations collected from farmers in Panama, 2005
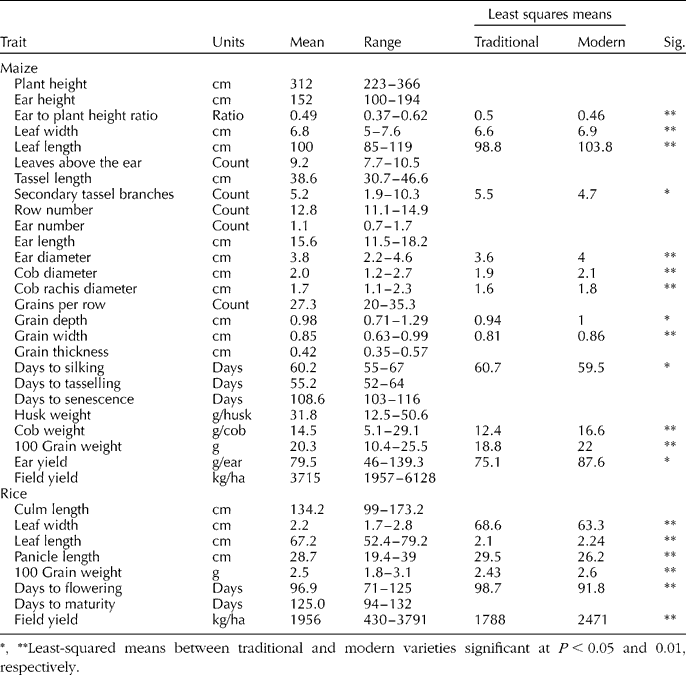
*, **Least-squared means between traditional and modern varieties significant at P < 0.05 and 0.01, respectively.
Table 2 The percentage of maize and upland rice populations exhibiting presence of nominal traits measured in an agromorphological trial in Panama, 2005

Molecular characterization
Only maize populations were characterized at the molecular level in CIMMYT's Applied Biotechnology Center using a bulked DNA technique for microsatellites (Warburton et al., Reference Warburton, Xianchun, Ambriz, Diaz, Villordo and Hoisington2001; Dubreuil et al., Reference Dubreuil, Warburton, Chastanet, Hoisington and Charcosset2006). In brief, the molecular methods included: (1) collection of tissue samples from 15 randomly selected individuals from each accession grown under greenhouse conditions; (2) extraction of DNA from individual samples using a modified cetyltrimethylammonium bromide method (CIMMYT, 2005) based on the protocol of Saghai-Maroof et al. (Reference Saghai-Maroof, Solima, Jorgenson and Allard1984); (3) quantification of individual DNA samples using Nanodrop® ND-1000 spectrophotometer (Nanodrop, Wilmington, DE); (4) bulking of equal quantities of DNA from each of 15 individual samples belonging to an accession to form a bulk DNA sample; (5) allele amplification using selected fluorescently labelled microsatellite primers; (6) detection of amplification products using an ABI™3100 sequencer (Perkin Elmer/Applied Biosystems, Foster City, CA); (7) identification of amplified alleles and correction of allele size, if necessary, on the basis of control samples using GeneScan® (Applied_Biosystems, 2001) and Genotyper® software; (8) estimation of allele quantities based on fluorescence intensity (maximum peak height) and calculation of allele frequencies using mathematical procedures described in Dubreuil et al. (Reference Dubreuil, Warburton, Chastanet, Hoisington and Charcosset2006) with R software; and (9) grouping of detected alleles into allele categories on the basis of marker repeat size and alleles detected in past studies.
The study used 11 microsatellite markers, spread across the maize genome, that had been optimized for amplification in bulk DNA samples. The microsatellites are publicly available online only at http://www.maizegdb.org/ and were: phi063, phi065, phi079, phi102228, phi299852, umc1161, umc1196, umc1447, umc1545, umc1917 and umc2250. Microsatellite data for the same marker set with the exception of phi065 were obtained for nine maize varieties from various parts of Latin America, used as diversity standards by the Applied Biotechnology Center at CIMMYT. These diversity standards were collected between 1943 and 1970 from Peru, Ecuador, Bolivia, Cuba, and Mexico at altitudes ranging from 30 to 2700 m.a.s.l. Four of the nine populations are of Mexican origin, while all other countries are represented by a single population.
Statistical analysis
Descriptive statistics were used to evaluate the occurrence of heterogeneous rice populations. Mixed-model analysis was used to compute phenotypic means for quantitative traits. Cluster analysis (Gower's distance, Ward's linkage method; Struyf et al., Reference Struyf, Hubert and Rousseeuw1997) was used to visualize populations' relationships on the basis of phenotypic data for rice and both phenotypic and molecular data for maize. Ordination (Euclidean distance, Non-metric multi-dimensional scaling; McCune and Grace, Reference McCune and Grace2002) was employed to visualize relationships for maize populations on the basis of molecular data only. A multiple response permutation procedure (Mielke and Berry, Reference Mielke and Berry2001) was used to assess the relationship between populations and grouping variables. Two groups were assessed: (1) population identity (modern or traditional) and (2) farming practices (swidden or transition).
A Bonferroni-adjusted mixed-model analysis was used to evaluate which phenotypic traits differed between levels within groups, and to estimate least-squared means for the traits of varieties belonging to each level. Straight-line geographical distances between collection site centroids were computed for all populations and a mantel test was used to correlate geographical distances with genetic distances. PC-ORD (McCune and Mefford, Reference McCune and Mefford1999), SAS system (SAS, 2005) and R (R_Development_Core_Team, 2007) statistical softwares were used to implement the above statistical procedures.
Results
Farmers provided a total of 71 maize populations and 54 rice accessions belonging to 10 and 20 distinctly named farmer varieties, respectively (Figs 1 and 2). Sufficient seed for a phenotypic characterization trial was available for 51 maize accessions and 52 rice populations, as well as the check varieties. Sixty-six maize accessions, including the check variety, were characterized at the molecular level. Both phenotypic and molecular data were available for 47 maize accessions.
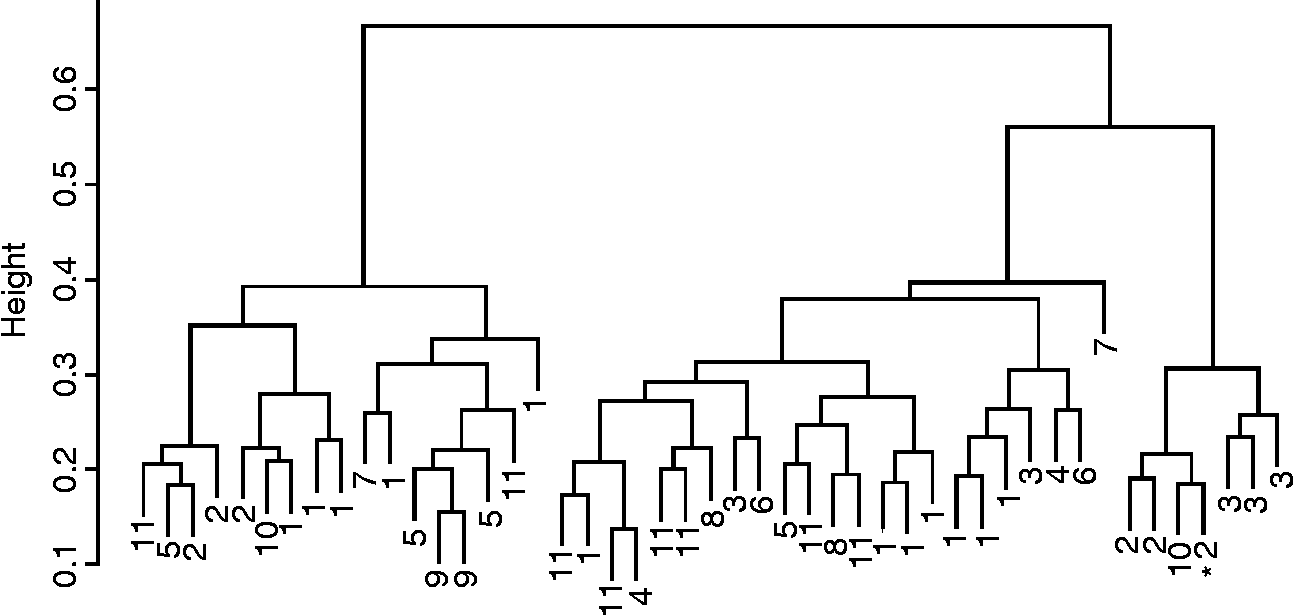
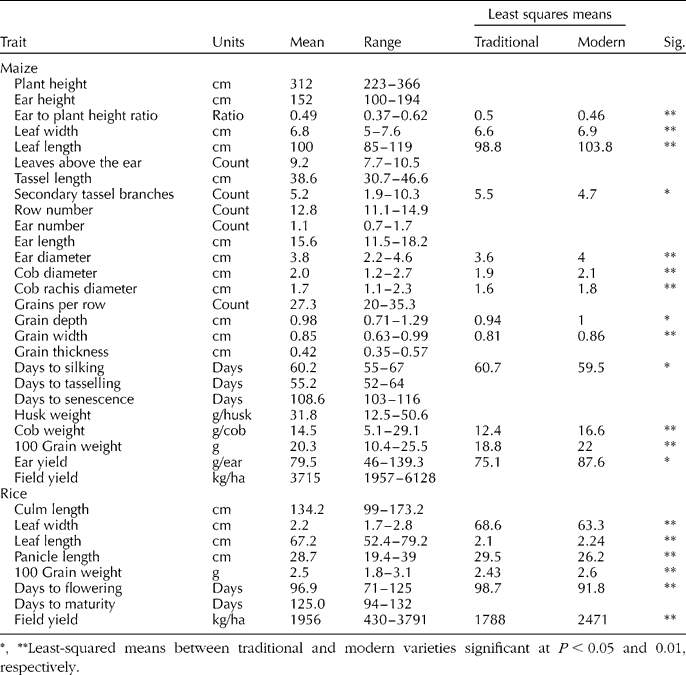
Fig. 1 Cluster dendrogram (Gower's distance, Ward's method and phenotypic and molecular data) of maize populations collected from farmers in Panama, 2005 (1, Isleño; 2, Guararé; 3, Blanco; 4, Maíz Perro; 5, Amarillo; 6, Colorado; 7, Palomita; 8, Tableño; 9, Capullo Morado; 10, no name). * Indicates check variety.
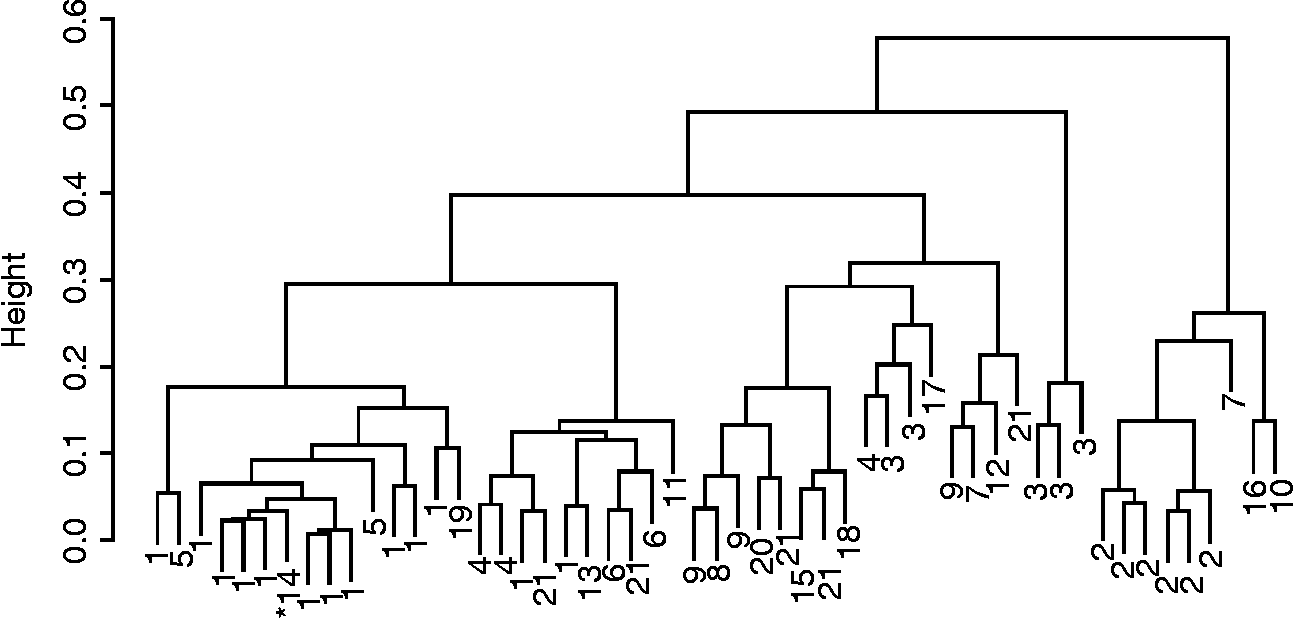
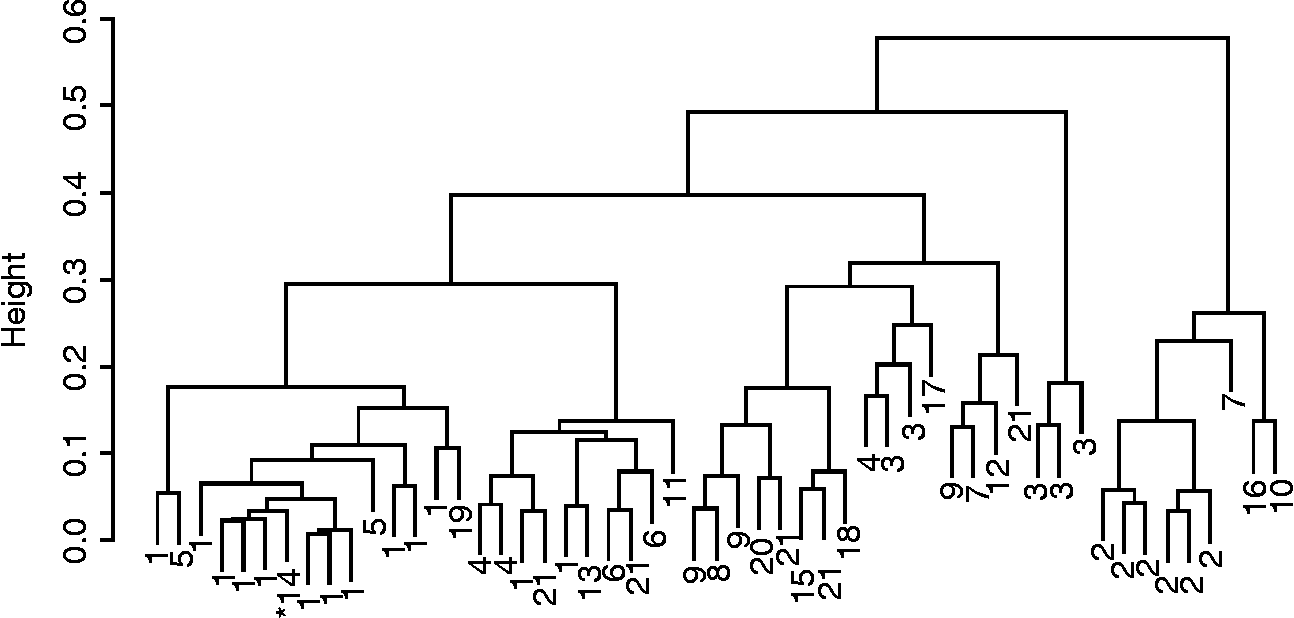
Fig. 2 Cluster dendrogram (Gower's distance, Ward's method and phenotypic data) of upland rice populations collected from farmers in Panama, 2005 (1, Lubón; 2, Culí Moreno; 3, Lijero; 4, Chino Blanco; 5, Tío Fulo; 6, Guayaquil; 7, Coría; 8, Colombia; 9, Cañadilla; 10, Zaíno; 11, Raízoro; 12, Plata; 13, Pajareño; 14, Orisíca; 15, Darién; 16, Chombo; 17, Chino Morado; 18 = Caballón; 19, Bonita; 20, Amarillo Pedregal; 21, no name). * Indicates check variety.
Variants were identified in 37% of rice accessions on the basis of seed morphology. In 68% of the cases where seed morphology variants were identified, the farmer providing the seed indicated that the variants were the result of admixture between recognized varieties. In the rest of the cases, variants were deemed to belong to the same variety that had been collected. Thus, on the basis of seed morphology, we found that few farmers (32%) declare a variety that is heterogeneous in seed morphology to be a single variety. Qualitative traits other than seed morphology also exhibited within accession heterogeneity, to varying degrees. The percentage of collected accessions exhibiting heterogeneity for these traits were: awns (63%), leaf pubescence (21%), leaf sheath colour (12%), collar colour (8%), node colour (4%), internode colour (4%) and anther colour (2%). In contrast to heterogeneity in seed morphology, all farmers indicated that heterogeneity in these traits was not the result of admixture.
Ranges indicated that maize accessions were uniform for flowering, silking, anthesis and maturity, but exhibited a wide variation for ear height, secondary tassel branching, leaves above the ear, ear diameter, cob diameter, cob rachis diameter, husk weight, cob weight, 100 grain weight, ear yield and yield (Table 1). By contrast, rice accessions were uniform for many variables and only panicle length and yield had a wide spread. Selected nominal traits for maize and rice (Table 2) exhibited significant variation. For maize, high levels of uniformity were only apparent for tillering and pubescence. Conversely, rice exhibited uniformity for a slightly larger number of traits: collar colour, auricle colour, ligule colour, ligule type, panicle branching and seed-coat colour.
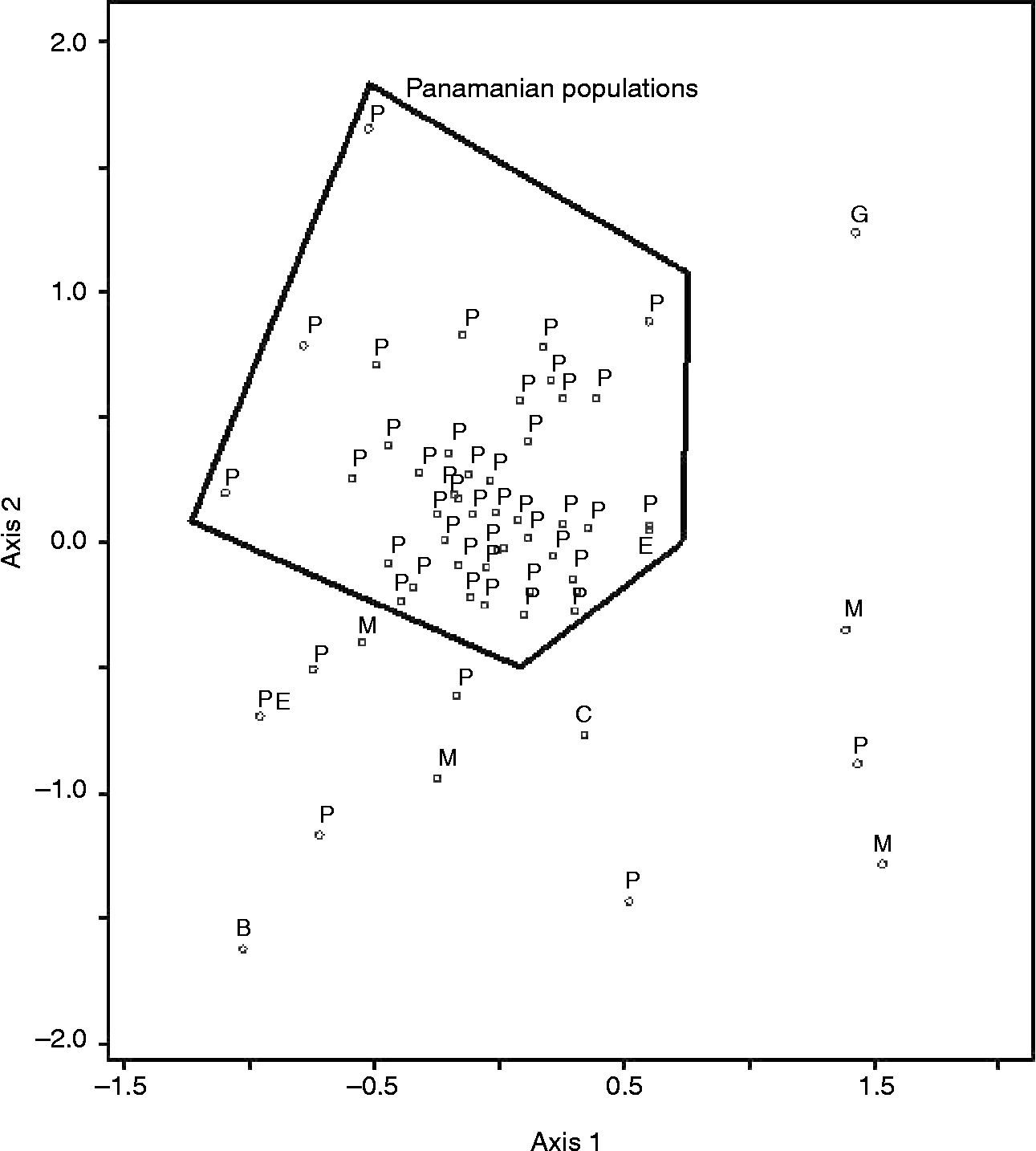
Farmers' naming practices only partially agreed with cluster analysis results and were the strongest for rice (Figs 1 and 2). The agglomerative coefficient (Kaufman and Rousseeuw Reference Kaufman and Rousseeuw1990) for rice clusters (0.86) was higher than that of maize (0.68). Ordination of molecular data explained 68% of the variance in the data (Axis 1 = 42%, Axis 2 = 26%) and indicated that Panamanian maize populations were generally molecularly distinct from populations used as diversity standards, with the exception of an Ecuadorian population. Only five Panamanian populations appeared to be molecularly similar to the diversity standards. These five populations were provided by farmers from different geographies and were known by different varietal names. In general, however, Panamanian populations were more similar to each other than the diversity standards (Fig. 3). Mantel tests comparing geographical and genetic distance were not significant for either rice or maize.
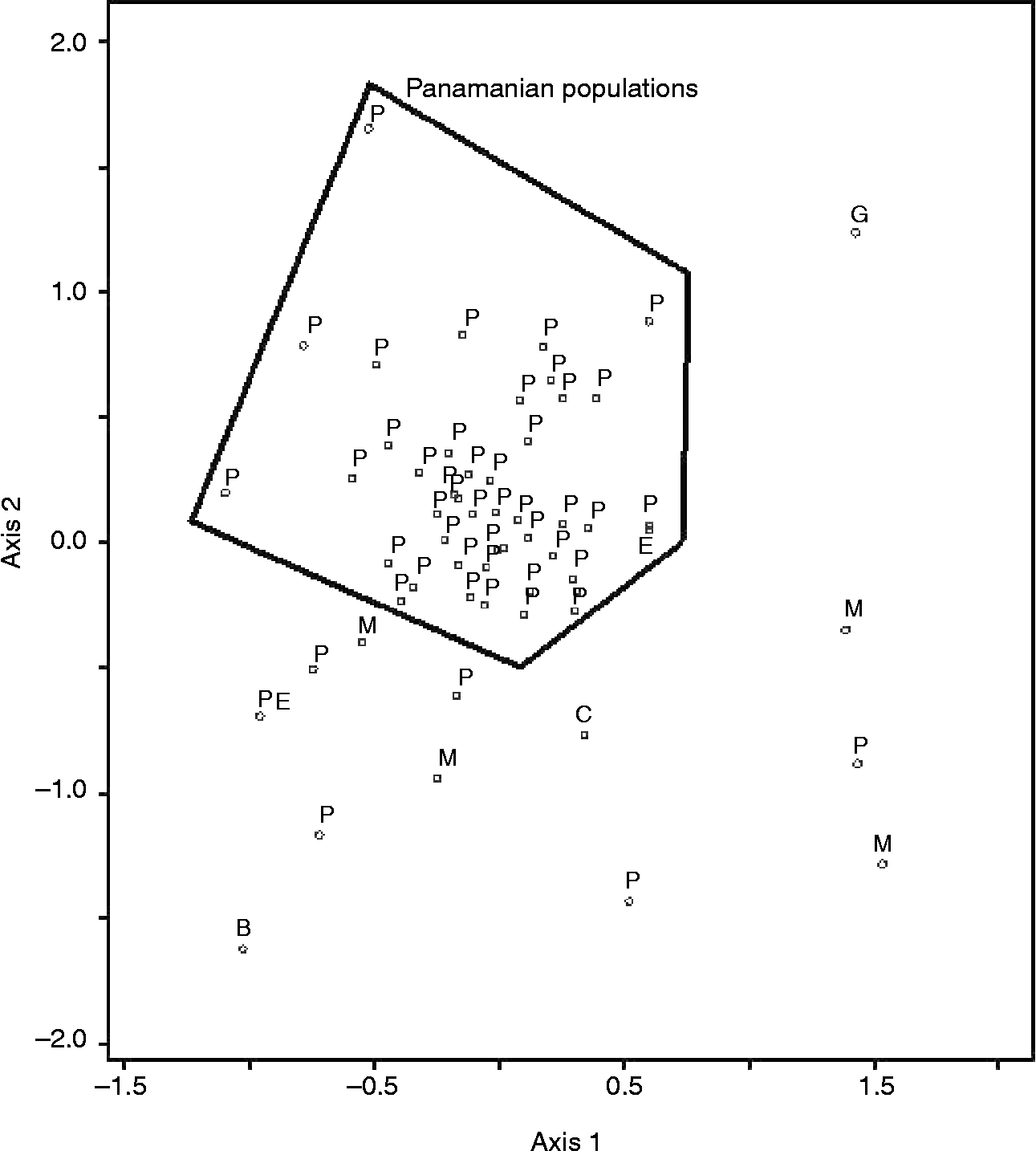
Fig. 3 Ordination (Euclidean distance, non-metric multi-dimensional scaling and molecular data) of maize populations collected in Panama in 2005 and populations collected elsewhere in Latin America (B, Bolivia; C, Cuba; E, Ecuador; G, Guatemala; M, Mexico; P, Panama; PE, Peru).
Multi-response permutation procedures indicated a grouping effect on the basis of accessions having a modern or traditional identity for both maize (P = 0.01) and rice (P < 0.001) phenotypic data. However, no grouping effect was found for molecular data on the basis of modern or traditional identity for maize (P = 0.29). No grouping effect was detected for farm management (swidden agriculture versus transition) for either maize (P = 0.50) or rice (P = 0.15).
Discussion
Poor Panamanian farmers have adopted modern maize and rice varieties, and these modern varieties are managed alongside traditional varieties. These farmer varieties contain substantial amounts of phenotypic diversity. The naming practices of farmers partially coincide with classifications based on a broad suite of traits. In a global study of plant genetic resources encompassing 27 crop species, Jarvis et al. (Reference Jarvis, Brown, Cuong, Collado-Panduro, Latournerie-Moreno, Gyawali, Tanto, Sawadogo, Mar, Sadiki, Hue, Arias-Reyes, Balma, Bajracharya, Castillo, Rijal, Belqadi, Rana, Saidi, Ouedraogo, Zangre, Rhirib, Chavez, Schoen, Sthpit, De Santis, Fadda and Hodgkin2008) found that naming practices of farmers could result in the misclassification of the actual varietal units being managed by them. In the present study, we believe that discrepancies between farmer nomenclature and statistical clustering are likely due to farmers using a few key characteristics (e.g. husk or hull colour, maturity, etc.) to classify populations, while cluster analyses employ a large number of characteristics to evaluate cluster membership. Thus, populations of the same name belonging to different clusters are evidence of variation existing within farmer varieties as well as some misclassification. The higher agglomerative coefficient for rice clusters suggests that those clusters are more distinct than those of maize. Higher levels of uniformity in rice may be due to the greater genetic isolation of self-pollinated crops, as well as the possibility of a genetic bottleneck upon introduction to the New World.
Heterogeneous upland rice accessions appear to be common in Panama. While farmers often recognize heterogeneity to be a result of admixture where grain traits are concerned, they tend to lump variants together into a single farmer variety where other forms of heterogeneity are concerned. This underscores the importance of seed traits where the classification of varietal groups is concerned.
Greater molecular similarity within Panamanian maize populations compared to diversity standards is expected, given the temporal and spatial similarity of Panamanian populations to each other. Interestingly, the diversity standard that was most similar to Panamanian maize populations was collected at an altitude of 2200 m.a.s.l in Ecuador. Despite this difference in altitude, Ecuador shares a long history of migration and interconnectedness with Panama (Kolman and Bermingham, Reference Kolman and Bermingham1997). The molecular distinctness that we report here suggests that molecular diversity of interest may exist in Panamanian populations. That the five Panamanian populations that diverged the most from other Panamanian populations were collected from different farmers and geographies and were assigned different varietal names underscores the importance of collecting broadly when historical diversity information is not available.
Varieties classified as modern and traditional were distinct phenotypically. A total of 13 and 6 phenotypic traits were significantly different between populations with modern and traditional identities for maize and rice, respectively (Table 1). In the case of maize, greater 100-grain weight, earlier silking, lower ear to plant height ratio, higher grain yield per ear and smaller tassels for populations of modern identity are consistent with the trends in modern maize breeding programs (Duvick, Reference Duvick2005). In the case of rice, shorter, narrower leaves, greater 100-grain weight, earlier flowering and higher yield are also consistent with the objectives of modern rice breeding programs (Peng et al., Reference Peng, Cassman, Virmani, Sheehy and Khush1999). This suggests that farmers' classification of varieties as modern and traditional accurately reflects the influence of modern breeding programs on crop morphology.
That ‘modern’ and ‘traditional’ effects were not detected in the molecular dataset is unsurprising given the small number of markers, which are likely selection neutral. Furthermore, gene flow can obscure divergence between populations at the molecular level (Ho et al., Reference Ho, Kresovich and Lamkey2005). Over the small geographical distances in this study ( < 40 km), we found that genetic distance was not correlated with geographical distance. This may also be due to gene flow (pollen and seed exchange) limiting genetic divergence over short distances.
In contrast to the classification of farmer varieties as modern or traditional, we found no association between phenotypic traits and farming practices (swidden versus transition to permanent agriculture). This indicates that swidden agriculturalists and those in transition to permanent agriculture appear to be cropping populations with similar phenotypic traits. This suggests that ‘modern’ phenotypes are of value under ‘traditional’ (e.g. swidden) agricultural management.
The farmers in this study retained a significant amount of maize and rice diversity by incorporating ‘modern’ germplasm and tolerating admixture, suggesting an actively dynamic management strategy. Continued collection and characterization of these materials will be required if plant breeding programs are to capitalize on this constantly evolving diversity.
Acknowledgements
This research was supported by a Natural Science and Engineering Research Council Canada Graduate Student Scholarship, a University of Alberta Dissertation Scholarship and the John G. Bene Fellowship of the International Development Research Centre of Canada in Community Forestry to B. Love. The authors would like to thank the University of Alberta's ALES Research Ethics Board for reviewing the proposed research and providing helpful suggestions. Many thanks to Dr Pedro Him of IDIAP, the Instituto Nacional de Agricultura and the National Seed Committee for supporting this research by allowing to use their facilities. A very special thanks to all the Azueran farmers who shared their invaluable time and seed.