INTRODUCTION
Size-at-maturity is widely used by managers as biological support for establishing legal size controls. The most common rationale behind the latter is that, in order to prevent recruitment overfishing individuals should be allowed to mate at least once before attaining legal size (Jamieson & Caddy, Reference Jamieson, Caddy, Jamieson and Bourne1984; Myers & Mertz, Reference Myers and Mertz1998). One problem with the design of size-based strategies is that discrimination between juveniles and adults varies depending on the criteria applied.
In the case of decapod crustaceans size-at-maturity has been defined on the basis of four criteria (Waddy & Aiken, Reference Waddy and Aiken2005): (1) physiological sexual maturity, which relates to the capacity of the gonads to produce gametes; (2) morphometrical sexual maturity, corresponding to the size at which individuals attain full development of secondary sexual traits; (3) behavioural sexual maturity, indicative of mating capacity; and (4) functional sexual maturity, at which the individuals effectively reproduce in nature.
Physiological maturity can be assessed through the macroscopic examination of the gonads (Cobo & Fransozo, Reference Cobo and Fransozo2005; Mura et al., Reference Mura, Orrù and Cau2005) and/or the histological analysis of tissue from the ovaries, testis or vasa deferentia (Minagawa & Higuchi, Reference Minagawa and Higuchi1997). Morphometrical maturity is usually detected through the statistical analysis of size-dependence in secondary sexual characters (Somerton, Reference Somerton1980; Hall et al., Reference Hall, Smith, de Lestang and Potter2006). Examples are the conspicuous changes that occur at the time of the puberty moult, such as the relative enlargement of the propodus of male chelae or the width of the female abdominal segments (Mura et al., Reference Mura, Orrù and Cau2005). Behavioural sexual maturity can be established by the presence of sperm plugs (Tallack, Reference Tallack2007; Ungfors, Reference Ungfors2007) and abdominal mobility (Fischer & Wolf, Reference Fischer and Wolff2006) in the case of females, and by direct observation of mate or guarding behaviour (Orensanz et al., Reference Orensanz, Parma, Armstrong, Armstrong and Wardrup1995; Goshima et al., Reference Goshima, Kanazawa, Yoshimo and Wada2000) or mating scars (Knuckey, Reference Knuckey1996) in the case of males. Functional maturity is unequivocally evidenced in females by the presence of ovigerous masses (Wolff & Soto, Reference Wolff and Soto1992; Tallack, Reference Tallack2007).
Regardless of the criteria and methods used, size-at-maturity is usually expressed as the size at which 50% of the individuals are found to be mature, and eventually by an associated error term (Udupa, Reference Udupa1986; Roa et al., Reference Roa, Ernst and Tapia1999).
Chilean crab fisheries, which are entirely artisanal, are supported by eight species, namely Cancer edwardsii (Bell, 1835), C. coronatus Molina, 1782, C. porteri Rathbun, 1930, C. setosus (Molina, 1782) (Cancridae), Homalaspis plana (Milne Edwards, 1834) (Platyxanthidae), Ovalipes trimaculatus (De Haan, 1833) (Portunidae), Taliepus dentatus (Milne-Edwards, 1834) and T. marginatus (Bell, 1835) (Majidae). The principal target species is C. edwardsii, which represents almost 90% of the catch reported over the last five years (SERNAPSCA, 2002–2006). Chilean crab fisheries are concentrated in the southern regions, between 40°S and 48°S, where 72% of the catch is landed (SERNAPESCA, 2002–2006).
In 1990 the Chilean fisheries authority introduced a ban on the landing of ovigerous females and a minimum legal size of 12 cm of carapace width (CW) for C. edwardsii all along the country's extended coastline, from approximately 18°S to 53°S. The rationale for this minimum legal size is not entirely clear because biological knowledge on this species is very scarce. Preliminary estimates are available only for morphometrical size-at-maturity (Pool et al., Reference Pool, Montenegro, Canales, Barahona and Vicencio1998; Olguín et al., Reference Olguín, Barahona, Bernal, Young, Orensanz, Montenegro, Quiroz, Toledo, Baez and Bahamonde2006). Minimum legal size should be reconsidered, incorporating additional biological criteria.
In this study, we describe the gonadal development of females and males, macro-and microscopically, using visual inspection and histological techniques. Size-at-maturity was estimated for both sexes of C. edwardsii based on gonadal development and morphometry; functional maturity is estimated only for females.
MATERIALS AND METHODS
Individuals of Cancer edwardsii were obtained using commercial traps from October 2006 to May 2007 in two fishing areas: Bahia Ancud (41° 51′56″S 73° 50′4″W) and Isla Cochinos (41° 50′50″S 73° 48′27″W), both located at the north end of Chiloé island. Given that the two sites were close to each other (3 km) and that catches are landed in the same port (Ancud), data were pooled for analysis. The catch was stratified by size (5 mm intervals) for crabs in the range 75–135 mm CW; an additional stratum was defined for crabs >135 mm CW. A minimum of six individuals in each size-class were measured and dissected for visual inspection of the gonads. A total of 743 individuals were analysed, including 381 females and 362 males. Morphometrical measurements (besides CW) included the length, width and height of both chelipeds in males, and the length and width of the 5th and 6th abdominal segments in females (Figure 1).

Fig. 1. Morphometric measurements of male cheliped and female abdomen. ChL, chela length; ChH, chela height; ChW, chela width; S5L, 5th abdominal somite length; S5W, 5th abdominal somite width; S6L, 6th abdominal somite length; S6W, 6th abdominal somite width.
Visual inspection of the reproductive system was standardized using photographs. Six and four stages of gonadal development were established by this method for females and males, respectively (Tables 1 & 2; Figures 2 & 3). The principal macroscopic character used to discriminate stages was the proportion of the cephalothoracic space occupied by the gonad. Additionally, colour and texture of the ovaries were considered for females, and the diameter of the vasa deferentia for males. Tissue from the ovary (N = 41 females) and the middle part of the vasa deferentia (N = 38 males) were fixed in buffered 5% formaldehyde solution, embedded in paraffin, sectioned, and stained with haematoxylin–eosin for microscopic description of each stage.

Fig. 2. Histological view of five macroscopic stages of ovary maturity. Gonad from first stage was undetectable. (A) Stage II primordial; (B) Stage III early development; (C) Stage IV late development; (D) Stage V mature; (E) Stage VI recovering; (F) macroscopic view of mature ovary (Stage V).

Fig. 3. Histological view of three macroscopic stages of the middle section of vasa deferentia. Tissue from first stage was undetectable. (A) Stage II primordial; (B) Stage III developing; (C) Stage IV mature; (D) macroscopic view of mature vasa deferentia. (Stage IV).
Table 1. Maturity stages established for female Cancer edwardsii using visual inspection and histology of the ovary.

Table 2. Maturity stages established for male Cancer edwardsii using visual inspection of testes and histology of vasa deferentia.

Stage of gonadal development was used to construct an index of maturity in order to estimate physiological size-at-maturity using criteria similar to Campbell & Eagles (Reference Campbell and Eagles1983). Full description of development stages is given in Tables 1 & 2. Females were considered mature when ovary stage was higher than Stage III (early development); the latter never has post-vitellogenic oocytes in histological analyses. Males were considered to be physiologically mature when they had attained Stage III, in which the testes are clearly distinguishable, the vasa deferentia are well developed and spermatophores occupy more than 50% of their lumen. The proportion of mature individuals (P mature) as a function of size-class was modelled with a sigmoid function (Neter et al., Reference Neter, Wasserman and Kutner1989):
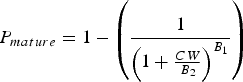
in which the asymptote is set to 1 because all individuals surviving through adulthood should eventually mature; B 1 is the regression coefficient and B 2 is the size at which 50% of the individuals have reached maturity. Parameters B 1 and B 2 were estimated by means of non-linear regression.
A piecewise linear regression model with a breakpoint was used to estimate morphometrical size-at-maturity. Parameters and their confidence limits were calculated by the least squares method using the Levenberg–Marquardt algorithm implemented in the STATISTICA software (Gill & Murray, Reference Gill and Murray1978). The independent variable was size, expressed as CW, and the dependent variables were measurements taken on the right chelae or the abdominal segment (only females). We used measurements from the left chelae when the right chela was absent. We found no significant differences between the size of right and left chelae (chela length: t1,569 = −0.33, P = 0.74; chela width: t1,569 = −0.34, P = 0.73; chela height, t1,569 = −0.12, P = 0.93). The breakpoint is considered a morphometrical indicator of maturity (Somerton, Reference Somerton1980). For model selection (one versus two regression lines) we used the test proposed by Somerton (Reference Somerton1980):
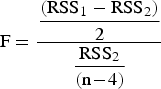
where RSS1 and RSS2 are the residual sums of squares for models with one and two allometric phases respectively, and n is the number of data points. The statistic is distributed as F with 2 and n−4 degrees of freedom.
Functional size-at-maturity was analysed only for females, using the sigmoid model and the presence of a clutch as the indicator of maturity. For this purpose we used the proportion of ovigerous females relative to the total number of females within each size interval during the month with the highest prevalence of ovigerous females. This procedure assumes that the sampling period covered the brooding season of the whole female population, which in the study region occurs principally in late autumn and early winter (Pool et al., Reference Pool, Montenegro, Canales, Barahona and Vicencio1998). Therefore, female functional size-at-maturity estimated here is only as a preliminary estimate.
RESULTS
Maturity stages
Six macroscopic stages were distinguished for females, based principally on ovary volume and colour (Table 1). In Stage I gonad tissue was undetectable, and therefore no histological analyses were conducted. In Stage II oocytes had large nuclei and conspicuous nucleoli; follicle cells were concentrated, principally in the periphery of the ovary lobules. Stage III presented oocytes in early vitellogenesis with follicular cells surrounding them; oocyte nuclei were easily visible in most cases. In Stage IV most oocytes were in late vitellogenesis, with round yolk granules distributed homogeneously; nuclei were not visible and follicular cells appeared flattened. Stage V was characterized by post-vitellogenic oocytes with large, lenticular yolk granules. Finally, Stage VI showed great diversity of cellular types: oogonia, follicular cells, and pre- and post-vitellogenic (remnant) oocytes (Figure 2).
The male reproductive system also showed conspicuous change during the course of development, and four maturity stages could be distinguished (Table 2). The aspect of the vasa deferentia changed from filamentous to highly convoluted, and the conduct thickened. Vasa deferentia and other parts of the male reproductive system were undetectable in Stage I, and therefore no histological analyses were conducted. Histological examination of vasa deferentia in Stage II showed nuclei of epithelial cells in a basal position, indicative of a juvenile stage (Johnson, Reference Johnson1980), with few spermatophores present. In Stage III the nuclei of epithelial cells became centric and many of them showed lobed nuclei; some spermatophores were surrounded by abundant seminal fluid (>50% of vasa deferentia lumen), spermatids inside spermatophores were small and peripherally located. Finally, Stage IV showed multinucleated cells close to the epithelial surface, spermatophores full of sperm, and seminal liquid occupying less than 20% of the vasa deferentia lumen (Figure 3).
Physiological size at maturity
The sigmoid curve fitted well the proportion of adults as a function of size, for both sexes (Table 3). No significant difference was detected in size at maturity (CW approximately 101 mm) between females and males based on observation of the gonads (t = 6.35, P = 0.1) (Figure 4).

Fig. 4. Physiological size at maturity. Sigmoid function fitted to mature fraction as a function of size (CW). (A) Females (N = 279); (B) males (N = 288).
Table 3. Estimated physiological size at maturity of Cancer edwardsii from the northern part of Chiloe Island.

Morphometrical size at maturity
A two-phase model fitted the relation between CW and male chelae height better than a one-phase model; this was not the case for other relations involving secondary sexual characters (Table 4). For that reason, all measurements of secondary sexual characters were integrated in a principal component analysis (PCA) for each sex. Although morphometric variables were highly correlated, PCA significantly improved the detection of allometric phases (Table 4; Figure 5), allowing the estimation of morphometrical size-at-maturity. Males reached morphometrical size-at-maturity at 118 mm CW (CI95%: 116–120) and females at 106 mm CW (CI95%: 102–109) (Table 5; Figure 5).

Fig. 5. Morphometric size at maturity. Piecewise linear regression. (A) Females (N = 381); (B) males (N =362).
Table 4. Relation between body size and sexual secondary characters. Statistical comparison between one- and two-line regression models. RSS, residual sum of squares; CW, carapace width; ASW, abdominal segment width; ASL, abdominal segment length; PCA, principal component analysis.

Table 5. Estimated morphometric size at maturity for Cancer edwardsii from the northern part of Chiloe Island.

Functional size at maturity of females
The sigmoid model was fitted to the data (r = 0.67), but the slope was non-significant (t = 2.66, P = 0.07). Size at functional maturity was estimated to be 103.3 mm CW (CI95%: 77.9 mm, 128.6 mm).
DISCUSSION
The multivariate approach used here to estimate morphometric maturity might be helpful when bivariate allometries are not obvious. At least in the case of females, integrating measurements of multiple secondary sexual characters (e.g. length and width of the 5th and 6th abdominal segments) into the first factor of PCA was the only effective way to detect allometric phases. Morphometric size-at-maturity was larger than physiological size-at-maturity in the Chilean commercial crab Cancer edwardsii; 95% confidence ranges overlap partially in the case of females. This implies that males and females are physiologically capable to reproduce before secondary sexual characters are fully expressed. The puberty moult (juvenile to adult transition) occurs within a size-range of 101–106 mm CW for females, and 101–118 mm CW for males. Whether individuals undergoing their puberty transition moult are effectively able to reproduce or not may depend on the size structure of the population, habitat quality, intensity of male–male competition, and female social interactions (Orensanz & Gallucci, Reference Orensanz and Gallucci1988; Ennis et al., Reference Ennis, Hooper and Taylor1990; Orensanz et al., Reference Orensanz, Parma, Armstrong, Armstrong and Wardrup1995; Wada et al., Reference Wada, Yoshimo, Sato and Goshima2000; Sainte-Marie et al., Reference Sainte-Marie, Sevigny and Carpentier2002).
A sensible recommendation to fisheries managers is that minimum legal size should be larger than the size at which individuals become effectively reproductive. In this study we offer a preliminary estimate of functional maturity for C. edwardsii based on the size of ovigerous females (103 mm CW), but confidence intervals (78–129 mm) were wide. Otherwise, functional size at maturity, being dependent on ecological context, is a moving target (Orensanz et al., Reference Orensanz, Parma, Armstrong, Armstrong and Wardrup1995). In exploited populations, male–male competition may be relaxed when large male crabs are targeted and small males get the opportunity to mate. In addition, estimation of functional size at maturity is methodologically difficult for males (Goshima et al., Reference Goshima, Kanazawa, Yoshimo and Wada2000), while for females it could be biased when traps are used for sampling, due to a well demonstrated reduction of foraging activity in ovigerous females (Howard, Reference Howard1982).
Under a precautionary approach, morphometric size-at-maturity should be recommended as biological support to establish a minimum legal size. The rationale is that: (1) functional maturity may be contingent upon ecological conditions; (2) morphometrical maturity is normally attained after gonadal maturity (Hartnoll, Reference Hartnoll and Abele1982; Mura et al., Reference Mura, Orrù and Cau2005; but see Tallack, Reference Tallack2007); and (3) accurate estimation of physiological and functional maturity usually present methodological difficulties (i.e. the requirement of a year-round sampling programme).
In the case of C. edwardsii, at least for males, the established minimum legal size (120 mm CW) would ensure that most individuals mate at least once before becoming available to the fishery. This size would be conservative in the case of females. However, in practice, scarcity of individuals larger than 120 mm CW (Olguín et al., Reference Olguín, Barahona, Bernal, Young, Orensanz, Montenegro, Quiroz, Toledo, Baez and Bahamonde2006) and poor enforcement by the fisheries authority render these regulations ineffective. A large fraction of the catch is below legal size: 20 to 71% for males and 70 to 93% for females at the main landing sites (Olguín et al., Reference Olguín, Barahona, Bernal, Young, Orensanz, Montenegro, Quiroz, Toledo, Baez and Bahamonde2006). More effective regulation is required to safeguard the reproductive potential of these harvested populations.
A 3S harvest strategy (size, sex and season) has been implemented in other cancrid species (Woll et al., Reference Woll, van der Meeren and Fossen2006). Seasonal closures could be implemented in the C. edwardsii fishery, but would not be necessarily effective in terms of improving the reproductive output of the harvested population (Shepherd, Reference Shepherd1993; Arendse et al., Reference Arendse, Govender and Branch2007). Considering that C. edwarsii is captured mainly with baited traps (Olguín et al., Reference Olguín, Barahona, Bernal, Young, Orensanz, Montenegro, Quiroz, Toledo, Baez and Bahamonde2006), control on gear selectivity appears to be a more suitable option for the C. edwardsii. Traps with escape vents to avoid capture of individuals under 100 mm CW could have better chances as an enforceable control. Trap designs with escape vents have been tested without success for Cancer setosus (Aguilar & Pizarro, Reference Aguilar and Pizarro2006), but have shown good results in other cancrid crab fisheries (Ungfors, Reference Ungfors2007). Experimental studies of trap design, coupled to research on crab reproductive ecology, should be a priority in management-oriented research for this valuable resource.
ACKNOWLEDGEMENTS
We thank IFOP's staff stationed at Ancud, especially Vivian Pezo, Dagoberto Subiabre and Pedro Alvarado for coordination and assistance in crab sampling. The Sindicato de Pescadores de Ancud was actively involved in the project. L.M.P. acknowledges the International Foundation for Science (IFS) and Dirección de Investigación y Desarrollo (DID) from UACh for financial support. We thank Dr Martin Thiel and an anonymous referee for their constructive comments and corrections on an early version of the manuscript. All experimental work was conducted in Chile and complied with existing laws and regulations.












