Management Implications
The decision to actively manage a plant infestation depends on accurate information about the impacts the invading plant poses, the plant’s life history, and its mode of invasion. The establishment stage of an infestation is one important determinant of how large and persistent an infestation may become. Salsola tragus (Russian-thistle), an annual forb, occurs throughout the West, and recent and historic infestations have been noteworthy. Managers of open lands lack sufficient information to determine which years will promote S. tragus growth and which locations, apart from roadsides and heavily disturbed areas, are susceptible to S. tragus infestation.
While S. tragus is considered drought tolerant once it becomes established, we show, on aeolian substrates, that it is sensitive to low precipitation in the early establishment stage. Given the high variability of rainfall in dryland areas, low precipitation early in the growing season may limit the years in which S. tragus can proliferate. Also, we found coppice mounds on aeolian substrates are susceptible to S. tragus infestation, potentially through capture of S. tragus seed by native plants associated with the mound. This may make sand dune–related plant communities more vulnerable to S. tragus infestations. Likewise, capture of S. tragus seed under litter, and potentially in grass clumps, may concentrate where S. tragus plants subsequently occur. Where wind is persistent, aeolian (windblown) sandy soils have a high level of sediment movement, and this naturally occurring erosion may promote persistent infestations. Managers can identify areas of expected high wind erosion on aeolian substrates and regularly monitor S. tragus infestation size and persistence to determine the need, if any, for any local management.
Introduction
Since the initial introduction in South Dakota in 1874 of Russian-thistle (Young and Evans Reference Young and Evans1972), one of the many nonnative annual species within the genus Salsola, infestations of Salsola species have been documented in all lower states in the United States, Hawaii, and the Canadian provinces (USDA 2018). While Salsola infestations are frequent on disturbed areas, infestations have also been observed in natural areas without apparent disturbance, where their invasion characteristics and potential impacts are less studied. Botanical sources vary in their treatment of Salsola spp. in different regions, and even in the same region, of the United States (Hrusa and Gaskin Reference Hrusa and Gaskin2008; Supplementary Table S1). In the arid Southwest, Russian-thistle [Salsola tragus L. sensu lato (= kali)] is the most extensively occurring species. Salsola tragus has iconic status among the public, yet land managers still must make decisions as to whether an infestation is potentially harmful and whether it should be actively managed.
The growing season for S. tragus is spring, generally after frost risk has diminished, until late fall, when in response to shorter days, the stem dries, and the plant remains are an open brittle structure (Young Reference Young1991). These stems readily break off at the base during the frequent and persistent winds experienced throughout the arid Southwest, and the “tumbleweeds” are blown across the landscape, scattering seed as they travel (Karpiscak and Grosz Reference Karpiscak and Grosz1979; Stallings et al. Reference Stallings, Thill, Mallory-Smith and Lass1995). Ultimately the dry weed remnants collect against barriers such as fences or houses or even pile up in roads; effects such as these have been reported and are commonly observed across the arid Southwest and southern Great Plains (e.g., Gabbert Reference Gabbert2014; Held 2018; Schwarz Reference Schwarz2014). The mounds of S. tragus obstructing infrastructure can impede animal movement and, in their dried state, pose increased fire risk (Young Reference Young1991).
Invasion of S. tragus has been described in agricultural systems (Stevens Reference Stevens1943; Young Reference Young1986; Young and Evans Reference Young and Evans1979), after restoration treatments (González et al. Reference González, Sher, Anderson, Bay, Bean, Bissonnete, Cooper, Dohrenwend, Eichhorst, El Waer, Kennard, Harms-Weissinger, Henry, Madarick, Ostoja, Reynolds, Robinson, Shafroth and Tabacchi2017), and in disturbed areas (Allen and Allen Reference Allen and Allen1988; Lodhi Reference Lodhi1979; Schmidt and Reeves Reference Schmidt and Reeves1989). In an examination of S. tragus in mine reclamation sites in Wyoming, Allen and Allen (Reference Allen and Allen1988) and Allen et al. (Reference Allen, Allen and Friese1989) showed that the plant is not mycorrhizal and will decline in abundance when mycorrhizal fungal populations increase in the soil. This finding has led some land managers to consider infestations as temporary and facilitative of succession on disturbed sites, while other managers have concern about the persistence of S. tragus infestations and their impacts.
Limited studies have been done on S. tragus impacts on native dryland plants. Allen (Reference Allen1982b) conducted greenhouse studies examining competition of S. tragus (as Salsola kali var. tenuifolia Tausch.) with two grasses native to the arid West, western wheatgrass [Pascopyrum smithii (Rydb.) Löve] (as Agropyron smithii Rydb.) and blue grama [Bouteloua gracilis (H.B.K.) Lag.]. The native grasses had lower biomass when grown with S. tragus, especially when water was a limiting resource. The investigators proposed that S. tragus’s drought tolerance may be a mechanism by which its colonization in native grasslands is enhanced during droughts (Allen Reference Allen1982a). In a different greenhouse study, Allen (Reference Allen1982a) examined competition among these same species under cool (10 /20 C night/day), warm (15.5/25.5 C night/day), and hot (21/31 C night/day) temperature regimes without water limitation and showed S. tragus to have the fastest germination under the high-temperature regime. That experiment also showed that the outcome of S. tragus competition with other species was dependent on the temperature at which it was grown and the species with which it was grown. Salsola tragus and other C4 photosynthesizing grasses had equal or superior competitive advantage at high temperatures, but at lower temperatures, a C3 photosynthesizing grass and annual forb had equal or superior competitive advantage.
Other studies have examined effects of S. tragus in field conditions. Vanier and Walker (Reference Vanier and Walker1999) studied the interference of S. tragus infestations on native seed dispersal in the Mojave Desert of California. Seeds of the native annual flatcrown buckwheat (Eriogonum deflexum Torr.), were more concentrated under S. tragus, particularly dead S. tragus, compared with living or dead E. deflexum; however, S. tragus seeds were not concentrated under the E. deflexum. The authors indicated that further study was required to determine whether such concentration would enhance or inhibit germination and growth of E. deflexum; however, they surmised that the concentration of native seed by S. tragus on a degraded site could be important in determining the success of the site’s restoration. Other studies have identified beneficial attributes of S. tragus. For example, Barrows (Reference Barrows1997) observed that S. tragus protected hatchlings of an endangered lizard in the western Sonoran Desert from predation. Another example, shown by Allen and Allen (Reference Allen and Allen1988), was the enhancement of grass cover (P. smithii) on an arid and windy site by standing S. tragus litter. This facilitation may have been through structural protection of emergent grass seedlings from wind and retention of snow that increased soil moisture. Other authors have noted that young S. tragus can serve as forage for livestock (Dwyer and Wolde-Yohannis Reference Dwyer and Wold-Yohannis1972; Young Reference Young1991).
Extensive aeolian (windblown) sand deposits cover much of the southern Colorado Plateau, where wind erosion risk is high (Nauman et al. Reference Nauman, Duniway, Webb and Belnap2018; Redsteer Reference Redsteer, Lancaster and Hesp2019) and sediment movement (i.e., air-borne dust or sand movement) can be substantial, particularly without sufficient stabilizing vegetative cover (Draut et al. Reference Draut, Redsteer, Amoroso, Giosan, Fuller, Nicoll, Flad and Clift2012). Within aeolian deposits of the plateau area, S. tragus has invaded native grasslands and mixed shrub–grass (steppe) communities (Thomas and Redsteer Reference Thomas and Redsteer2016). For example, an invasive plant survey conducted at Petrified Forest National Park, AZ, from 2003 to 2005 (Thomas et al. Reference Thomas, Hunt, Arundel and Guertin2009a) documented S. tragus occurrences on 1-ha sampling units. At least 1% cover of S. tragus was observed in 56% to 77% of the sampling units in areas that varied by sample year from 983 to 2,624 ha. The highest cover of S. tragus (50% to 75%) was in sampling units in the disturbed areas immediately adjacent to paved roads, but S. tragus was also consistently observed on grasslands and steppe more than 1 km from paved roads and with no human-derived disturbance. Earlier vegetation mapping at Petrified Forest National Park documented S. tragus throughout the park in 77 of 149 (51%) relevé plots in 2003 and in 172 of 662 (26%) relevés in 2006 (Thomas et al. Reference Thomas, McTeague, Cully, Schulz and Hutchinson2009b). On the adjacent Navajo Nation, grasslands on sandy soil have become destabilized over large areas, resulting in moving sand dunes where S. tragus is often the only plant observed growing on the shifting sand (Redsteer et al. Reference Redsteer, Bogle and Vogel2011).
On the aeolian surfaces of the southern Colorado Plateau and elsewhere, it is not known under what conditions a S. tragus infestation will be minimal or prolific, how persistent infestations will be over time, or what the impacts of an invasion on native plant species and dune stability are. Various analytic frameworks have been proposed to explain the invasion dynamics of plant species (Catford et al. Reference Catford, Jansson and Nilsson2009; Gurevitch et al. Reference Gurevitch, Fox, Wardle and Inderjit2011; Richardson Reference Richardson2000). Blackburn et al. (Reference Blackburn, Pyšek, Bacher, Carlton, Duncan, Jarošík, Wilson and Richardson2011) suggested a framework comprising four stages (transport, introduction, establishment, and spread), each with characteristic barriers the plant must surmount to pass to the next stage. To better understand the establishment stage of S. tragus, we studied its emergence and early establishment on two aeolian substrates, a stabilized sand sheet with previous human disturbance and a semistabilized sand dune, both with a high level of natural disturbance through wind movement of sediment. We measured seedling emergence of S. tragus in the field to investigate the seasonality of seedling emergence and how removal of seedlings affected subsequent emergence. In growth chamber assays, we measured S. tragus emergence to investigate the spatial characteristics of S. tragus in the soil seedbank.
Materials and Methods
Study Area
Our field sites comprised a flat stabilized sand sheet (SS) (34.8242°N, 109.8928°W), with some microtopography caused by small mammal burrowing, and the ridgetop of a large, stabilized sand dune (SD) (34.9475°N, 109.7786°W) with noticeable coppice mounds at Petrified Forest National Park in the southeastern part of the southern Colorado Plateau ecoregion (Figure 1). The sites were characteristic of topographies occurring at the Park.
Figure 1. Location of Petrified Forest National Park, the park boundaries are current to 2007 (park boundaries were in the process of expanding during the study). A star indicates approximate location of each study site. The sand sheet site is to the south and the sand dune site to the north.
The SS site, at the southwest portion of the park, had been disturbed at its western edge. A barbed-wire fence ran parallel to the site. In previous years, strong winds accumulated sand against the fence. This area of concentrated sand ran parallel to the fence and was bladed to level the sand 3 to 4 yr before the study. The disturbed area graded at its eastern edge into a grassland co-dominated by B. gracilis and James’ galleta [Pleuraphis jamesii Torr.; syn. Hilaria jamesii (Torr.) Benth] with occasional fourwing saltbush [Atriplex canescens (Pursh) Nutt.] shrubs (Figure 2). The site had a prolific infestation of S. tragus in the fall of 2014.

Figure 2. The sand sheet site at Petrified Forest National Park, AZ, looking north. The site runs parallel to the fence line shown in the picture and represents a disturbed sand surface that grades into native grassland steppe. The olive-brown plants in the photo are Salsola tragus individuals. Photo credit: U.S. Geological Survey.
The second site, SD (Figure 3), was on the ridge of an east–west-oriented, semistable echo duneFootnote 1 located about 22 km N/NE of the SS Site. Topography along the dune ridge consisted of low coppice mounds (0.5 to 1 m) with sparse vegetation and either barren or sparsely vegetated interspaces. Coppice mounds typically were oblong in shape and oriented parallel to the predominant wind direction from the southwest. Native grasses occurring on the site were mainly P. jamesii and less frequently Indian ricegrass [Achnatherum hymenoides (Roem. & Schult.) Barkworth] and giant dropseed (Sporobolus giganteus Nash); shrubs were dominated by common dunebroom (Parryella filifolia Torr. & A. Gray ex A. Gray), snakeweed (Gutierrezia spp.), and mound saltbush (Atriplex obovata Moq.). The grasses were observed often anchoring the shorter coppice mounds, with shrubs, or woody remains of shrubs, anchoring the taller mounds. Salsola tragus was observed on the echo dune ridge and sides in previous years.

Figure 3. The sand dune (SD) site at Petrified Forest National Park, AZ, looking north (A). Vegetation seen on the windward side of the dune includes native grasses, shrubs, and dried Salsola tragus. Plots were placed along the ridgeline (B) of the large echo dune. Vegetated coppice mounds and barren interspaces were sampled. Photo credit: U.S. Geological Survey.
Regional and Local Precipitation
Precipitation occurs bimodally on the southern Colorado Plateau, with peaks during winter storms (December to March) and the American Southwest monsoon season (mid-June through mid-September). Normally approximately 45% of annual precipitation occurs during the summer monsoon season (Redsteer Reference Redsteer, Lancaster and Hesp2019). These two wet seasons are separated by a dry, windy spring (April to June) when most aeolian sediment movement occurs, causing the dominant wind and precipitation seasons regionally to be out of phase with each other (Bogle et al. Reference Bogle, Redsteer and Vogel2015; Draut et al. Reference Draut, Redsteer, Amoroso, Giosan, Fuller, Nicoll, Flad and Clift2012). Arid regions, such as the southern Colorado Plateau, are characterized by press-droughts (long-term reduction of available precipitation; Hoover et al. Reference Hoover, Duniway and Belnap2015) and pulse-droughts (short-term and intense reduction of available precipitation; Hoover et al. Reference Hoover, Duniway and Belnap2015).
Using precipitation and growing degree days (base 50) data from daily summary records of measurements taken at a NOAA Cooperative meteorological station situated immediately adjacent to the SS site (Petrified Forest NP Station 026468; Western Regional Climate Center 2018), we summarized cumulative precipitation and growing degree days for the two water years (October 1 to September 30) encompassing the study (Figure 4). The weather station provided highly accurate precipitation data for the SS site but only an estimate of precipitation experienced at the SD site. As summer moisture at the park often arrives from the south and diminishes northward, we expected the pattern of rainfall at the SD to covary with that recorded at the SS weather station but to be somewhat less in quantity.

Figure 4. Precipitation and accumulated growing degree days (GDD) for the 2014–2015 and 2015–2016 water years (October 1 to September 30) as recorded adjacent to the sand sheet site. While the accumulated GDD were nearly equivalent between the years, 2016 was substantially drier during the summer growing season.
Soil Characterization
We characterized the soil texture at each site using sediment subsamples collected in June 2015 from within 2 to 4 cm of the surface at representative areas between vegetation (i.e., without grass cover and not underneath shrubs). Two samples were collected at SS and four samples were collected at the SD with half from coppice mounds and the other half from the interspace between vegetated areas. Samples were analyzed for particle size distributions by optical diffraction (Gee and Or Reference Gee, Or, Dane and Topp2002). The range of measurement was 0.04 to 2000 microns divided into 116 bins. Representative samples were put into suspension (filtered water is routinely used) within a fluid module attached to a device containing a light source and detectors. The pattern of scattered light intensity is a function of scattering angles produced as the light is deflected off particles of various sizes within a sample. Using this information, a distribution of particle sizes was deduced using a mathematical model based on Fraunhoffer diffraction theory. Each sample was sonicated before measurement, run through the device for 90 s, and analyzed twice to obtain an average.
Seedling Emergence and Growth
Measures of seedling emergence and establishment at the SS and SD sites were made in 2015 and 2016 during four sampling intervals (May, June, late July or early August, and September). At each interval, we tallied the number of S. tragus plants (seedlings and established plants) and made estimates of the S. tragus cover. In 2016, we estimated S. tragus cover but did not count seedling emergence, because seedlings were present at each sampling interval, but they were usually dried out and brittle. As the persistent and often strong winds at the sites could have blown away dry sprouts, we did not believe a count would be representative of actual emergence that year.
At each site, we established plots (1 m2 with a minimum 0.6 m separation between each) using a randomized block design. Within each block, we designated plots for removal of S. tragus throughout the growing season and other plots for no removal. At SS, 12 plots were established in 2015 (8 removal and 4 nonremoval) over an area approximately 10-m wide and 100-m long. Four additional nonremoval plots were added in 2016 for a total of 8 nonremoval plots in 2016. At SD, we established 18 plots (12 removal and 6 nonremoval) in 2015 over an area approximately 50-m wide and 300-m long, with an additional plot established nonrandomly to target the top of a coppice mound with numerous S. tragus already present in the spring of the first growing season.
In 2015, we counted the number of S. tragus plants (seedlings in removal plots and seedlings and established plants in nonremoval plots) and made visual estimates of S. tragus cover in nonremoval plots. For the visual cover estimates, we used the Domin-Krajina scale with intervals of <1%, 1% to 5%, >5% to 10%, >10% to 25%, >25% to 33%, >33% to 50%, >50% to 75%, >75% to100% cover (Mueller-Dombois and Ellenberg Reference Mueller-Dombois and Ellenberg2002). We also tallied the native plant species in each plot at the first sampling interval in 2015 and made a visual estimate of the cover of each using the Domin-Krajina scale.
In 2016, we measured S. tragus cover in nonremoval plots using a gridded frame placed over each plot and determined percent cover of S. tragus by the number of grid intersection points (100 total) that overlay S. tragus. Point counts were converted into percentages and categorized by the same Domin-Krajina scale used in 2015. Conversion of the grid cover estimates to the Domin-Krajina scale was consistent with the cover estimates made visually the previous year. We evaluated cover estimates at both sites using a Student’s t-test implemented in Excel.
We also measured windblown sediment movement at each plot at SS and SD in 2016. At two opposite corners of each plot, a thin, 4-cm-diameter metal washer was set against the soil surface and secured by a metal skewer. The skewer was pushed into the ground until its upper curved end just touched the washer. Sediment erosion caused by the lateral force of wind on the sand between measurements caused the washer to fall below the skewer’s curved end, and we measured the distance (mm) below the curved end to the top of the washer as an approximation of the amount of sediment lost. Deposition of sediment on the washer was measured as the distance between the top of the washer to the top of any sediment accumulated on the washer. We also noted whether the washer was completely covered or only partially covered with sediment (i.e., 0.75, 0.5, 0.25, or trace). We made a conservative summary of sediment movement within the plots by tallying all observations of accumulation or erosion of 2 mm or more (and on at least one-half of the washer for accumulation) by sampling interval.
Seedbank Assay
We assessed emergence of S. tragus seedlings from soil samples at SS and SD and from plant litter samples taken from SS, all collected in May 2016. Four plots that had S. tragus cover the previous year were chosen as sample foci at SS. Three soil samples (bare soil, near a grass clump, and under the closest shrub) were collected near each plot and near a point 5 m east of the plot in native grassland. At each sampling site, we collected two cylindrical cores of soil, 8-cm diameter by 1- and 6-cm deep, for a total of 46 soil samples at SS (one plot had no nearby shrubs, which reduced the total by two). Samples were stored in a labeled paper bag and sealed until being processed for assessment in a growth chamber. Also, at the SS site, we selected five patches of S. tragus litter within clumps of actively growing S. tragus. Within each litter patch and on adjacent bare soil, a 0.1-m2 area was flagged. Surface litter was collected with a hand trowel and stored in a paper bag. Using the same soil-collection technique as described for the soil samples, we took a 1-cm soil sample from under the litter and in the adjacent bare soil patch for a total of three samples for each of the five patches.
At SD, we collected soil samples around four coppice mounds with S. tragus growing on them. At each mound, a sample was collected at the interspace just to the southwest (windward) of the coppice mound, at the southwest foot of the mound, the top of the mound, and to the northeast (lee) foot of the mound. Samples were collected using the same technique as at SS, with samples collected to both 1- and 6-cm depths for a total of 32 samples at SD. The soil in some spots was too well-indurated to press the sampling container into the ground. In these cases, we excavated samples with a hand trowel, attempting to reach the same dimensions and volume as the cylindrical core.
We assayed seeding emergence of each sample in a growth chamber. Growing pots (9-cm diameter) were filled with 250 ml of sterilized sand, and a mixture of a 20-ml subsample of the soil sample and 60 ml of sterilized sand was added to create a top layer about 1-cm thick. Before being mixed with sand, soil samples were lightly crushed with a mortar and pestle if they were compacted and contained large soil clumps. For the SS litter samples, large pieces of litter (i.e., stems, leaf matter) were removed, and the remains were placed in a growing pot using the same method as the soil samples. Control pots had 25 S. tragus seeds each of seed collected from a mature, standing plant at SS in November 2015. Control seeds were mixed with 80 ml of sand and added to the top of each control pot. Each sample was replicated at least twice in trays placed in a growth chamber maintained at 20 C. Pots were watered with 100-ml distilled water initially and with 50-ml water when the soil appeared to dry at its surface. We counted and plucked emerging seedlings from the pots at 3- to 4-d intervals for up to 25 d.
Results and Discussion
Growing Environment
Both SD and SS had a sandy substrate of 70% to 85% sand in bulk composition. Soils at SS were 5% to 10% siltier and were less well sorted in texture than at SD (Supplementary Figure S1). At SD, coppice dune sands were finer grained, while interspace locations tended to have the coarsest sediment, likely due to wind winnowing of finer-grained silts and sands. Measurement of sediment accumulation and erosion revealed active sediment movement within each site and among plots within a site (Supplementary Table S2). Measured sediment accumulation and erosion could occur within the same plot, or either sedimentation or erosion could occur, or neither. There was no consistent pattern, except for an indication of continuous wind movement of sediment throughout the growing season, which we expected given the history of wind patterns in the region. Salient is the dynamic nature of the growing environment in these aeolian contexts, where surface sediment movement provides a natural level of disturbance.
Our earliest sampling interval was in mid-May, when we observed small seedlings each year. Precipitation accumulation since the beginning of the water year (October of the previous year) until May of the growing year was nearly equal in both 2015 and 2016 (Figure 4), as were the growing degree days. However, the 2016 growing season had little precipitation from May until August. Most of the growing season precipitation occurred in August of 2016.
Seedling Emergence and Growth in the Field
At SS, seedling emergence was greatest during the spring (May and June) sampling intervals. However, we observed newly emerged seedlings in all sampling intervals on both the removal and nonremoval plots, although with declining numbers as the growing season progressed. The average number of S. tragus seedlings on nonremoval plots was higher than the average on the removal plots throughout the four sampling intervals (Table 1). While all nonremoval plots had seedlings at each sampling interval, removal plots may have had one or more sampling intervals without seedlings. As shown in our removal plots and indicated by other researchers, S. tragus is capable of germinating and producing seedlings throughout the growing season (Allen Reference Allen1982a).
Table 1. Average number of Salsola tragus seedlings per square meter in plots with seedlings removed (removal) or not (nonremoval), the number of plots with seedlings (n), and SD of seedling number in 2015.a

a The sand sheet site had 8 removal plots and 4 nonremoval plots. The sand dune site had 12 removal and 6 nonremoval plots. The targeted plot on the coppice mound was not included in the averages for the sand dune site, as it was an outlier to the other plots in that sampling season. The coppice mound had more than 68 stems of established plants at the first sampling interval in May.
The total number of S. tragus counted in the removal and nonremoval plots at SS was significantly different (P < 0.05, Student’s t-test). The mean total number of seedlings in the removal plots in 2015 was 15.4 m−2 (SD 11.4) and the mean for the nonremoval plots was 31.3 m−2 (SD 9.9). Emergence of new S. tragus seedlings in the nonremoval plots did not appear to be competitively constrained by seedlings already established. It is possible that the larger, established S. tragus on the nonremoval plots provided a more favorable microenvironment for new S. tragus emergence throughout the growing season.
At the SD site, the average number of emerging S. tragus was also greater on the nonremoval plots than the removal plots, except at the first sampling interval (Table 1). One removal plot had a notable 104 seedlings at the initial sampling interval, but only one plot had a new seedling in September at the end of the sampling interval. One nonremoval plot had no seedlings in 2015.
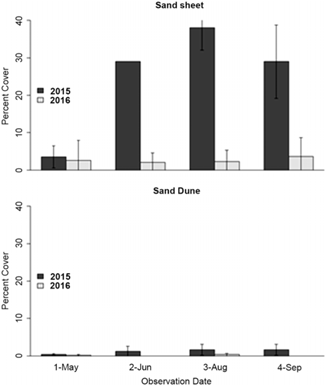
Salsola tragus cover on the nonremoval plots varied yearly between sites and within sites (Figure 5; Supplementary Table S3). In both 2015 and 2016, cover was significantly different between the sand sheet and sand dune site (P < 0.001). Plot cover values were greatest at SS in 2015 (P < 0.001). In 2015, mean S. tragus cover was highest in August (38%, SD 6.9), and all four nonremoval plots had S. tragus cover at each sampling interval. However, in 2016, only three of the now eight nonremoval plots at the SS site had any S. tragus cover, and the highest cover on any plot that year was only 11% in September. There was no apparent pattern between S. tragus cover in the nonremoval plots and the dominant native plant cover of B. gracilis and P. jamesii. Cover of these perennial grasses among the plots ranged between 18% and 26%, while the highest S. tragus cover was >33% to 50% in three plots and >25% to 33% in the plot with 18% grass cover.

Figure 5. Average cover and standard deviation of Salsola tragus on nonremoval plots at the sand sheet and sand dune sites. The targeted coppice mound plot at the sand dune site (with 33%–75% cover over the four sampling intervals in 2015) was not included in the cover statistics for 2015, as it was an outlier that year. It was included in 2016 statistics, in which its cover was <1% for the first three sampling intervals and zero at the last interval.
With one exception, the targeted plot, S. tragus cover at SD was low with no significant difference in plot cover values between 2015 and 2016 (P = 0.165). In 2015, the highest mean cover on the nonremoval plots at the SD site was in September (Figure 5), 1.7% (SD 1.5). The one targeted plot, not counted in the 2015 nonremoval plot average, had S. tragus cover of greater than 33% in May and greater than 75% in September. In 2016, there was very little S. tragus cover in the nonremoval plots; the highest cover that growing season was measured in August with less than 1% cover in four plots and none in the others. The targeted plot had less than 1% S. tragus cover at the first three sampling intervals and none at the last sampling interval in 2016. There was no discernible pattern between the cover of S. tragus or number of seedlings and the cover of native plants in the SD plots.
We attribute between-year differences in S. tragus cover at each site to the differences in summer precipitation between 2015 and 2016. At the May 2016 sampling interval, S. tragus cover at both sites was nearly equal to that measured in 2015, except on the targeted coppice mound at SD. In subsequent sampling intervals in 2016, cover was much less than in the previous year, as was summer precipitation. Salsola tragus, a C4 photosynthesizing plant, has been characterized as having high water-use efficiency and being potentially more drought tolerant than co-occurring native plants (Wallace Reference Wallace1970). Our study implies that, at least at the early seedling stage, pulse-drought, such as seen in the 2016 growing season, is an impediment to S. tragus yearly growth. This result is consistent with a study conducted by Wallace (Reference Wallace1970), in which he concluded that S. tragus may be drought resistant once established but sensitive to soil moisture in early growth. It is also consistent with Dwyer and Wolde-Yohannis’s (Reference Dwyer and Wold-Yohannis1972) findings in greenhouse studies that for S. tragus seeds in sandy medium to germinate and grow to at least 3 cm in height, they needed initial watering equivalent to 12.7 ml water (= 0.75 inches of rainfall). These authors also measured productivity of S. tragus, as expressed by height of the experimental plants, over 90 d at four different water levels. They found reduced productivity of S. tragus at less than 50% field capacity, and at 10% of field capacity, productivity was reduced to half of that at full field capacity. These findings corroborate our interpretation that a critical level of soil moisture is needed for early establishment stage of S. tragus despite the species’ apparent drought hardiness when the plant is larger.
While low precipitation in 2016 may in part explain the low cover (0% to 5%) of S. tragus on the targeted mound SD compared with its high cover (>75%) the previous year, microtopography may also have been a factor. Our plot placement at SD was random, except for the targeted plot, and plot placement frequently captured interspace and mound edges combined. In contrast, the plot on the coppice mound encompassed the top and sides of the 1-m-high mound. In addition to the low to nonexistent cover in 2016 of S. tragus on the targeted coppice mound, standing remains of dead shrubs from the previous year were absent in 2016. Throughout the 2016 growth season, the mound was nearly bare and completely exposed to wind erosion. We observed other larger mounds anchored by shrubs to have more S. tragus than surrounding interspace areas. These microtopographic influences on S. tragus distribution within the dune field suggest S. tragus growth and potential impacts on coppice mounds should be monitored separately from interspaces; however, our study design did not a priori account for this potential source of variation. As we observed most vegetation on the ridgeline of this echo dune being associated with the coppice mounds, the role of S. tragus in native plant persistence on the coppice mounds, and potentially the role of S. tragus infestation on dune dynamics, needs further clarification.
Seedbank Assay
Seedling emergence was low from soil samples taken around the plots at SD and SS (Table 2). Among the soil-sampling combinations at SD, soil from one sample area had two sprouts taken from the top of the mound at 6-cm depth and six sprouts taken from the back of the mound at 1-cm depth. Soil samples from only two areas at SS had seedling emergence; both were from bare soil with one near a plot and the other 5-m from a plot. Total seedling emergence was greater from the samples of litter and surface soil beneath the litter (Table 2), with a mean of 4.40 (SD 3.17) and 1.0 (SD 1.41) seedlings per replicate pot respectively. Adjacent to each litter patch, seedlings emerged from three of five samples with an overall mean of 0.70 (SD 1.64) per replicate pot. More than 60% of the control seeds in each assessment emerged.
Table 2. Emergence of Salsola tragus seedlings in the growth chamber assay of field-collected soil and litter.a

a Field samples were divided among growing pots and, for most field samples, were replicated, with pooled means reported in the table. Control pots contained 25 seeds each.
The low seedling emergence from the soil samples around plots at SS and coppice dunes at SD could indicate that there were few S. tragus seeds in the samples, the seed had an earlier germination signal that season and emergence had failed, or conditions were not suitable for seed germination and emergence. Salsola tragus seed requires afterripening before germination (Evans and Young Reference Evans and Young1972). As we saw adequate seedling emergence with the control S. tragus seed, collected in the fall of the preceding year and presumably of the same cohort of S. tragus seed found in the soil samples, we assume lack of afterripening was not a barrier to seedling emergence. The higher number of seedlings that emerged from samples in and under the litter also provides evidence that seeds were capable of emerging from the collected samples and that seed capture and concentration within litter could be of importance in local distribution of S. tragus. Seeds within and under litter were protected from wind that could have blown them away, and the microenvironment under the litter may have been more favorable for emergence. Evans and Young (Reference Evans and Young1972) found higher seedling emergence of S. tragus (as S. kali var. tenuifolia) from soil and litter field collected underneath intact S. tragus plants compared with soil samples taken at a distance from the plants. In the greenhouse, they also saw higher seedling emergence in pots with grass litter applied. They suggested that seedlings germinating on a bare surface may not be able to penetrate the soil with their root tip before desiccating (Evans and Young Reference Evans and Young1972).
We did not sample seed within grass clumps, yet while counting seedling emergence at the plots, we observed many seedlings emerging within grass clumps, particularly at the SS site. It may be that within patches of vegetation, as well as under litter, S. tragus seeds tend to collect and will germinate and emerge under favorable conditions. As growth of native grasses is seasonal and germination and establishment of grass perhaps even less frequent, the question is raised of the degree to which establishment and growth of S. tragus within native grass clumps impedes the growth and reproduction of these grasses. Visual estimation of plant cover in each plot was too coarse to detect the position of S. tragus with respect to grass or shrubs. We expect that use of a gridded sampling frame or a line-intersect transect approach could provide the spatial resolution to measure the cover and location of S. tragus emergence with respect to individual plants.
In summary, we found emergence and early establishment of S. tragus to be precipitation sensitive. Young seedlings failed to establish without sufficient precipitation postemergence. However, with proper conditions, seedlings could emerge and establish throughout the growing season, albeit at a lower rate than in the spring. Our growth chamber examination of seedling emergence from field-collected soil and litter showed highest emergence where the seeds were protected under litter. This, in combination with our observations of seedlings emerging within grass clumps and established S. tragus on coppice mounds suggest that existing vegetation and litter may be important factors in where S. tragus emerges and establishes locally. Study of the competitive effects of S. tragus with co-occurring vegetation over a longer term than our study could provide more insight on the impact of this invasive on the aeolian surfaces of the southern Colorado Plateau and elsewhere. This study also suggests that, in future research, the placement of study plots needs to take into consideration how vegetation patches occur on the landscape and that sampling in plots should be at a resolution to detect the spatial relationship of S. tragus and co-occurring native plants. On aeolian soils, the high level of windblown sediment movement is a source of natural disturbance that may encourage persistent S. tragus infestations in which the extent of infestation will vary yearly depending on the timing and amount of precipitation. While the question of the need for and means of managing S. tragus infestations remains open, our measurements of the species’ early establishment characteristics are informative in part toward that overarching management question and pose directions for future research.
Data generated from this study are available from the USGS ScienceBase-Catalog (Thomas et al. Reference Thomas, Redsteer, Stuaffer and Matias2019).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2019.7
Author ORCIDs
Kathryn Thomas 0000-0002-7131-8564; Margaret H Redsteer 0000-0003-2851-2502
Acknowledgments
We thank the staff at Petrified Forest National Park for their logistical assistance with the project: William Parker, Andrew Bridges, and the housing staff. Kimberlie Perkins, USGS Hydrologist (Menlo Park, CA) provided the soil particle size analysis. Brett Stauffer provided field assistance in the 2016 field season and Sergio Mathias provided technical support for the soil seedbank analysis. McKenna Zandarski assisted with data entry and quality control. Thanks also to volunteer Barbara Phillips, who assisted with sampling in 2015, and Martin Karpiscak, who provided valuable input on Salsola biology. The article was improved by the input of Dan Winkler and three anonymous reviewers. Tanya Quist of the School of Plant Sciences, University of Arizona, facilitated use of the growth chamber and provided technician support. This research was conducted under Petrified Forest National Park permit PEFO-2014-SCI-0007. The Hydrologic Extremes Project of the U.S. Geological Survey’s Global Research and Development program and CESU Cooperative Agreement G15AC00455 with the University of Arizona funded the project. No conflicts of interest are declared. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.