Published online by Cambridge University Press: 03 March 2004
Transmission of the malaria parasite Plasmodium is influenced by many different host, vector and parasite factors. Here we conducted a field study at Mbita, an area of endemic malaria in Western Kenya, to test whether parasite transmission to mosquitoes is influenced by the severity of malaria infection in its human host at the time when gametocytes, the transmission forms, are present in the peripheral blood. We examined the infectivity of 81 Plasmodium falciparum gametocyte carriers to mosquitoes. Of these, 21 were patients with fever and other malaria-related symptoms, and 60 were recruited among apparently healthy volunteers. Laboratory-reared Anopheles gambiae s.s. (local strain) were experimentally infected with blood from these gametocyte carriers by membrane-feeding. The severity of the clinical symptoms was greater in febrile patients. These symptomatic patients had higher asexual parasitaemia and lower gametocyte densities (P=0·05) than healthy volunteers. Ookinete development occurred in only 6 out of the 21 symptomatic patients, of which only 33·3% successfully yielded oocysts. The oocyst prevalence was only 0·6% in the 546 mosquitoes that were fed on blood from this symptomatic group, with mean oocyst intensity of 0·2 (range 0–2) oocysts per mosquito. In contrast, a higher proportion (76·7%) of healthy gametocyte carriers yielded ookinetes, generating an oocyst rate of 12% in the 1332 mosquitoes that fed on them (mean intensity of 6·3, range: 1–105 oocysts per mosquito). Statistical analysis indicated that the increased infectivity of asymptomatic gametocyte carriers was not simply due to their greater gametocyte abundance, but also to the higher level of infectivity of their gametocytes, possibly due to lower parasite mortality within mosquitoes fed on blood from healthy hosts. These results suggest that blood factors and/or conditions correlated with illness reduce P. falciparum gametocyte infectivity.
Studies of malaria parasites (Plasmodium sp.) in humans (Rogier, Cammenges & Trape, 1996; Chotivanich et al. 2000) and rodent models (Mackinnon & Read, 1999) indicate that disease severity is positively correlated with asexual blood-stage parasite density. The successful transmission of Plasmodium from the human host to the mosquito vector (Anopheles sp.) depends, however, upon the presence of sexual stages (gametocytes) and their density in the peripheral blood (McKenzie et al. 2001). Gametocytogenesis is thought to begin after the onset of asexual infection, peaking after asexual blood-stage parasites have reached their maximum density (Sinden, 1983). This developmental shift from asexual to gametocyte production is likely triggered by a host response to infection. As gametocyte production and infectivity determine parasite fitness, understanding its relation to disease is crucial to understanding the epidemiological significance of parasite virulence.
Successful transmission of malaria parasites from humans to mosquitoes may depend not only on the availability and the density of mature, infectious gametocytes, but also on host blood factors and quality. Quantification of non-gametocyte related factors to transmission would be of great interest to both clarify transmission biology and evaluate control prospects. Many epidemiological studies have attempted to quantify the contribution of individuals within a population to malaria transmission (Muirhead-Thomson, 1954; Gamage-Mendis et al. 1991; Githeko et al. 1992; Boudin et al. 1993; Tchuinkam et al. 1993; Drakeley et al. 2000), with most indicating that there is substantial heterogeneity in host infectiousness to mosquitoes. Factors responsible for this heterogeneity are complex (Woolhouse et al. 1997) and may include host immune response (Graves et al. 1990; Mendis, David & Carter, 1990; Mulder et al. 1994; Lensen et al. 1996; Roeffen et al. 1996), age (Githeko et al. 1992), and exposure to antimalarial drugs (Klein, Lima & Toda, 1992; Hogh et al. 1998; Robert et al. 2000), among others as reviewed by Beier (1998). One factor that has not, to our knowledge, been explicitly examined is the role of specific symptoms and disease intensity on transmission.
Studies of rodent malaria indicate that disease severity is positively associated with transmission to mosquitoes (Mackinnon & Read, 1999). Comparable studies on humans are not possible as it would be unethical to deny treatment to infected volunteers in order to monitor the extent of disease severity and gametocyte production through time. It is possible, however, to conduct point surveys of the infectivity of carriers with varying degrees of symptoms. Such studies are useful to demonstrate if illness at the time of mosquito biting impedes infection, and may be useful to infer the long-term transmission consequences to parasites that generate persistent illness. Here we conduct a field study on P. falciparum, to test whether the severity of disease at the time of mosquito feeding influenced transmission to the Anopheles gambiae vector.
The study was conducted at Mbita Point, in the Suba district of Western Kenya. Mbita Point lies on the shore of Lake Victoria and is inhabited by approximately 8000 people. Most Mbita residents are fishermen and/or traditional farmers who live adjacent to the lake due to the accessibility of fresh water. The major malaria vectors in the region are Anopheles funestus, An. gambiae and An. arabiensis, which yield an average exposure rate of about 5 infective bites per year per person (Petrarca et al. 1991; Minakawa et al. 1999). In Mbita, P. falciparum malaria is a leading cause of morbidity, accounting for 50% of all illness clinically diagnosed at the local health centre.
The Kenyan and United State National Institute of Health (NIH) ethical review committees approved the recruitment procedures reported in this study. Plasmodium falciparum gametocyte carriers were detected during surveys of patients attending the local dispensary and of healthy children attending school in the community. Surveys of gametocyte carriers reporting to the clinic were conducted from June 2000–2001, and those of healthy children from August 2001–June 2002 (simultaneous surveillance of carriers from both the clinic and the community was not possible due to protocol restrictions). Prior to the surveys, the school head teachers were informed of the purpose of the study and communicated the information to parents through a letter and through their children. During cross-sectional parasitological surveys, finger-prick blood samples were taken from which thick blood films were made. At the local clinic, only patients that presented with clinical symptoms and were diagnosed as having malaria were sampled. Blood smears were Giemsa stained and examined microscopically for the presence of asexual and sexual stages of the malaria parasite P. falciparum. In addition, a verbal report of fever, body temperature and other symptoms of febrile disease (headache, joint pain, chills, vomiting and diarrhea) were recorded for all study participants. Volunteer carriers were enrolled upon signing an informed consent form. All children unable to give consent were volunteered to participate by parents or guardians. Exclusion criteria from the study included mixed-species infections, any symptoms indicating severe clinical malaria and other concomitant diseases requiring hospitalization or follow-up, and children less than 3 years old because of concerns of the vulnerability of these patients to treatment delay. All malaria-positive cases at the clinic were referred to the clinician, and during community surveys, all individuals with asexual parasiteamia (>1000 parasites/μl) were referred to the local health centre for treatment with Fansidar. Plasmodium falciparum gametocytes were counted in microscopical fields that cumulatively contained 500 leukocytes and an estimate of gametocyte density was obtained by assuming a standard number of 8000 leukocytes/μl of blood.
We used a local strain of An. gambiae s.s. from a laboratory colony originating from the Mbita area. These mosquitoes were previously selected and adapted to feed on Parafilm® membrane. Batches of 50–100 females, 3 days post-emergence, were put in paper cups and starved for 6–8 h prior to the blood feeding. For experimental infections, the clinical officer or health technician withdrew 2 ml of venous blood from each volunteer and transferred it into a heparinized tube. This blood was immediately fed to mosquitoes using pre-warmed (37 °C) artificial membrane mini-feeders as previously described (Tchuinkam et al. 1993). Mosquitoes were allowed to feed for 15 min after which unfed mosquitoes were removed and those remaining were maintained in cages under constant insectary climatic conditions (27±1 °C, 70±5% RH), and given ad libitum access to a 6% glucose solution.
Subsequent to each blood feed, a subsample of 5 fed mosquitoes was dissected after 24 h and their midguts examined under an epi-fluorescence microscope (×40, glycerin immersion lens). Ookinetes were detected using a specific FITC-labelled monoclonal antibody against 25 kDa sexual surface antigens (Gouagna et al. 1998). Seven days later, all remaining mosquitoes were dissected and their midguts stained with 2% mercurochrome in distilled water to facilitate detection of oocysts by light microscopy (10×). Mosquitoes that had ookinetes after 24 h or oocysts on day 7 post-infection were considered as ‘infected’, and their parasite load was counted.
Statistical analyses were performed using Excel 2000® and SPSS® ver.10 packages for Windows. Differences between groups in the prevalence of specific symptoms and parasite types (asexuals and gametocytes) were quantified using chi-square tests. Analysis of variance (ANOVA) was used to compare quantitative measures of parasite density between experimental groups.
General linear models (GLM) were used to test the association between human clinical status and infectivity to mosquitoes. The response variable was oocyst infection rate (arising from each person), and the independent factors were experimental group (asymptomatic or symptomatic), gametocyte density, asexual presence and density, patient sex, age, reported anti-malarial drug use and the prevalence of symptoms (chills, headache, cough, body temperature >37·5 °C, diarrhea and joint pain). In addition to these main effects, the GLM model also tested for interactions between experimental group and all other explanatory variables. Prior to analysis, infection rate data were arcsin transformed, and all parasite density measures from gametocytes to oocysts were log-transformed to increase their fit to the normal distribution. Investigation of possible determinants of differential mosquito infectivity (e.g. inter-stage parasite mortality) was performed using life table analysis. We computed the mortality coefficient (k) for parasites as they developed from gametocytes to oocysts via the ookinete stage (Vaughan et al. 1992; Gouagna et al. 1998), and tested whether this parameter varied in response to clinical status.
Over the course of this study, a total of 3987 patients attending the clinic with malaria-like symptoms (symptomatic) were examined, of which 45·7% had asexual parasites in their blood smears (Table 1). A parallel screening of 1159 apparently healthy subjects in the local community found 34·7% had P. falciparum asexual parasites. At the time of screening, no significant differences were observed between the gametocyte prevalence in the clinic (3·1%) and community (4%) populations (Table 1). Absolute densities of asexual and sexual parasites were only estimated for gametocyte carriers enrolled in infection experiments (Table 2).

Table 2. Characteristics of Plasmodium falciparum gametocyte carriers recruited for infection experiments with Anopheles gambiae (SP, Sulfadoxine pyrimethamine; CQ, chloroquine. Both asexual parasite and gametocytes densities are estimated per μl of blood.)

The rate of recruitment was higher in the community than in the clinic group. Of 94 P. falciparum gametocyte carriers detected in the asymptomatic group, 60 agreed to participate in the study. By contrast, only 21 out of 47 gametocyte carriers from the clinic were able to participate, with several being excluded on account of severe clinical symptoms. Of all gametocyte carriers that were recruited, asexual parasites were observed in 76% and 50% of the symptomatic and asymptomatic population respectively (χ12=3·8, P=0·04). Mean asexual parasite density was significantly higher in the clinic group than among the asymptomatics (F1,79=13·30, P<0·01, Table 2). The mean density of circulating gametocytes in individuals recruited from the community was marginally higher than in volunteers from the clinic (F1,79=4·12, P=0·05).
The distribution of symptoms among each group is shown in Fig. 1. There was a clear difference in reported symptoms between the two groups. The reported prevalence of several symptoms including headache (χ12=10·7, P<0·01), fever (χ12=11·2, P<0·01), joint pain (χ12=14·0, P<0·01) and vomiting (χ12=7·9, P<0·01) was substantially higher in the clinic than in the community population. There was no significant difference in the reported use of anti-malarial drugs between the two groups (Table 2). With regard to body temperature, a higher proportion of gametocyte carriers from the clinic had fever ([ges ]37·5 degree Celsius) at presentation as compared to the asymptomatic group (χ12=13·8, P<0·01). Reported fever combined with the presence of asexual parasites was also strongly associated with clinical status (χ12=17·5, P<0·01).

Fig. 1. Frequency distribution of reported malaria-like symptoms reported by symptomatic and asymptomatic Plasmodium falciparum gametocyte carriers.
There was no difference between the average numbers of mosquitoes dissected per trial in each group (mean, 22·7 and 22·4 mosquitoes dissected in the symptomatic and asymptomatic experimental groups respectively, F1,78=0·002, P=0·96). Only 2 (10%) symptomatic individuals infected mosquitoes, while 53% of the 60 asymptomatic gametocyte carriers yielded at least one mosquito infection (χ12=11·53, P<0·01, n=80). The proportion of mosquitoes that became infected per trial was substantially higher for asymptomatics than patients (F1,78=12·19, P<0·01, Table 3), as was the mean oocyst intensity (F1,78=8·46, P<0·01, Table 3).

General Linear Models were used to investigate the influence of gametocyte density, presence and density of asexual parasites, patient sex, reported anti-malarial drug use and symptoms on the outcome of experimental mosquito infections. Analyses were performed in which all of the above factors were fitted simultaneously, with non-significant terms being eliminated to yield a minimal statistically significant model of mosquito infection rate. The minimal statistically significant model for infection rate included both gametocyte density (F1,76=7·60, P<0·01) and health status (F1,76=7·96, P<0·01). Overall, infection rate increased with gametocyte density, and for a given gametocyte density, asymptomatics were more infectious than those with clinical symptoms (Fig. 2A). The slope of the relationship between gametocyte density and oocyst infection rate did not vary between groups (gametocyte density*health status interaction: F1,73=1·06, P=0·31). Neither asexual parasite prevalence, density, subject age, sex, disease symptoms, nor previous drug were significantly related to mosquito infection rate.

Fig. 2. Relationships between gametocyte density and (A) infection outcome and (B) total parasite mortality, in mosquitoes fed blood from symptomatic and asymptomatic Plasmodium falciparum gametocyte carriers. (○) Data from asymptomatic carriers; ([bull ]) carriers reporting to the clinic. The grey and black lines give the regression lines for the gametocyte density relationships for the asymptomatic and symptomatic populations respectively.
Mechanisms underlying the difference in infection rates and intensities between the two groups were further investigated using life table analysis. At 24 h post-infection, ookinetes were found in only 33·3% of experiments with blood from symptomatic patients. Of these, 67% failed to yield infection 7 days later at the oocyst stage. In contrast, a higher proportion (80·7%) of experimental healthy gametocyte carriers yielded ookinete infections, a prevalence that was only slightly higher than that observed at the oocyst stage (69·5%). There was strong evidence of density-dependent mortality of parasites within the mosquito, with survival from gametocyte to oocyst decreasing with initial gametocyte density (F1,72=41·06, P<0·01). Accounting for variation in initial gametocyte density (by including this factor in the model), there was an additional effect of patient health status on mortality. Both total parasite mortality (F1,71=6·55, P=0·01, Fig. 2B) and the mortality from gametocyte to ookinete (F1,72=36·8, P<0·01) was higher in the symptomatic group.
Overall parasite mortality (KT) was significantly related to the proportion of mosquitoes that became infected (F1,70=156·02, P<0·01). When added to the minimal statistically significant model of infection rate (that included only gametocyte density and patient health status, Fig. 2A), it eliminated the effect of patient health status (effect of health status in presence of KT and gametocyte density: F1,70=0·79, P=0·38). Thus the minimally statistically significant model of mosquito infection rate included only gametocyte density (F1,71=159·33, P<0·01, positive association) and overall parasite mortality (F1,71=178·06, P<0·01, negative association), suggesting that the effect of patient health status on mosquito infection outcome can be explained by group-specific variation in these two factors.
In this study, we found that P. falciparum is substantially more infectious to An. gambiae mosquitoes when the human hosts are asymptomatic, in contrast to when they are clinically ill. Experimental infections using blood from symptomatic gametocyte carriers were 5 times less likely to infect mosquitoes and produced lower parasite burdens than infections from asymptomatic individuals. There are several explanations for the poor infectivity of blood from symptomatic carriers. First, gametocyte density was substantially lower in patients reporting to the clinic than in those detected in the community. Additionally, parasite mortality within mosquitoes was higher for the symptomatic group even after controlling for variation in initial gametocyte density. This difference in parasite mortality could be due to variation in the degree of maturation (Jeffery & Eyles, 1955; Ponnudurai et al. 1989; Sinden, 1991) and/or the sex ratio (Read et al. 1992; Robert et al. 1996) of gametocytes between groups. Certainly, if symptomatic individuals are at an earlier stage of their infection than healthy individuals, it is likely that their gametocytes are at an earlier developmental stage and perhaps less efficiently fertilized.
Asexual parasite densities were substantially higher in symptomatic individuals, a result that other studies have found to be associated with reduced infectivity of gametocytes (Eyles, 1952; Rutledge, Gould & Tantichareon, 1969; Ong et al. 1990; Sinden, 1991). We suspect that the infectivity of gametocytes at the time of clinical manifestation of malaria disease may be inhibited by serum factors, such as cytokines (TNF and IFN-γ) (Naotunne et al. 1990) and nitric oxide (Motard et al. 1993; Cao Ya-Ming, Tsuboi & Torii, 1998) which are released in response to asexual infection in laboratory models (Eyles, 1952; Butcher, Mitchell & Cohen, 1978; Mendis et al. 1987; Graves et al. 1990; Motard, Boccam & Landau, 1990; Naotunne et al. 1993; Bate & Kwiatkowski, 1994). These previous studies suggested that peaks of parasitaemia are associated with ‘crisis’ in Plasmodium infections and there is a simultaneous reduction of gametocyte infectivity to mosquitoes, which may persist for 5–7 days (Naotunne et al. 1990). In P. falciparum, gametocytes appear in the blood circulation 10–14 days after the peak of asexual parasitaemia (Hawking, Wilson & Gammage-Mendis, 1971; Sinden, 1993). Thus, any reduction of gametocyte infectivity may not be accounted for a priori by these previously studied factors released at crisis following an initial single infection. It is, however, possible that other more long-lasting factors that have not yet been studied are responsible for this longer-term reduction in infectivity.
A final factor that could have, at least in theory, influenced infectivity between groups is variation in self-administered antimalarial chemotherapy by patients prior to the recruitment (Hogh et al. 1978; Robert et al. 2000; Targett et al. 2001). However, this is not likely to explain our results, as the reported use of drugs prior to recruitment did not vary between groups. Also, given that the gametocyte carriers we studied came from the same transmission focus, and that the two groups were similar in age, we assume that it is unlikely that they exhibited different transmission blocking immune activities as demonstrated in previous studies (Butcher et al. 1978; Graves et al. 1988, 1990; Baird et al. 1991; Mulder et al. 1994; Lensen et al. 1996).
Although the presence of symptoms themselves was a good indicator of infectiousness (negative association), we could not detect any finer scale correlations between specific symptoms and transmission. Malaria related complaints (2 weeks prior to recruitment) were common in almost all gametocyte carriers, with headache, fever and joint pain being the most common. However, individually, none of the symptoms reported was significantly related to infectivity. This is consistent with previous observations in P. falciparum models (Tchuinkam et al. 1993; Haji et al. 1996). However, we caution that as most of the reported symptoms were self-described, non-specific and therefore imprecise, we cannot entirely rule out the possibility that other symptoms influence parasite transmission.
Caution should be exercised when interpreting these results with respect to general relationships between malaria disease severity and transmission. There are at least three alternative explanations for our results other than evidence of a transmission cost to inducing symptoms. First, ethical considerations made it necessary to exclude those with very severe disease from participating in this study. It is therefore possible that the range of disease severities we examined was not sufficient to accurately represent severity-transmission relationships. Second, as it would be unethical to deny treatment to infected volunteers in order to monitor the extent of disease severity and gametocyte production through time, we conducted point surveys of the infectivity of carrier groups with varying degrees of symptoms. It is thus possible that the differences we report between groups could be due to variation in the parasite population at different time-points, and not clinical status. However, we note that the differences we found between subgroups were not simply due to variation in gametocyte density (a factor that can vary substantially through time), but per gametocyte infectivity. Although it is possible to suppose our findings are a mere artifact of temporal variation in the parasite strains, we do not feel this is the most parsimonious explanation for our results.
A final and more plausible alternative explanation for our results is that instead of testing people with different levels of disease severity, we may have simply been comparing the infectivity of people at different stages of infection. Malaria-associated illness usually manifests when asexual parasite densities are high (Sinden, 1993), which is often several days or weeks before the peak of gametocyte density (Hawking et al. 1971). Thus, the higher gametocyte densities in asymptomatic individuals may be a result of their being in a later stage of infection than those sampled at the clinic. Interestingly, however, the increased infectiousness of asymptomatic carriers could not simply be explained by parasite density. Statistical analysis showed that for a fixed gametocyte density, oocyst infections were much more likely to arise from asymptomatic than symptomatic carriers; indicating that ‘per-gametocyte’ infectivity is reduced in clinically ill hosts.
In conclusion, the key finding of this study is that not only is gametocyte density lower in patients with symptomatic as opposed to asymptomatic P. falciparum malaria, but also that gametocytes produced in symptomatic carriers are less infectious than those generated in their healthy counterparts. The reduced infectivity of symptomatic infections could not be associated with the presence of any specific malaria-symptoms (e.g. fever, headache, joint pain, chill and vomiting), nor to the previous use of antimalarial drug. The reduced ‘per-gametocyte’ infectivity we observed was due, at least in part, to the higher mortality rates of parasites from symptomatic individuals as they pass from the gamete to ookinete stage of development. The reason for this increased parasite mortality is uncertain, but may be due to the presence of toxic blood factors that both cause illness and inhibit parasite development, to variation in the maturity of gametocytes in symptomatic versus asymptomatic infections, or parasite genetic differences that modulate both disease severity and infectivity. Further investigation of these factors would be of great interest to help illuminate whether the reduced infectivity of symptomatic P. falciparum infections is simply a product of parasite life-history (e.g. infectiousness to mosquitoes increases from the time of crisis), or a manifestation of a fitness cost to being virulent (Read & Mackinnon 1999; Chotivanich et al. 2000).
We are grateful to S. Ombonya and A. Ouko at the Mbita Health Centre, Suba district, L. Omukuba, P. Obare, and H. Akelo, at the Mbita Point Research Training Center of ICIPE, all the village leaders and all the patients for their support, assistance and cooperation. This paper is submitted for publication with the permission of Director, Kenya Medical Research Institute (KEMRI) and Director General, International Centre of Insect Physiology and Ecology (ICIPE). This study was supported by grants from National Institutes of Health (U19 AI45511, D43 TW01142, TW00920 and TW 01505) and ICIPE ARPPIS scholarships.

Table 1. Characteristics of individuals surveyed in the village community (asymptomatic) and in the local clinic (symptomatic) for the recruitment of gametocyte carriers

Table 2. Characteristics of Plasmodium falciparum gametocyte carriers recruited for infection experiments with Anopheles gambiae
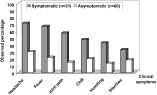
Fig. 1. Frequency distribution of reported malaria-like symptoms reported by symptomatic and asymptomatic Plasmodium falciparum gametocyte carriers.

Table 3. Parasite prevalence and burden in infected Anopheles gambiae following experimental feeds on Plasmodium falciparum infected blood from asymptomatic and symptomatic subjects

Fig. 2. Relationships between gametocyte density and (A) infection outcome and (B) total parasite mortality, in mosquitoes fed blood from symptomatic and asymptomatic Plasmodium falciparum gametocyte carriers. (○) Data from asymptomatic carriers; ([bull ]) carriers reporting to the clinic. The grey and black lines give the regression lines for the gametocyte density relationships for the asymptomatic and symptomatic populations respectively.