Introduction
Life-history theory predicts that the juvenile growth rate should be maximized under natural selection because organisms can reach a large size in a short time, and shorter juvenile periods also result in a higher probability of surviving to reproduction (Stearns, Reference Stearns1992; Roff, Reference Roff1993). The fact is growth rates are rarely maximized in nature. The concept of a trade-off is a major explanation, as fast growth may have some physiological costs because individuals have a limited pool of resources to allocate among traits (Zera & Harshman, Reference Zera and Harshman2001), that is, trade-off occurs when organisms have to divert energy to growth at the expense of other traits related to survival and fecundity (Niewiarowski et al., Reference Niewiarowski, Angilletta and Leaché2004). For example, the increased growth rate may have negative effects on maintenance efficiency and the repair of cellular damage, resulting in a shorter adult lifespan and decreased stress tolerance (Arendt, Reference Arendt1997; Metcalfe & Monaghan, Reference Metcalfe and Monaghan2003). Hence, organisms always adjust their growth trajectories to balance the benefits and costs of a high growth rate (Gotthard, Reference Gotthard, Atkinson and Thorndike2001; Dmitriew, Reference Dmitriew2010).
However, this hypothesized negative relationship between juvenile growth rate and adult traits related to survival has proven to be very difficult to test robustly because the trade-off may be quite mild and detectable only under stressful conditions (Dmitriew & Rowe, Reference Dmitriew and Rowe2007). Other variables, particularly nutrient availability and final size, can ameliorate the trade-off or make it more pronounced (Gotthard, Reference Gotthard, Atkinson and Thorndike2001; Zera & Harshman, Reference Zera and Harshman2001). Because of this, studies from the even same species show contrasting results. For example, some artificial selection for different pre-adult growth experiments in Drosophila suggested a negative correlation between fast larval development and adult lifespan (Lints & Soliman, Reference Lints and Soliman1977; Partridge & Fowler, Reference Partridge and Fowler1992; Djawdan et al., Reference Djawdan, Sugiyama, Schlaeger, Bradley and Rose1996), while many other studies in Drosophila showed a contrasting result or no correlation (Economos & Lints, Reference Economos and Lints1984; Zwaan et al., Reference Zwaan, Bijlsma and Hoekstra1991). Studies in two butterflies found that decreased lifespans under starvation conditions are a cost of a high larval growth rates (Stockhoff, Reference Stockhoff1991; Gotthard et al., Reference Gotthard, Nylin and Wiklund1994), while in another species, this cost was not found (Gotthard, Reference Gotthard1998). The trade-off between the larval growth rate and adult cold tolerance was found in a damselfly (Stoks & De Block, Reference Stoks and De Block2011) but not in other insects (Karl et al., Reference Karl, Stoks, Bauerfeind, Dierks, Franke and Fischer2013). Taken together, this suggests that the expression of a trade-off between juvenile growth and adult traits related to survival may depend on the experimental conditions and/or the genetic architecture of the founder populations (Yadav & Sharma, Reference Yadav and Sharma2014).
We set out to test the hypothesis that there is a trade-off between the pre-adult growth rate and adult lifespan. Because the trade-off can be affected by environmental factors, we also test whether there is a condition-dependent trade-off by changing the temperature and nutrient availability. In addition, life-history trade-offs play important roles in local adaptation, but most of the studies have been done in laboratory selected lines. It is important to understand how life-history trade-off is involved in the natural populations. We used a widely distributed moth, the cotton bollworm, Helicoverpa armigera, collected from different geographic regions as our model organism. This moth is distributed from almost the southernmost to the northernmost regions of China, with an impressive ability to adapt to different environments. Because of the ability to adapt to extremely different environments, testing how this insect balances the allocation of the energy pool to larval growth and the following adult fitness traits is important for us to understand the mechanism of insect distribution. Many of its life-history traits show geographical variation, such as cold tolerance and diapause, due to local adaptation (Chen, Reference Chen2012; Chen et al., Reference Chen, Chen, He, Xia and Xue2013). We noticed that the northern China population grew faster than the southern China population when we performed an experiment to study the geographical variation of diapause (Chen et al., Reference Chen, Chen, He, Xia and Xue2013). Therefore, we believe there may be some genetic variations in larval growth among populations, which may be a result of adaptation to the length of the growing season.
We first examined the pre-adult development time, growth rate, and pupal weight in three geographical populations and found that the pre-adult development time is significantly shorter, and the growth rate is significantly higher in the northern China population than in the southern China population. Then, we performed another experiment to examine the adult lifespan with only the southern and northern China populations, which differed most significantly. To test how environmental factors can influence trade-offs, the adult lifespan was tested at different temperatures and after being provided with different levels of nutrient supplements. The result suggests that there is a strong correlation between the larval growth rate and adult lifespan, but this correlation is highly environment-dependent, as indicated by significant population–temperature and population–nutrient interactions.
Materials and methods
Insect culture
Fully grown larvae were collected from three geographical populations that represent populations from northern, central, and southern China along a latitudinal gradient from three locations in China [southern China population: Huizhou, Guangdong Province (23°07′N, 114°21′E); central China population: Hefei, Anhui Province (31°49′N, 117°13′E); northern China population: Langfang, Hebei Province (39°32′N, 116°40′E)]. Collected larvae were reared on an artificial diet (Wu & Gong, Reference Wu and Gong1997) and maintained in the rearing room under L:D 16:8 at 25°C until adult eclosion. These adults were transferred to cages (40 × 25 × 18 cm) with a removable gauze cloth top to mate and for egg collection. The adults were fed a 10% sucrose solution. Eggs were collected on days 4, 5, and 6 after eclosion. After hatching, every 3–5 newly hatched larvae were reared together in 24-well cell culture plates (for each well: diameter: 1.5 cm; height: 2 cm) on an artificial diet until the third instar. Third instar larvae were individually transferred to new cell culture plates with food until the fifth instar. Then, the fifth instar larvae were individually reared in plastic plates with 21 wells (length: 2.5 cm; width: 2.5 cm; height: 2.5 cm) until pupation. The third laboratory-reared generation was used for the experiment. All the experiments were carried out in incubators (LRH-250-GS, Guangdong Medical Instrument Manufacturer, Guangdong, China) equipped with six fluorescent 30 W tubes. The light intensity during photophase was approximately 2.0 W m−2, and the variation of temperatures was ±1°C.
Pre-adult life-history traits
For each of the three geographical populations, the newly hatched larvae collected from the three different days were treated as three blocks (represent three replicates), and each block of newly hatched larvae was randomly divided into three groups that were reared under L:D 16:8 at three temperatures of 20, 25, and 30°C. The rearing methods for larvae were the same as described above. For all individuals, we recorded the larval development time from hatching to pupation and the pupal development time from the day of pupation to adult eclosion. Together, these measurements indicated the total development time. All pupae were weighed within 24 h after pupation using an electric balance (AUY120 produced by SHIMADZU Corporation, Japan). Adults were weighed by place into a pre-weighted tube on the day of eclosion after having excreted meconium, so the adult weight is calculated by using the total weight of tube and adult to substrate the weight of tube. A measure of the growth rate was calculated for each individual according to the equation: Growth rate = ln (pupal weight)/larval development time (Gotthard et al., Reference Gotthard, Nylin and Wiklund1994). Weight loss during pupal development was also calculated for each individual according to the equation: weight loss rate = ln (pupal weight – adult weight)/pupal time. Because the results on development time and mass are not the focus of this paper, they are presented as a supplementary file (figs S1 and S2).
Adult lifespan
According to the larval developmental result, we select the southern and northern China populations to study the adult lifespan because these two populations show the largest differences in pre-adult growth rate. For each population, the newly hatched larvae from eggs collected from three different days were treated as three blocks (represent three replicates), and each block of newly hatched larvae was divided into three groups that were reared under L:D 16:8 at three temperatures of 20, 25, and 30°C. The rearing methods for larvae were the same as described above. After pupation, pupae were picked out from the artificial diet to cages for eclosion and kept at the same temperature as the larvae. Adult eclosion was checked every day. The newly emerged adults were sexed and individually placed in a 40 ml polystyrene tube covered with gauze and provided with one of the three nutritional conditions: (1) standard rearing condition (fed with 10% sucrose solution); (2) starvation (fed with water only); (3) desiccation (no water or any other supplements). All adults were kept at the same temperature as the larva to test the adult lifespan. Therefore, there were three temperature treatments and three nutritional treatments for each population. For each treatment, the adult lifespan was scored every day, and the lifespans of 85–124 adults were successfully recorded for each treatment.
Statistical analyses
We analyzed the development time, pupal weight, and growth rate in linear mixed-effect models with temperature, population, and sex as the fixed effects and the block as a random effect using the lme4 library (Bates et al., Reference Bates, Maechler, Bolker and Walker2015), and χ2 tests of fixed effects were performed using the CAR package. Longevity was analyzed by a mixed-effects Cox model with the coxme library (Therneau, Reference Therneau2015), with the sex, population, and temperature treated as fixed effects and the block as a random effect. Pairwise comparisons were also performed to compare the differences between the two populations under each treatment. All statistical analyses were performed with R 3.02 (R Core Team, 2016).
Results
Pre-adult life-history traits
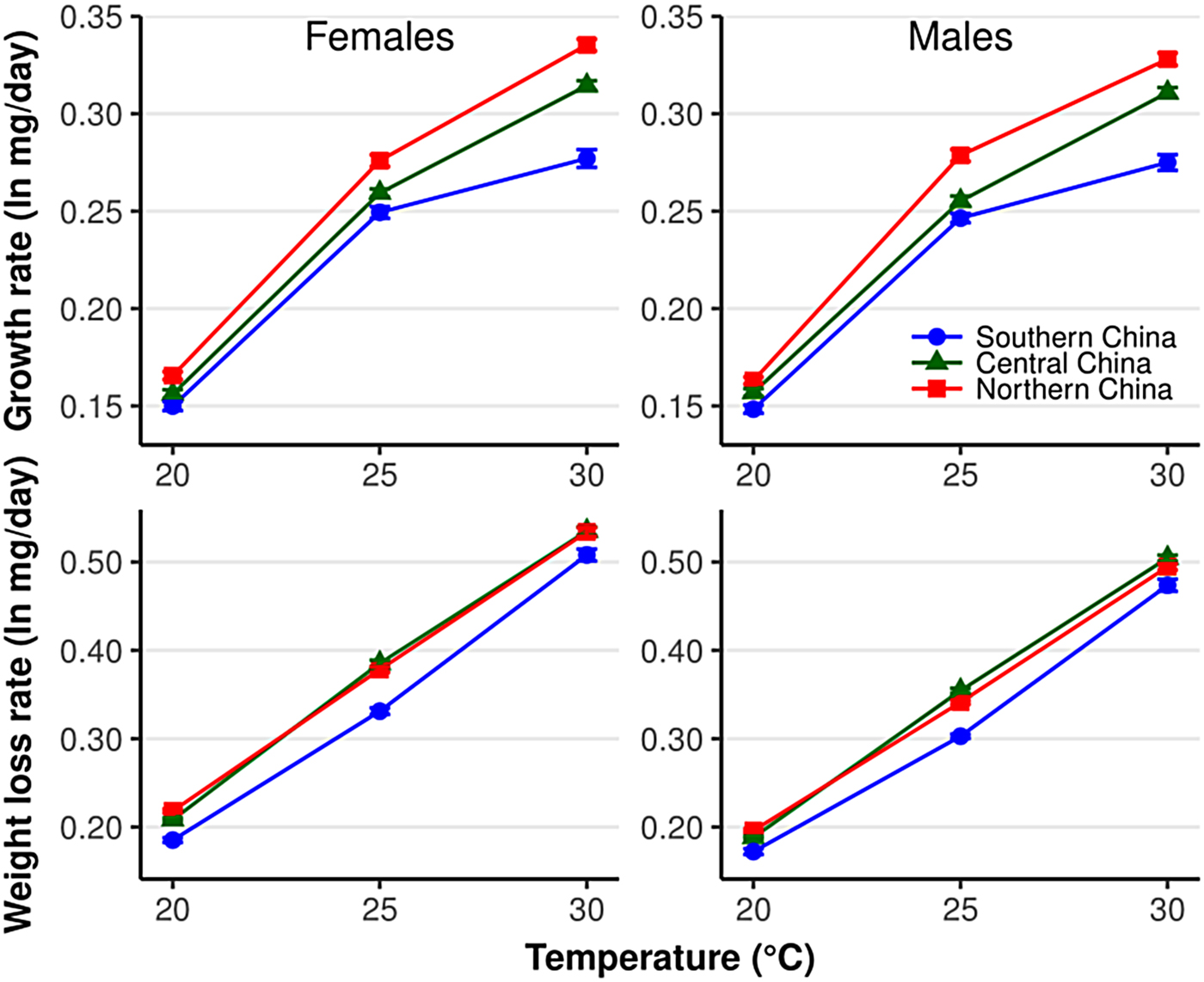
The growth rate was significantly different among populations (χ2 = 145.07, df = 2, P < 2.2×10−16), and the difference was significantly affected by temperature (population × temperature: χ2 = 36.5, df = 4, P = 2.27×10−07) (fig. 1). For example, the growth rate was significantly different between the central and southern China populations at 30°C, but not at 20 and 25°C (fig. 1). All populations show an increase in the growth rate with increasing temperatures (χ2 = 3393.9, df = 2, P < 2.2×10−16). The growth rate was significantly higher in males than females (χ2 = 6.5, df = 1, P < 0.01), and the differences between sexes were not influenced by either temperature (sex × temperature: χ2 = 1.8, df = 2, P = 0.41) or population (sex × population: χ2 = 1.3, df = 2, P = 0.52).
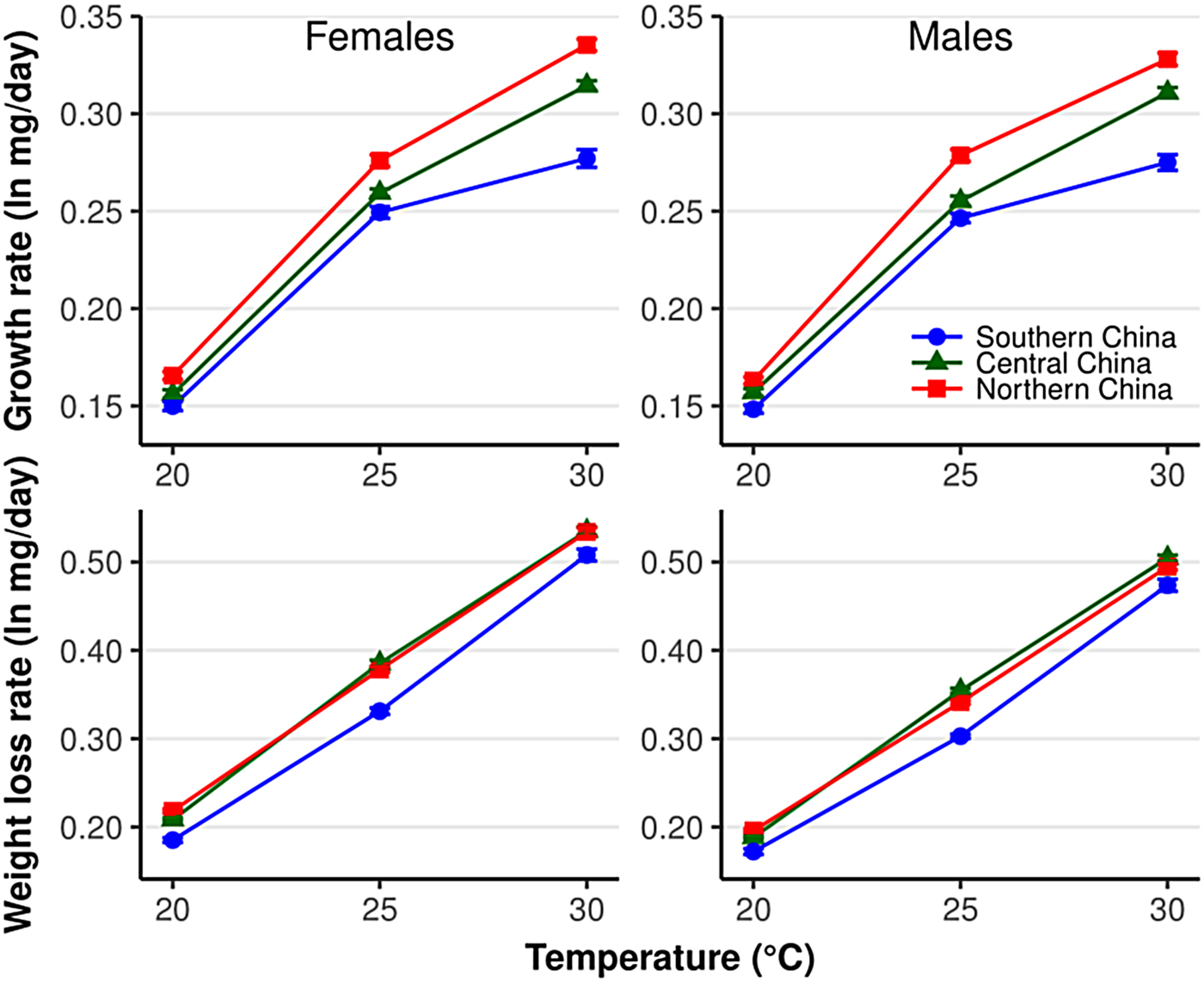
Fig. 1. Larval growth rate and pupal weight loss rate in Helicoverpa armigera females and males in relation to temperature and population. Error bars indicate SE.
The weight loss rate was significantly affected by population (χ2 = 41.40, df = 2, P = 1.03×10−09), sex (χ2 = 32.54, df = 1, P = 1.17×10−08), and temperature (χ2 = 6278.7, df = 2, P < 2.2×10−16) (fig. 1). The weight loss rate in the northern China population was significantly higher than that in the southern China population in all treatments (all P < 0.05) but was only significantly higher than the central China population at 25°C. This indicates a higher metabolic rate in the northern China population than in the southern China population.
Adult lifespan
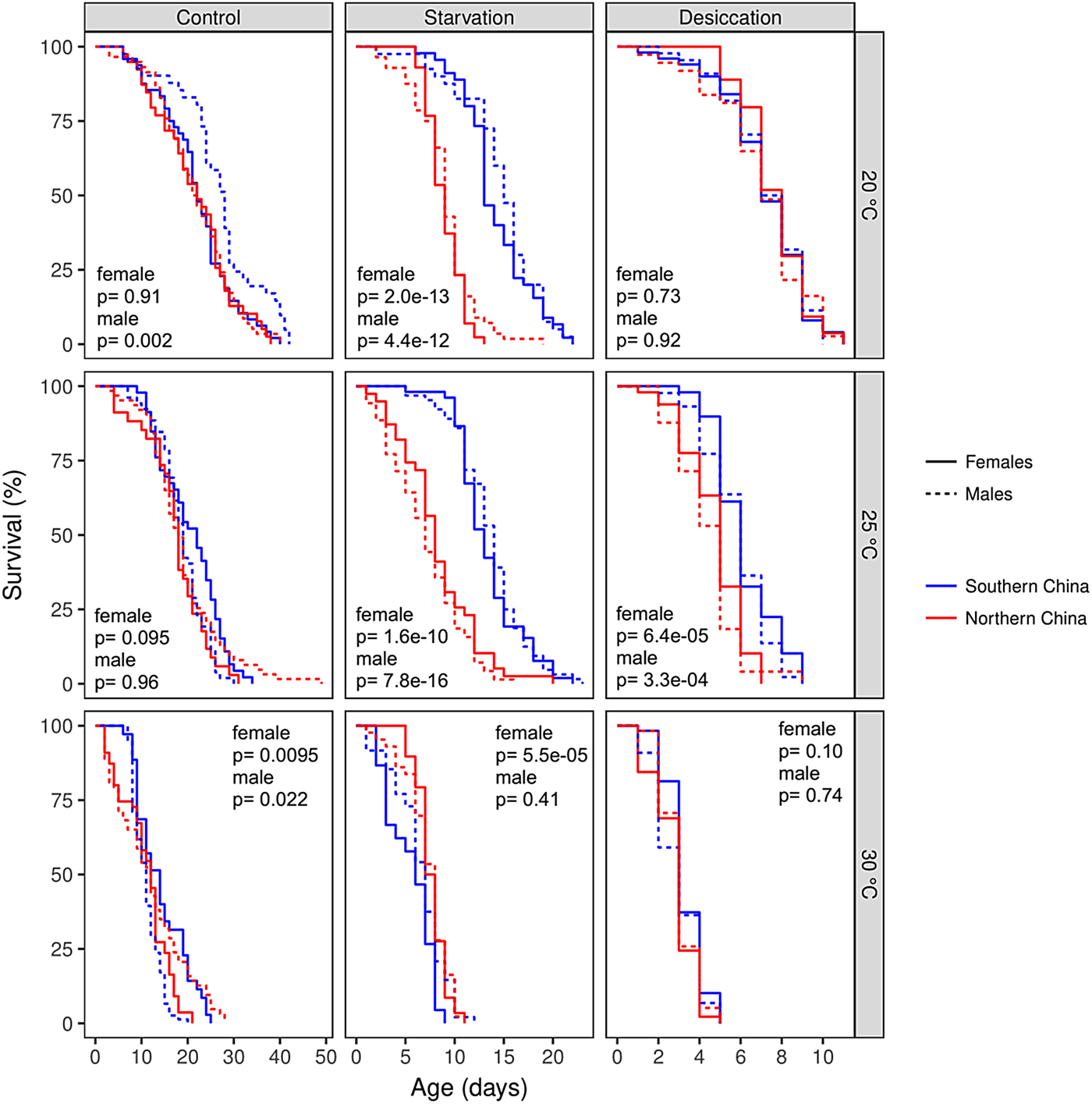
Differences in the adult lifespan between the southern and northern China populations were condition-dependent, which is indicated by significant interactions between population and food (population × food: χ2 = 71.8, df = 2, P = 2.6×10−16) and the three-way interactions among population, food, and temperature (population × food × temperature: χ2 = 115.09, df = 4, P < 2.2×10−16) (fig. 2). At 20°C, females from southern China population (higher larval growth rate) lived significantly longer than that of northern China population (lower larval growth rate) only in starvation condition (z = 0.35, P = 7.30×10−01); males from southern China population lived significantly longer than that of northern China population in both control (z = 3.09, P = 0.002) and starvation conditions (z = 6.92, P = 4.40×10−12) (fig. 2). At 25°C, both females and males from southern China population lived significantly longer than that of northern China population in starvation (female: z = 6.39, P = 1.60×10−10; male: z = 8.05, P = 7.80×10−16) and desiccation conditions (female: z = 4.9, P < 0.001; male: z = 3.7, P = 0.0002), but not in control condition (z = 1.51, P = 0.13). At 30°C, females from Southern China population lived significantly longer than that of northern China population in control condition (z = 2.59, P = 0.0095), but lived significantly shorter in starvation condition (z = 4.03, P = 5.5×10−05); males from southern China population lived significantly shorter than that of northern China population in control condition (z = 2.29, P = 0.022), but a significant difference was not found in any other conditions.
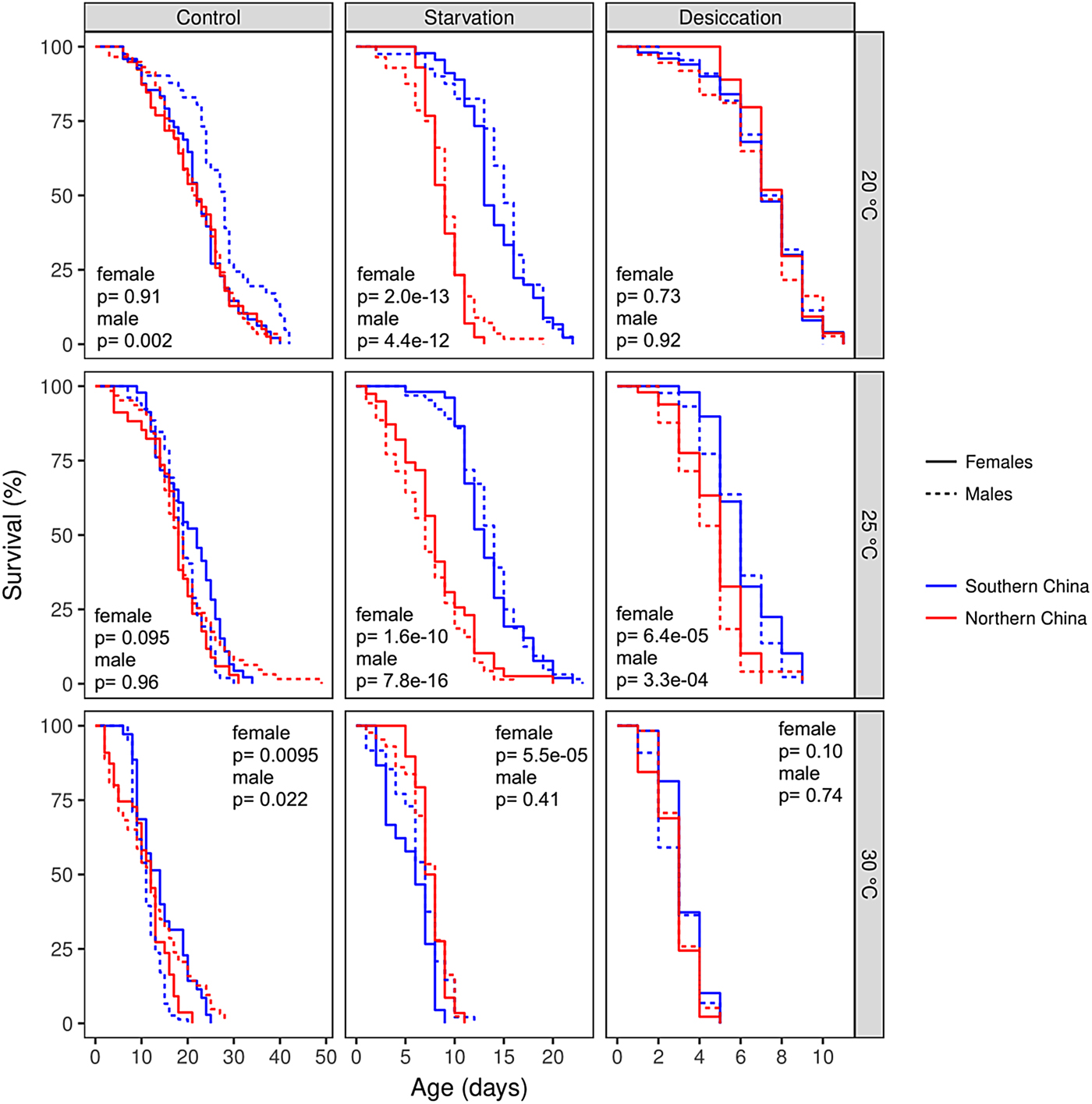
Fig. 2. Survival curves of Helicoverpa armigera from southern and northern China populations under the control conditions (fed with 10% sucrose solution), starvation feeding condition (fed with water only), and desiccation feeding condition (no water or any other supplements were supplied) at three different temperatures of 20, 25, and 30°C. P-values are results of pairwise comparisons between populations under a specific treatment.
Sex difference in lifespan was also condition-dependent (sex × temperature: χ2 = 27.5, df = 2, P = 1.1×10−06; sex × temperature × food: χ2 = 26.3, df = 4, P = 2.7×10−05). Under the standard condition, a significant sex difference was found at 20 and 25°C for the southern population (20°C: z = 2.70, P = 0.007; 25°C: z = 1.74, P = 0.08; 30°C: z = 4.07, P < 0.001) and at 30°C for the northern population (20°C: z = 0.12, P = 0.9; 25°C: z = 1.71, P = 0.09; 30°C: z = 2.97, P = 0.003). Under the starvation condition, a significant sex difference was only found at 30°C for the southern population (z = 2.65, P = 0.008). No sex difference was found under the desiccation condition (all P > 0.05).
Discussion
In this study, we show that the population of H. armigera collected from northern China has a higher growth rate (fig. 1) and shorter developmental time (fig. S1) than the population collected from southern China, indicating faster pre-adult growth and development. We speculate that the faster growth is a result of the adaptation to shorter and cooler growing seasons in the north. Then, we use the northern and southern China populations with different growth rates to test for a trade-off between pre-adult growth and adult lifespan. The results demonstrated that there may be trade-offs between the growth rate and lifespan, which was suggested by a negative relationship between juvenile growth rate and adult life span in stressed conditions, such as starvation and desiccation; however, the trade-offs can be ameliorated or even offset by supplying sugar. The trade-offs can also be confounded by adult body size because a larger body size is expected to have more nutrient reserves (Gotthard, Reference Gotthard2000; Gotthard, Reference Gotthard, Atkinson and Thorndike2001). Our results partially support the resource-mediated mechanism underlying the trade-off between growth rate and lifespan, which hypothesized that the high growth rates associated with high metabolic rates would deplete the stored resources faster during a period of exposure to sublethal conditions, such as during a period of starvation (Gotthard, Reference Gotthard, Atkinson and Thorndike2001).
Geographical variation in pre-adult life-history traits
We found very consistent patterns of development and growth variation among geographical populations in all treatments. The development time is shorter, and the growth rate is higher in the northern China population compared with the southern China population in all experimental temperatures and both sexes. We think this pattern is a result of local adaptation to the length of growing season, even though voltinism is involved in our study organism because the seasonal time was thought to be sharply decreased where new generations are added to the phenology of a species (Kivelä et al., Reference Kivelä, Välimäki, Carrasco and Oksanen2011). H. armigera can complete 3–4 generations in northern China, 4–5 generations in central China, and 6–7 generations in southern China (Guo, Reference Guo1998), overwintering as pupae in the soil. The estimation of seasonal length for each generation is complex for this insect. However, we can expect that for the diapause generation in central and northern China, the larvae that hatched earlier could have a better chance to develop to the diapause stage because of the sharp temperature decrease in the autumn to below the developmental threshold (~12°C) (Chen, Reference Chen2012) of this insect (fig. S3). Actually, we can find young larvae in mid-September for the northern population when the average temperature is below 15°C, and these larvae generally cannot develop to the diapause pupal stage (self-observation). For the southern China population, the situation is completely different, as the temperature never drops below the developmental threshold (fig. S3), and the larvae of the diapause generation that hatched very late can still develop to the diapause pupal stage. Therefore, the seasonal time constraint is relatively relaxed for the southern China population compared with central and northern China populations. The patterns of pre-adult life-history traits in our insect are consistent with many latitudinal studies in other insects, which are greater time constraints in the north (Blanckenhorn & Demont, Reference Blanckenhorn and Demont2004; Kivelä et al., Reference Kivelä, Välimäki, Carrasco and Oksanen2011).
Condition-dependent trade-offs between the growth rate and adult lifespan
Because variation in the growth rate was found in our study insect, it is reasonable to hypothesize that there may be some variation in adult lifespan because of potential effects from the larval growth rate. Thus, the main aim of this study is to investigate whether there is a genetic trade-off between the growth rate and adult lifespan in natural selected populations that differ in growth rate and how this trade-off can be affected by environmental factors. Two widely accepted mechanisms underlying the trade-offs are the free-radical-mediated trade-offs and resource-mediated trade-offs. Free-radical-mediated trade-offs hypothesized that an increased growth rate is associated with higher oxidative stress and the organism accumulates cellular damage faster, resulting in a decreased lifespan (Metcalfe & Monaghan, Reference Metcalfe and Monaghan2003; Mangel & Munch, Reference Mangel and Munch2005). The resource-mediated trade-offs hypothesized that the increased growth rate is associated with a higher metabolic rate, which would consume stored resources during a period of exposure to sublethal conditions, such as a period of starvation (Gotthard, Reference Gotthard, Atkinson and Thorndike2001). We found that changes in nutrient availability and energy reserves can obscure the negative relationship between the growth rate and lifespan in our study insect, which supports the second hypothesis in particular.
We found that the difference in growth rates between the southern and northern populations increased with the rearing temperature (fig. 1). If there is a trade-off between the growth rate and adult lifespan, we also expected that the difference in adult lifespan should be larger at higher temperatures. However, our results did not show such a covariation. The largest and smallest differences in the growth rate between populations are at 30 and 20°C, respectively. However, the lifespan, under the standard laboratory condition and desiccation condition, was not different between populations in most of the treatments. Under the starvation condition, the largest difference in the adult lifespan between populations is at 20°C and not at 30°C. We speculate that this can be explained by two reasons. First, there may be some other fitness traits also involved in the trade-offs; for example, the insects reared at higher temperatures may invest more energy to be heat-resistant, and those reared at lower temperatures may allocate some energy to cold tolerance, so the changes in the allocation of energy to different fitness traits may affect the current study of trade-offs between the growth rate and lifespan. Another possibility for this result may be because of the confounding of the final size. We found that adult weight in the northern population is significantly larger than that of the southern population at 30°C but is significantly smaller than that of the southern population at 20°C (fig. S2). Therefore, the hypothesized negative relationship between the growth rate and lifespan is potentially affected by adult body size, because the larger size is expected to have more nutrient reserves and may have some positive effects on lifespan (Gotthard, Reference Gotthard, Atkinson and Thorndike2001). For example, under the starvation condition at 30°C, the northern population was expected to live for a shorter time because of the higher growth rate; however, the accelerated growth rate also resulted in a larger body size, which always correlated with more energy reserves (Gotthard, Reference Gotthard, Atkinson and Thorndike2001; Karl & Fischer, Reference Karl and Fischer2008), thereby offsetting the costs arising from the high growth rate. Thus, the northern population actually benefited from the higher growth rate to be more resistant to starvation stress. Under the starvation condition at 20°C, the difference in lifespan was expected to be small because of the small difference in growth rate. Interestingly, the lifespan was significantly longer in the southern population than in the northern population (fig. 2). This may also be a result of the difference in body size, because the northern population had a significantly smaller body size, which means fewer energy reserves, and then the costs of the high growth rate become more pronounced.
Thus, the adult weight seems to be positively related to the lifespan, a large body size can offset and small body size can amplify the cost of a high growth rate in some conditions, and the potential effects of body size on the lifespan cannot be neglected (Lee et al., Reference Lee, Monaghan and Metcalfe2012; Lind et al., Reference Lind, Chen, Meurling, Guevara Gil, Carlsson, Zwoinska, Andersson, Larva and Maklakov2017). In our study, the results from both 20 and 30°C cannot help us establish a solid negative relationship between the growth rate and lifespan because of the potential effect of body size. Therefore, it is important to consider the effect of body size when testing the growth rate and lifespan because the body size may obscure the relationship between the growth rate and lifespan.
At 25°C, testing for a trade-off is no longer confounded by body size because no significant differences in body size were found between the two populations. The correlation between the growth rate and lifespan can be established based on the result from 25°C. The most interesting result in this respect is that we do find a negative relationship between the growth rate and adult lifespan, but the presence of the negative relationship is only under stressed conditions. Under starvation and desiccation conditions, adults from the southern population with lower growth rate lived much longer than those of the northern population with higher growth rate, which suggested there may be a trade-off between growth rate and adult lifespan. However, there was no clear correlation between the growth rate and lifespan found under the standard laboratory condition; that is, no trade-off was found as the lifespans between the populations were not significantly different in both females and males. Similar results were found in the ladybird beetle, Harmonia axyridis, in which accelerated growth had no effects on female fecundity or survivorship when offered food; however, individuals who underwent accelerated growth died significantly sooner when deprived of food late in adult life (Dmitriew & Rowe, Reference Dmitriew and Rowe2007). Both results suggest that the costs of a high growth rate may be quite mild and detectable only under stressful conditions. Our results also support the resource-mediated mechanism underlying the trade-off, which hypothesized that the high growth rate is associated with a high metabolic rate would consume the stored resources faster during a period of exposure to sublethal conditions, such as a period of starvation (Gotthard, Reference Gotthard, Atkinson and Thorndike2001). This has been shown in a damselfly, where the fast-growing damselflies that had a higher metabolic rate depleted their stored reserves, such as glycogen and triglycerides, faster (Stoks et al., Reference Stoks, De Block and McPeek2006). In our case, under starvation and desiccation conditions, the insect can only rely on their energy reserves to survive, and because the northern population grew faster, which is associated with a higher metabolic rate, this will deplete the energy reserves faster and finally make lives shorter. However, under standard laboratory conditions, the energy source becomes unlimited, the lifespan is less affected by metabolic rate, and the trade-off finally disappeared.
In our study, we did not directly measure the metabolic rate, but a relatively higher metabolic rate is always expected in ectotherms from high latitudes/altitudes in terms of metabolic cold adaptation theory (Addo-Bediako et al., Reference Addo-Bediako, Chown and Gaston2002). We may also infer a higher metabolic rate in the fast-growing population from the result of pupal weight loss rate. During the pupal stage, the weight loss rate should be only directly correlated with the metabolic rate, as the insect is not feeding and growing and only consumes stored reserves. The weight loss rate in the northern population is higher than in the southern population in both females and males at all the experimental temperatures, indicating a higher metabolic rate in the northern population. Therefore, our results support the hypothesis of a higher metabolic rate in the population with faster growth.
In conclusion, testing for a trade-off, especially mild trade-offs, is always hard because of many potential confounding variables, and the expression of a trade-off is highly dependent on the environment. In our study, the trade-off between the growth rate and lifespan is only found under stressed conditions. Because development and growth are often under strong selection in environments with strong seasonality, our results suggest that the trade-off between growth and lifespan may play an important role in the evolution of lifespan in natural populations. Understanding how this widely distributed insect balances the costs and benefits of a high growth rate is critical for us to understand the mechanisms that determine the distribution of the insect. However, our result about rapid growth decreases adult lifespan under adverse conditions did not indicate low fitness consequences since the whole life cycle should be considered when thinking about fitness. Even though fast growth has some negative effects on adult lifespan, fast growth is always beneficial in most situations. Thus, other life-history parameters such as the intrinsic rate of natural increase are needed to determine whether a decreased adult lifespan can affect the overall fitness. In addition, only two populations were used in the present study, and further studies with more populations are still needed to test for the generality of this pattern. Testing other fitness traits is also necessary for us to unlock the framework of life-history evolution since multiple-trait trade-off is involved in many situations.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485318001001.
Acknowledgements
We thank all the students in the laboratory for helping with the experiment. This study is financially supported by National Natural Science Foundation of China (31260430) to FS Xue.