INTRODUCTION
The snail Buccinanops monilifer (Kiener, 1834) is a common representative of the malacofauna of the south-western Atlantic Ocean. It is one of the seven species grouped in the genus Buccinanops d'Orbigny, 1841, endemic in the south-western Atlantic Ocean. In the Argentine Malacological Province Buccinanops monilifer is widely distributed in shallow waters up to 50 m deep, from Rio de Janeiro in Brazil (23°S) to San Matías Gulf (42°S) in Patagonia, Argentina (Rios, Reference Rios1994; Gallardo & Penchaszadeh, Reference Gallardo and Penchaszadeh2001). In Mar del Plata (Buenos Aires Province), B. monilifer lives in subtidal sandy bottoms of temperate waters. It is usually collected with bottom trawling nets between 5 and 20 m in depth, as by-catch of the shrimp Artemesia longinaris fisheries, representing 1–2% of the biota (Scelzo et al., Reference Scelzo, Arca and Lucero2002).
Buccinanops monilifer was situated within the Dorsanum genus for a long time, because the adult's external aspect differs from other adult Buccinanops by the possession of a tuberculed shell; Pastorino (Reference Pastorino1993) included the species into the Buccinanops genus based on radular, morphological and reproductive characters (see also Penchaszadeh, Reference Penchaszadeh1971a, Reference Penchaszadehb). As in all other Buccinanops species, B. monilifer attaches a variable number of egg capsules to the callous region of the adult's shell where it carries them (Penchaszadeh, Reference Penchaszadeh1971a).
In a previous study in this species Penchaszadeh et al. (Reference Penchaszadeh, Averbuj and Cledón2001) reported for the first time the occurrence of the imposex phenomenon in Argentina. Imposex is a worldwide distributed phenomenon first cited by Blaber (Reference Blaber1970), and it has been used extensively to monitor tributyltin (TBT) and other organic pollutants in seawater environments (Gibbs & Bryan, Reference Gibbs, Bryan and Kramer1994; Oehlmann et al., Reference Oehlmann, Fioroni, Stroben and Markert1996b; Minchin & Minchin, Reference Minchin and Minchin1997; Gooding et al., Reference Gooding, Gallardo and Leblanc1999; Castro et al., Reference Castro, Matthews-Cascon and Fernandez2000; Bigatti & Penchaszadeh, Reference Bigatti and Penchaszadeh2005; Miloslavich et al., Reference Miloslavich, Penchaszadeh and Bigatti2007; Bigatti et al., Reference Bigatti, Primost, Cledón, Averbuj, Theobald, Gerwinski, Arntz, Morriconi and Penchaszadeh2009). Furthermore, the effect of imposex on individuals may have undesirable effects for the population, e.g. sterilization of the females that may lead to population decline (Oehlmann et al., Reference Oehlmann, Stroben, Schulte-Oehlmann, Bauer, Fioroni and Markert1996a).
In Mar del Plata a ‘beach nourishment’ (beach filling with sediment from a distant site) took place between November 1998 and April 1999 (Rubén Lopez, personal communication). Sediment was pumped from a sand bank located in the southern breakwater of the Mar del Plata harbour (Marcomini & Lopez, Reference Marcomini and Lopez2006), where previous studies evidenced the presence of organotin (TBT) compounds (Penchaszadeh et al., Reference Penchaszadeh, Averbuj and Cledón2001; Goldberg et al., Reference Goldberg, Averbuj, Cledón, Luzzatto and Nudelman2004; Bigatti et al., Reference Bigatti, Primost, Cledón, Averbuj, Theobald, Gerwinski, Arntz, Morriconi and Penchaszadeh2009). Teso & Penchaszadeh (Reference Teso and Penchaszadeh2009) demonstrated the effect of the beach filling in an endemic Olividae, Olivancillaria deshayesiana (Ducros de Saint Germain, 1857).
Embryos in this genus complete their development within the egg capsule by ingestion of nurse eggs, hatching as crawling juveniles through an opening area opposed to the peduncle. Commonly one embryo hatches from each egg capsule in Buccinanops species, including B. monilifer. Buccinanops cochlidium is the only exception, hatching five embryos per egg capsule on average, and with variable sizes (Penchaszadeh, Reference Penchaszadeh1971b, Reference Penchaszadeh1973; Averbuj, Reference Averbuj2009; Averbuj & Penchaszadeh, Reference Averbuj and Penchaszadeh2009). The intracapsular embryonic development of B. monilifer was also studied in order to search for possible malformations or other TBT consequences.
The aim of this paper was to assess the impact of imposex on the reproductive activity of a population of B. monilifer from Mar del Plata, in the period when the beach filling process occurred, and to compare with an undisturbed location (Mar Chiquita).
MATERIALS AND METHODS
Sampling
Specimens of Buccinanops monilifer were obtained in monthly samples taken from December 1997 to December 2001. The material was collected in waters off Mar del Plata (MdP) (38°00′S 57°33′W) and in one sample from off Mar Chiquita (MCh) in December 2000, which is an area of low marine traffic situated 22 km north of MdP (37°S43′57°29′W) (Figure 1). Collection was performed from fishing boats by bottom trawling between 5 and 20 m in depth, with a bottom trawling net with 25 mm mesh size.
Fig. 1. Sampling sites: Mar del Plata and Mar Chiquita.
Reproductive biology
Individuals were measured, dissected and sexed by the presence or absence of a vagina and accessory glands. The penis was measured from the base to the top, in cold-relaxed males and females (with imposex). The sex-ratio was calculated; juveniles were not used in this study. Total shell lengths were measured with a 0.1 mm precision Vernier caliper. Whenever present, the attached spawn was fixed in a 5% formalin solution. Damaged spawn were not included in this study.
In order to search for malformations in the embryonic development and other abnormalities in the polluted site, the following reproductive parameters were studied: (1) egg capsules were counted in each spawn; (2) two egg capsules were randomly chosen within each spawn, detached from the shell and dissected; the number of nurse eggs per capsule was counted under a Zeiss light microscope whenever there were not any embryos with a velum (the stage when the embryos start ingesting eggs) within the egg capsules; (3) the intracapsular egg diameter was measured before cellular cleavage; for this purpose 10 eggs were randomly chosen from each of the dissected egg capsules; and (4) the embryos were described and counted, at each of the different stages of development (see Table 1). All measurements were made under a Zeiss stereoscopic microscope with a 0.1 mm precision ocular micrometer.
Table 1. Embryo stages, sizes, and number of embryos accompanying the successful embryo at each stage in the egg capsules, in Mar del Plata.

Imposex
The imposex percentage and the relative penis size index (RPSI) (Gibbs & Bryan, Reference Gibbs, Bryan and Kramer1994) were calculated in both sites. The imposex percentage and RPSI were compared between the months previous (March–June 1998), during (November 1998–February 1999) and posterior (May and November 1999) to the nourishment episode in Mar del Plata. Comparisons of imposex percentage and RPSI were also made between females from the polluted area with attached egg capsules and those without them. Sex-ratio, female shell size, and reproductive parameters such as the number of egg capsules per female and the number of eggs per capsule, were compared between sites in order to search for evidence of reduction in the reproductive activity.
Statistical analysis
The female size, number of egg capsules per female, and number of eggs per capsule were compared, by a t-test (between sites). Normality and homogeneity of variances were tested with Lilliefors test and Levene's test, respectively. The sex-ratio, proportion of female carrying egg capsules (between sites) and imposex percentage (between sites, along the nourishment event and between spawning and non-spawning females) were compared by Chi-square (χ2) test. Statistical analyses were made with the Statistica 7.0 package.
RESULTS
The sex-ratio in the population of Buccinanops monilifer inhabiting MdP waters did not differ significantly from 1:1 (χ = 0.0144; P = 0.904; N = 270) as a whole, with 56% being males, although it varied among months. In the MCh population the result was similar (χ = 0.064; P = 0.937; N = 84), but only 46% of the snails were males.
Reproductive biology
We found egg capsules from October to February. All individuals carrying egg capsules were identified as females that measured between 38.0 and 53.9 (N = 20) mm in shell length in MdP, and 38.5 to 49.3 (N = 19) mm in MCh.
In MdP the percentage of females that carried egg capsules (considering months from October to February) was 20.4% (N = 98), and in particular none of the 13 females collected in December 2000 (MCh sampling month) carried egg capsules; while in the MCh sample the percentage of females that carried egg capsules was 42.2% (N = 45).
The number of egg capsules per female was 17.9±8.0 (average±SD; N = 19) in MdP and 22.6±9.0 (N = 18) in MCh. The average number of eggs per egg capsule in MdP and MCh was 1409.8±482.1 (N = 38) and 1120.3±252.6 (N = 18), respectively. The uncleaved eggs measured 275.5±25.5 (N = 387) in MdP and 291.2±36.8 (N = 185) in MCh.
We found no evidence of malformations (abnormal sizes, the apparition of organs in unexpected stages, a malformed shell in the later stages, abortive embryos, etc.) neither in the egg capsules nor in the embryos of B. monilifer.
Within each egg capsule (Figure 2A) a variable number of eggs (up to 12) began cleavage without simultaneity (Figure 2B). When the embryos developed a velum, began to eat the eggs one by one (Figure 2C), and did so continuously throughout development until nurse eggs were not available anymore. It was observed by transparency that no more than one single egg was found in the stomach, at any stage of development (Figure 2C). As the result of starting cleavage at different times, embryos of different sizes and stages of development coexisted within an egg capsule.
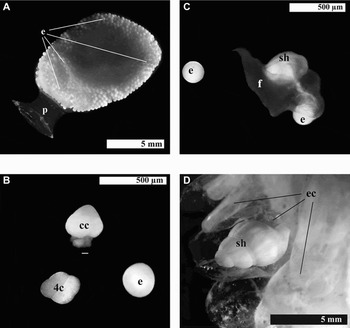
Fig. 2. Embryonic development. (A) Detail of an egg capsule with peduncle (p) and full of eggs (e); (B) uncleaved egg (e), 4 cell cleavage stage (4c) and advanced cell cleavage stage (cc); (C) shell (sh) ‘pediveliger’ ingesting an egg, with developed foot (f); (D) hatchling stage with coiled shelled (sh) in the egg capsule (ec) without eggs, other egg capsules are hatched.
The number of embryos found within an egg capsule varied depending on the developmental time; a decrease in number was observed at the later stages (pre-hatching and hatching), when a single individual was found at each egg capsule. In this study each hatchling (Figure 2D) ate all the nurse eggs, the cleaved cell stages (up to 13), morulae (Figure 2B), early ‘veligers’ (a maximum of 8) and/or shelled ‘veliger’ (4) embryos (Figure 2C), within the egg capsule that contained it. A single hatchling finally emerged leaving an empty egg capsule (Figure 2D). Table 1 shows the embryo stages, its sizes and number of embryos accompanying the successful embryo at each stage in the egg capsules.
The hatchling shell size was 5.75±0.7 mm, and ranged between 4.8 and 6.5 mm in total length (N = 6) in MdP. In MCh we did not find any hatchling-stages within the collected egg capsules (in December). Figure 3 includes mean size and standard deviation of each developmental stage found.
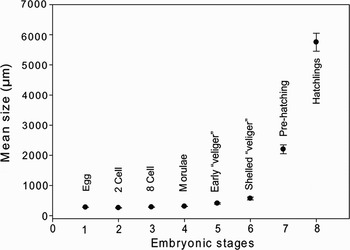
Fig. 3. Mean size (µm) and standard error (SE) of each developmental stage.
Imposex
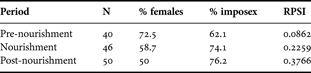
Table 2 shows the comparison of the percentage and RPSI between sites. MdP showed high imposex percentage through the year (although the RPSI was moderate); while in MCh there were no traces of imposex. The imposex percentage in MdP was very variable between months (10–100%); and when data were pooled in periods (before, during and after the beach nourishment) the percentages showed a tendency to increase through time, but without significant differences (χ = 0.33; P = 0.565; N = 54). The RPSI increased over four times throughout the beach nourishment episode. These results are expressed in Table 3.
Table 2. Comparison of imposex parameters between Mar Chiquita versus Mar del Plata (whole study period).
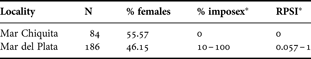
RPSI, relative penis size index; *range.
Table 3. Comparison of imposex parameters through the nourishment event in Mar del Plata (1998–2000).
RPSI, relative penis size index.
When comparing the percentage of imposex and RPSI between females with and without egg capsules, in the MdP area, both parameters were significantly higher in non-spawning females (χ = 14.75; P = 0.0001; N = 97). Results are expressed in Table 4.
Table 4. Comparison of imposex parameters between females with and without egg capsules, in Mar del Plata (October to February 1997–2001).

RPSI, relative penis size index; *range.
The proportion of spawning females was significantly larger in MCh than in MdP (df = 1; χ = 7.4; P = 0.0066; N = 143). No differences were observed in the spawning female shell sizes between the MdP and MCh sites (t = 1.51; P = 0.138; N = 38), while the number of egg capsules per female was significantly different; MCh > MdP (t =−2.432; P = 0.02; N = 38). No differences were observed in the number of eggs per capsule between sites (t = 2.25; P = 0.1463; N = 26).
DISCUSSION
Reproductive biology
Females of Buccinanops monilifer carrying egg capsules are not common in Mar del Plata when compared to nearly close unpolluted areas, probably due to the effect of imposex on the population's reproduction. No malformations were observed on the egg capsules or embryos in the present study.
Adelphophagy, common to all Buccinanops species (Penchaszadeh, Reference Penchaszadeh1971a, Reference Penchaszadehb, Reference Penchaszadeh1973), represents a modality of embryonic nutrition that leads in some species to hatching as large crawling juveniles. Intracapsular cannibalism represents an additional intracapsular nutritional resource, avoiding the disadvantages of sharing/competing for the nurse egg stock. Thus, the hatching sizes will be different from a theoretical optimal hatching size, as reviewed by Spight (Reference Spight1976), and also discussed by other authors (Pechenik, Reference Pechenik1986; Miloslavich & Penchaszadeh, Reference Miloslavich and Penchaszadeh2001).
The intracapsular ‘early veliger’ stage embryos of Buccinanops monilifer began to eat the nurse eggs one by one, without accumulating them in the digestive tract, while they developed to the hatching stage. This coincided with previous observations (Penchaszadeh, Reference Penchaszadeh1971a) that suggested that the embryos rotated the eggs and ingested particles or entire eggs, as in Buccinum undatum (Fioroni, Reference Fioroni1967). In Buccinanops monilifer the average hatching size was 5.8±0.7 mm in total shell length; while in B. cochlidium from Patagonia with a variable number of hatchlings per egg capsule the average hatching shell length was 4.0±0.6 mm, although a tendency towards a larger hatching size was observed when the number of embryos within the egg capsule was smaller. The average hatching size of B. cochlidium in egg capsules with a unique embryo increased to almost 6 mm (Averbuj, Reference Averbuj2009; Averbuj & Penchaszadeh, Reference Averbuj and Penchaszadeh2009).
The nassarid closely related genus Bullia reviewed by Brown (Reference Brown1982), includes reproductive types with adelphophagy (nurse egg feeding) with complete intracapsular development as the main developmental path, intracapsular cannibalism of other embryos, and species with planktotrophic development (Ansell & Trevallion, Reference Ansell and Trevallion1970). Cannibalism by one embryo (probably the first to develop a velum and mouth), feeding on other developing embryos may be a complementary food source to adelphophagy in B. monilifer. This modality of intracapsular nutrition by adelphophagy together with cannibalism could explain the fact that in all the other studied Buccinanops species (except in B. cochlidium) a single hatchling per egg capsule is the rule. This is supported by extensive research on B. cochlidium embryonic development (Averbuj, Reference Averbuj2009; Averbuj & Penchaszadeh, Reference Averbuj and Penchaszadeh2009) where no evidence of cannibalism was found at any developmental stage.
Imposex
The population of B. monilifer inhabiting waters off the Mar del Plata coasts is proved to suffer from imposex (Penchaszadeh et al., Reference Penchaszadeh, Averbuj and Cledón2001). This phenomenon was related to the presence of TBT in the water column (Goldberg et al., Reference Goldberg, Averbuj, Cledón, Luzzatto and Nudelman2004) and sediments (Bigatti et al., Reference Bigatti, Primost, Cledón, Averbuj, Theobald, Gerwinski, Arntz, Morriconi and Penchaszadeh2009). The consequence in marine snails of exposure to organotin compounds used in anti-fouling paints is the imposition of male characters, namely a penis or a vas deferens, in the female body. Species under exposure to high levels of TBT might suffer a population decline due to the impossibility of the females to lay the egg capsules, as a consequence of gonopore obstruction (Bryan et al., Reference Bryan, Gibbs, Hummerstone and Burt1986; Oelhmann et al., 1996a,b), especially in species such as B. monilifer with direct development in which larval importation is not possible as reported in Thais haemastoma from Brazil (Fernandez et al., Reference Fernandez, Limaverde, Castro, Martins-Almeida and de Luca Rebello Wagener2002). The results in this work show a very low spawning frequency in samples from the polluted area of MdP: only 20 females in the whole period (4 years); while 19 females carried egg capsules in a single sample in a close non-polluted locality. In the population of B. monilifer inhabiting MdP, the percentage of imposex and the RPSI were remarkably lower within the females that succeeded in laying egg capsules (compared to those that have no egg capsules attached to the shell). This result suggests that only those females that were not affected by TBT (without imposex) were able to reproduce, even though affecting its fecundity (number of egg capsules/female) when compared with the MCh population. These results contrast with an experimental study in laboratory aquaria, in Hexaplex trunculus the number of spawning females decreased with TBT concentration, while TBT had no effect on the number of egg-capsules laid per female, nor on the number of eggs per capsule (Abidli et al., Reference Abidli, Lahbib and Trigui el Menif2009).
According to our results, the beach nourishment event (Marcomini & Lopez, Reference Marcomini and Lopez2006) had a direct effect on the biota during and after it finished in April 1999. Although the increase in imposex percentage showed no significant differences through time (always high), it did show a high impact on each individual; this is evidenced by the remarkable increase in the female penis sizes (RPSI). This increase in imposex is probably associated with rising TBT levels in the water column, due to sediment resuspension. Teso & Penchaszadeh (2008) monitored imposex with the sentinel caenogastropod species Olivancillaria deshayesiana, during the same nourishment event. The results are coincident with this work, and even a more marked increase was observed, suggesting a different degree of sensibility to TBT for the two species.
ACKNOWLEDGEMENTS
This research was partially supported by UBACyT X-171, PICT-01869 and PIP-5301. The authors are members of CONICET. We thank Dr Scelzo and the late Mr Ungarelli for collaborating with sample collection and Jennifer Antonides for suggestions on an early version of the manuscript. All research work including sampling and laboratory researches comply with Argentine current laws.









