Introduction
Green turtles (Chelonia mydas) (Linnaeus 1758) have a unique and complex life history (Carr, Reference Carr and Bjorndal1982; Meylan et al., Reference Meylan, Bowen and Avise1990), marked by an ontogenetic shift from omnivorous during the pelagic phase (straight carapace length about 20–30 cm) to herbivorous in coastal areas (Mortimer, Reference Mortimer and Bjorndal1982; Bjorndal, Reference Bjorndal, Lutz and Musick1997; Limpus & Limpus, Reference Limpus and Limpus2000; Meylan & Meylan, Reference Meylan, Meylan, Eckert, Bjorndal, Abreu-Grobois and Donnelly2000; Bolten, Reference Bolten, Lutz, Musick and Wyneken2003; Arthur et al., Reference Arthur, Boyle and Limpus2008). In early life stages, this species takes up residence along the coast, occupying developmental habitats and returning to specific areas for foraging, only leaving after reaching sexual maturity in order to perform breeding migrations (Musick & Limpus, Reference Musick, Limpus, Lutz and Musick1997; Chaloupka et al., Reference Chaloupka, Limpus and Miller2004; Limpus et al., Reference Limpus, Limpus, Arthur and Parmenter2005). Juvenile green turtles are very common along the Brazilian coast accounting for a high proportion of occurrences (e.g. stranding, sighting and bycatch) and they can also be found in pelagic areas, especially on the north-east coast (Sales et al., Reference Sales, Giffoni and Barata2008; Santos et al., Reference Santos, Almeida, Santos, Gallo, Giffoni, Baptistotte, Coelho, Lima, Sales, Lopez, Stahelin, Becker, Castilhos, Thomé, Wanderlinde, Marcovaldi, Mendilaharsu, Damasceno, Barata and Sforza2011).
Studies on the growth rate of wild green turtles, especially in feeding areas, are necessary to understand demographics and to create successful management and conservation plans (Bjorndal et al., Reference Bjorndal, Bolten and Chaloupka2000). According to Thomson et al. (Reference Thomson, Burkholder, Heithaus and Dill2009), body condition assessment provides valuable data on ecology and conservation biology. Furthermore, it impacts on behavioural decisions and physiological processes that directly or indirectly influence fitness.
Studies on haematological and biochemical parameters have indicated reference intervals for green turtles, and contribute to the determination of their health status; a helpful tool in decision-making regarding rehabilitation and release (Bolten & Bjorndal, Reference Bolten and Bjorndal1992; Aguirre et al., Reference Aguirre, Balazs, Spraker and Gross1995; Hasbún et al., Reference Hasbún, Lawrence, Naldo, Samour and Al-Ghais1998; Samour et al., Reference Samour, Hewlett, Silvanose, Hasbún and AlpGhais1998; Hamman et al., Reference Hamann, Schauble, Simon and Evans2006; Flint et al., Reference Flint, Morton, Limpus, Patterson-Kane, Murray and Mills2010; Fong et al., Reference Fong, Chen and Cheng2010; Osborne et al., Reference Osborne, Jacobson, Bresette, Singewald, Scarpino and Bolten2010; Labrada et al., Reference Labrada-Martagón, Méndez-Rodriguez, Gardner, López-Castro and Zenteno-Savin2010a, Reference Labrada-Martagón, Méndez-Rodriguez, Gardner, Cruz-Escalona and Zenteno-Savin2010b; Anderson et al., Reference Anderson, Harms, Stringer and Cluse2011; Hirama et al., Reference Hirama, Ehrhart, Rea and Kiltie2014; Lewbart et al., Reference Lewbart, Hirschfeld, Denkinger, Vasco, Guevara, García, Muñoz and Lohmann2014). Variability in haematological and biochemical values may also occur in healthy turtles according to their geographic location, habitat, genetics, maturity, sex, breeding and migratory status, and diet (Herbst & Jacobson, Reference Herbst, Jacobson, Lutz, Musick and Wyneken2003; Stamper et al., Reference Stamper, Harms, Epperly, Braun-McNeill and Stoskopf2005; Deem et al., Reference Deem, Dierenfeld, Sounguet, Alleman, Cray, Poppenga, Norton and Karesh2006; Hamann et al., Reference Hamann, Schauble, Simon and Evans2006; Whiting et al., Reference Whiting, Guinea, Limpus and Fomiatti2007). A few studies have combined parameters from the same geographic subpopulation of marine turtles using the results to identify unhealthy individuals (Flint et al., Reference Flint, Morton, Limpus, Patterson-Kane, Murray and Mills2010; Lewbart et al., Reference Lewbart, Hirschfeld, Denkinger, Vasco, Guevara, García, Muñoz and Lohmann2014).
Body condition index (BCI) is an objective and accurate indicator, based on the length–body mass relationship of the individual, previously used in several wildlife species to evaluate individual health (Stevenson & Woods, Reference Stevenson and Woods2006). It is used as part of green turtle routine health evaluations and/or field assessments, and may correlate with biochemical changes and to the general health condition of green turtles with and without fibropapillomatosis (Flint et al., Reference Flint, Morton, Limpus, Patterson-Kane, Murray and Mills2010; Santos et al., Reference Santos, Martins, Baptistotte and Work2015).
Fibropapillomatosis (FP) is a neoplastic disease included in the list of sea turtle priority research issues (Hamann et al., Reference Hamann, Godfrey, Seminoff, Arthur, Barata, Bjorndal, Bolten, Broderick, Campbell, Carreras, Casale, Chaloupka, Chan, Coyne, Crowder, Diez, Dutton, Epperly, FitzSimmons, Formia, Girondot, Hays, Cheng, Kaska, Lewison, Mortimer, Nichols, Reina, Shanker, Spotila, Tomás, Wallace, Work, Zbinden and Godley2010). It is characterized by skin tumours that can reach a large enough size to hamper mobility and foraging of affected individuals (Aguirre & Lutz, Reference Aguirre and Lutz2004). It has been suggested that associated herpesvirus, either as a group of close viruses or variants with regional distributions, had diverged long before FP emerged as a panzootic, suggesting that environmental or ecological factors are relevant to the global occurrence of FP (Hargrove et al., Reference Hargrove, Work, Brunson, Foley and Balazs2016). Molecular diagnostic data suggest that an alphaherpesvirus, currently known as Chelonid alphaherpesvirus 5 (ChHV5), is the aetiological agent of FP (Lackovich et al., Reference Lackovich, Brown, Homer, Garber, Mader, Moretti, Patterson, Herbst, Oros, Jacobson, Curry and Klein1999; Quackenbush et al., Reference Quackenbush, Casey, Murcek, Paul, Work, Limpus, Chaves, Dutoit, Vasconcelos Peres, Aguirre, Spraker, Horrocks, Vermeer, Balazs and Casey2001; Ene et al., Reference Ene, Su, Lemaire, Rose, Schaff, Moretti, Lenz and Herbst2005; Work et al., Reference Work, Dagenais, Balazs, Schumacher, Lewis, Leong, Casey and Casey2009; Duarte et al., Reference Duarte, Faisca, Loureiro, Rosado, Gil, Pereira and Tavares2012; Patricio et al., Reference Patricio, Herbst, Duarte, Veles-Zuazo, Loureiro, Pereira, Tavares and Toranzos2012; Rodenbusch et al., Reference Rodenbusch, Baptistotte, Werneck, Pires, Melo, Ataíde, Reis, Testa, Alieve and Canal2014; Gattamorta, Reference Gattamorta2015; Monezi et al., Reference Monezi, Mehnert, Moura, Müller, Garrafa, Matushima, Werneck and Borella2016). However, environmental pollutants may also play a role in tumour development and they need to be investigated (Aguirre & Lutz, Reference Aguirre and Lutz2004; Van Houtan et al., Reference Van Houtan, Hargrove and Balazs2010; Keller et al., Reference Keller, Balazs, Nilsen, Rice, Work and Jensen2014; Hargrove et al., Reference Hargrove, Work, Brunson, Foley and Balazs2016; Vilca et al., Reference Vilca, Rossi, Olinda, Sánchez-Sarmiento, Prioste, Matushima and Tornisielo2018). In Brazil, FP is considered an important threat to sea turtles, especially for green turtles, the most affected species (Herbst et al., Reference Herbst, Greiner, Ehrhart, Bagley and Klein1998; Matushima et al., Reference Matushima, Filho, Di Loretto, Kanamura, Sinhorini, Gallo and Baptistotte2001; Santos et al., Reference Santos, Martins, Torezani, Baptistotte, Farias, Horta, Work and Balazs2010). FP prevalence in green turtles in Brazil varies according to geographic regions: higher in Ceará state, followed by Rio Grande do Norte, Espírito Santo and Sergipe states (Baptistotte, Reference Baptistotte, Hargrove, Work, Brunson, Foley and Balazs2016).
We compared BCI in FP-free and FP-affected green turtles from the Brazilian coast according to their origin (intentional capture, fishery, stranding and afloat), sex and FP score to evaluate the potential of BCI as a tool for future FP studies in this species.
Materials and methods
Data collection
Individual biometric data (CCL and body mass – BM) were recorded in order to calculate the BCI, according to Bjorndal et al. (Reference Bjorndal, Bolten and Chaloupka2000): BCI = BM (kg)/SCL3 (cm); SCL = straight carapace length. Since BCI is calculated with SCL and we had data on CCL, we needed a linear regression (appropriate for individuals found in Brazil) in order to predict an individual's SCL based on its CCL. Therefore, CCL and SCL were recorded from another 107 green turtles captured in Espírito Santo state, and evaluated by a single person in order to avoid measurement variations. The obtained linear regression model was used to analyse the relationship between CCL and SCL: SCL = 0.93 * CCL – 0.28 (R-squared = 97.6%, P-value < 0.0001, root-mean-square error = 1.1) (Figure 1). This allowed calculation of SCL for green turtles with only CCL values. A flexible tape was used to measure CCL, while an anthropometric caliper was employed for SCL measurements. The 887 examined individuals presented SCL ranging from 20.1 to 103.2 cm (37.3 ± 7.5 cm), therefore classified as juveniles and adults, and their body mass ranged from 1.2 to 160 kg (8.1 ± 7.7 kg).

Fig. 1. Linear regression between curved carapace length (CCL) and straight carapace length (SCL) from green turtles studied along the Brazilian coast (R-squared = 97.6%, P-value < 0.0001).
Plastron shape visual assessment was employed for Subjective Body Condition (SBC) in a subset of the total FP-free green turtles (N = 454). These individuals were found dead, thus it was easier to manipulate and fully visualize the plastron. Based on this method, individuals were classified into three categories according to the plastron descriptions made by Thomson et al. (Reference Thomson, Burkholder, Heithaus and Dill2009): good (convex), regular (flat) and poor (concave).
The sex of live green turtles was not determined because they were juveniles, therefore with no external sexual dimorphism. The sex was only determined in dead individuals during carcass examination and based on the analysis of gonads.
FP tumours were individually counted and classified into four size categories based on their diameter: A (<1 cm), B (1–4 cm), C (>4–10 cm) and D (>10 cm) (Work & Balazs, Reference Work and Balazs1999). Then, the total number of tumours was calculated for each size category (NA, NB, NC and ND; N = number of tumours in each A–D size category), and classified according to the South-west Atlantic Fibropapillomatosis Score (FPSSWA): mild, moderate and severe (Rossi et al., Reference Rossi, Sánchez-Sarmiento, Vanstreels, Santos, Prioste, Gattamorta, Grisi-Filho and Matushima2016).
This study followed the Ethical Principles in Animal Research adopted by the Ethics Committee in the use of animals (Comissão de Ética no Uso de Animais) of the Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo (697/2005, 1932/2010, 2116/2010 and 2555/2012), and was approved by the Chico Mendes Institute for Biodiversity Conservation (ICMBio) – Ministry of the Environment through the Biodiversity Information and Authorization System (SISBIO) numbers 22751, 26667, 21802 and 32636.
Data analysis
Individuals were analysed according to (1) the presence or absence of FP, (2) FPSSWA, (3) study site, (4) origin (intentional capture, fishery, stranding and afloat) and (5) sex (female or male, when known).
The Mann–Whitney and Kruskal–Wallis non-parametric hypothesis tests were used to detect differences between BCI among individuals in different category variables (FP status, FPSSWA, study site, origin and sex); the first test to compare two groups and the second to compare three or more groups. The Dunn's test was used to perform the post-hoc analysis. Simple linear regression was employed to analyse the relationship between quantitative variables. Results were considered significant when P-value < 0.05. Logistic regression was applied to study differences in the proportion of FP-affected individuals among study areas. Mean and standard deviation are presented in the following notation: mean ± standard deviation. Analyses were performed using R software (R Core Team, 2016), with readxl (Wickham & Bryan, Reference Wickham and Bryan2017), ggplot2 (Wickham, Reference Wickham2009), EnvStats (Millard, Reference Millard2013), dunn.test (Dinno, Reference Dinno2017) and ggpubr packages (Kassambara, Reference Kassambara2017).
Results
Examined green turtles
We studied a total of 887 green turtles (518 FP-free and 369 FP-affected), either captured, rescued or found stranded dead along the coastal feeding areas of five Brazilian states by the Projeto TAMAR, between 2005 and 2014 (Figure 2). The curved carapace length (CCL) ranged from 26.1 to 111.3 cm in FP-free, and between 30.2 and 68.5 cm in CCL of FP-affected individuals.
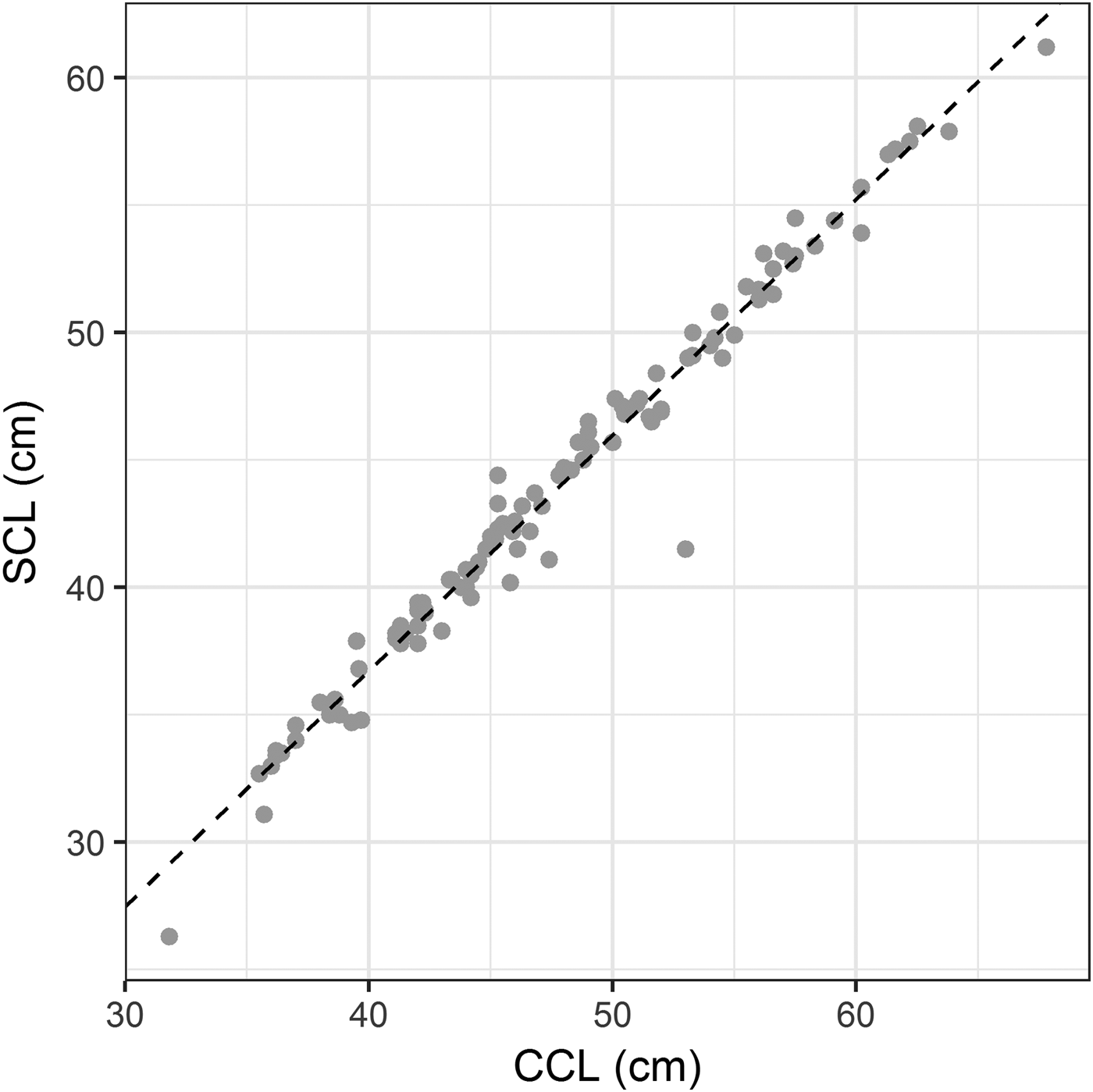
Fig. 2. Geographic distribution of study area. In parentheses: total number of green turtles per state.
Body condition index and Subjective Body Condition
The BCI of FP-free green turtles ranged from 0.70 to 2.24 (1.32 ± 0.24). According to the SBC, we classified 280 individuals as good, 68 as regular and 106 as poor. Visual analysis of the relationship between BCI and SBC from 454 FP-free individuals revealed that the visual assessment provides a clear classification between the regular and poor, but not between the regular and good scores (Figure 3A).

Fig. 3. (A) Relationship between Subjective Body Condition (SBC) and body condition index (BCI) from FP-free green turtles studied along the Brazilian coast. (B) BCI from green turtles with and without fibropapillomatosis (FP). (C) BCI according to South-west Atlantic Fibropapillomatosis Score (FPSSWA). (D) BCI of green turtles from different study areas. (E) BCI among different origins. (F) BCI according to sex.
Body condition index and fibropapillomatosis
Tumour counts and anatomical distribution were evaluated in 221 out of 369 FP-affected green sea turtles (59.89%). The BCI of FP-free individuals ranged from 0.70 to 2.24 (1.32 ± 0.24), and between 0.73 and 2.0 (1.41 ± 0.22) in FP-affected individuals. There was a significant statistical difference in the mean BCI scores between FP-free and FP-affected green turtles (Mann–Whitney test, P < 0.0001; Table 1, Figure 3B).
Table 1. Body condition index of green turtles studied in Brazilian feeding areas between 2005 and 2014
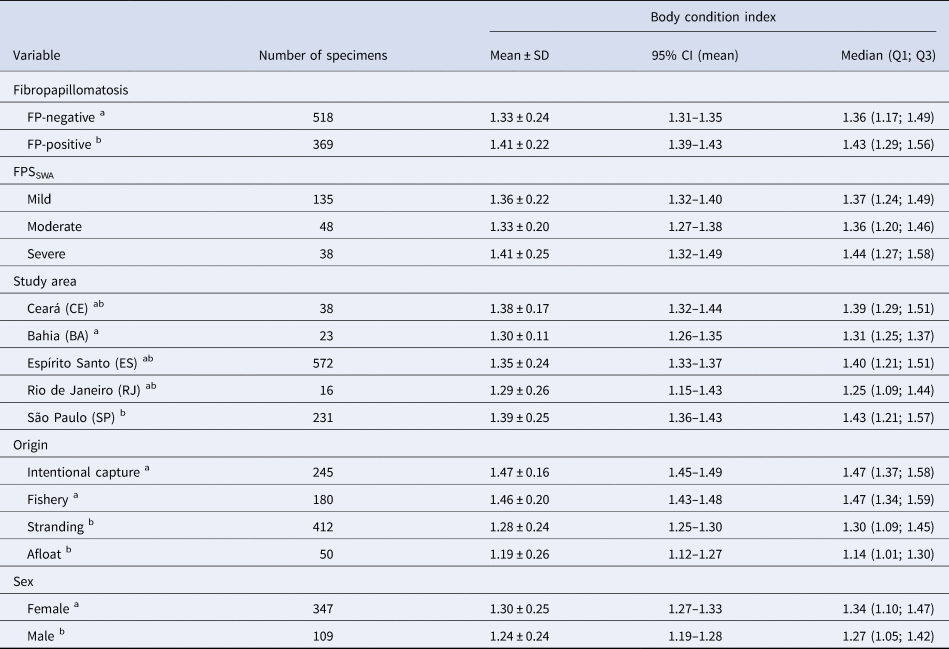
FP, Fibropapillomatosis; FPSSWA, South-west Atlantic Fibropapillomatosis Score; SD, standard deviation; CI, confidence interval; Q1, 25th percentile; Q3, 75th percentile. Superscript letters indicate categories for which BCI was statistically different.
A more detailed analysis splitting the data between different origins revealed statistically significant differences between FP-affected and FP-free individuals are present in the intentional capture (P = 0.008) and afloat (P = 0.019) groups, but not in the fishery (P = 0.125) or stranding (P = 0.138) groups. In all comparisons, FP-affected individuals had a higher BCI than FP-free individuals (Figure 4).

Fig. 4. Body condition index (BCI) from green turtles with and without fibropapillomatosis (FP) among different origins.
There was no statistical difference in BCI among green sea turtles classified according with FPSSWA: mild (1.36 ± 0.22; N = 135), moderate (1.33 ± 0.20; N = 48), and severe (1.41 ± 0.25; n = 38) (Kruskal–Wallis test, P = 0.22) (see Table 1). There was also no correlation between BCI and FPI (linear regression analysis, P = 0.10). Figure 3C shows that BCI distribution is similar among green turtles in spite of FP and FPSSWA.
Study areas and fibropapillomatosis
The proportion of FP-affected individuals varied among different geographic locations (Figure 1). Higher proportions of FP-affected turtles were found on Espírito Santo (44.6%; 255/572) and São Paulo (41.6%; 96/231), followed by Rio de Janeiro (31.3%; 5/16), Ceará (23.7%; 9/38), Bahia (17.4%; 4/23), Sergipe (0%; 0/3) and Santa Catarina (0%; 0/4). A logistic regression analysis (excluding Sergipe and Santa Catarina due to the small number of examined green turtles) showed that individuals from Espírito Santo (OR = 3.8, IC 95% = 1.42–13.3, P = 0.016) and São Paulo (OR = 3.4, IC 95% = 1.22–11.93, P = 0.032) were more likely to be affected by FP than individuals from Bahia (used as reference).
Body condition index among study areas
The Kruskal–Wallis test showed a significant statistical difference in turtle BCI among study areas (P = 0.02), and the post-hoc Dunn's test multiple comparison analysis revealed one statistically significant difference, between São Paulo and Bahia states (P = 0.047). Visual (Figure 3D) and numerical (Table 1) analyses suggest that this difference is very small, since the biggest BCI difference among study areas is around 0.1, while standard deviation is often between 0.1 and 0.3. We removed the states of Santa Catarina and Sergipe from this analysis due to the small number of individuals from these areas (4 and 3, respectively).
Body condition index and origin of green turtles
The highest BCI was found in the intentional capture group (N = 245; 1.47 ± 0.16), followed by fishery (N = 180; 1.46 ± 0.20), stranding (N = 412; 1.28 ± 0.24) and afloat groups (N = 50; 1.19 ± 0.26) (Table 1).
There was a significant statistical difference in the BCI values among green turtles with different origins (Kruskal–Wallis test, P < 0.001). Post hoc analysis revealed no differences between fishery and intentional capture (P = 1.000), and between stranding and afloat groups (P = 0.053); fishery and intentional capture had BCI values higher than the other groups (P < 0.001; Figure 3E).
Body condition index and sex
BCI values were higher in females (N = 347; 1.30 ± 0.25) than in males (N = 109; 1.24 ± 0.24) (Mann–Whitney test, P < 0.017) (Figure 3F).
Discussion
Rapid visual-assessment techniques of physical condition characterization are useful in field studies of wildlife health status (Thomson et al., Reference Thomson, Burkholder, Heithaus and Dill2009); however, we observed that such techniques are adequate to differentiate between regular and poor, but not between good and regular body conditions, in which the employment of BCI techniques is indicated. Preliminary studies on green turtles captured in Brazilian feeding areas revealed the same results; that the subjective assessment is reliable for individuals in poor condition but is unclear for green turtles classified as regular and good (Sánchez-Sarmiento et al., Reference Sánchez-Sarmiento, Rossi, Vanstreels, Santos, Marigo, Bertozzi, Baptistotte, Becker and Matushima2012). Another study carried out in Brazil demonstrated that the subjective body condition had a good relationship with BCI when examined by a single observer, avoiding inter-observer variation (Santos et al., Reference Santos, Martins, Baptistotte and Work2015).
Body condition index of FP-free individuals ranged between 0.50 and 2.59 (1.33 ± 0.25). A study conducted in Baja California, Mexico, from 1995 to 2002, demonstrated that green turtles (probably immature based on SCL measurements) presented BCI ranging from 1.03 to 2.19 (1.42 ± 0.015; N = 102) (Seminoff et al., Reference Seminoff, Jones, Resendiz, Nichols and Chaloupka2003). A long-term monitoring of green turtles in the same area revealed a BCI between 0.67 and 2.30 (from 1.27 ± 0.23 to 1.38 ± 0.13; N = 1169), including both juvenile and adult individuals (López-Castro et al., Reference López-Castro, Koch, Mariscal-Loza and Nichols2010). We found similar results, and conclude that the few observed differences may be due to food availability in their studied habitat (Mexico and Brazil) and their metabolism.
Depending on the size, number and anatomical position, cutaneous tumours may interfere with movement, food ingestion, growth, reproduction, vision and the ability to avoid predators (Herbst, Reference Herbst1994; Adnyana et al., Reference Adnyana, Ladds and Blair1997; George, Reference George, Lutz and Musick1997). We expected affected individuals to have a lower BCI than non-affected ones. However, we found higher BCI values in FP-affected individuals in comparison with FP-free, and a significant difference between the two categories (P < 0.0001); in contrast with previous studies that revealed no significant difference in BCI values according to the presence and absence of FP or FP severity scores (Santos et al., Reference Santos, Martins, Torezani, Baptistotte, Farias, Horta, Work and Balazs2010, Reference Santos, Martins, Baptistotte and Work2015). Studies carried out in the Georgia Sea Turtle Center revealed no significant difference between mean BCI of turtles that developed FP during rehabilitation (1.06 ± 0.07) and turtles that already presented FP (1.15 ± 0.04) (Page-Karjian et al., Reference Page-Karjian, Norton, Krimer, Groner, Nelson and Gottdenker2014). There was no statistical difference among green turtles in regards to FPSSWA; however, individuals classified as severe presented the highest mean values (1.42 ± 0.25). The higher BCI values found in these individuals could be due to the presence of numerous and/or large tumours, which increases body mass and may promote differences in our results. On the other hand, according to Santos et al. (Reference Santos, Martins, Baptistotte and Work2015), severely afflicted individuals have a lower BCI. A study carried out in the effluent discharge channel of a steel plant in Espírito Santo state – Brazil, captured 640 green turtles and verified that 420 individuals were FP-free: 378 presented normal body condition, and 42 were either underweight or emaciated, while the 220 FP-affected individuals presented different levels of tumour severity score but were in normal health condition (Torezani et al., Reference Torezani, Baptistotte, Mendes and Barata2010). Studies carried out in three different ecosystems in the USA revealed low severe FP frequency (9.3%) in 310 examined green turtles (38.4% free-FP) captured in the Indian River Lagoon (Lagoon); FP prevalence at 14.8% (38/256) in the Nearshore Reef (Ocean); and no evidence of FP (N = 82) in the Trident Submarine Basin (Port) (Hirama & Ehrhart, Reference Hirama and Ehrhart2007).
We found a significant difference among study areas (P = 0.003). López-Castro et al. (Reference López-Castro, Koch, Mariscal-Loza and Nichols2010) studied black sea turtles from different coastal foraging areas in Baja California – USA, and observed that the BCI was significantly lower in Laguna San Ignacio than in all other sites, which showed no variations amongst one another. Hirama & Ehrhart (Reference Hirama and Ehrhart2007) also observed BCI variations among the three study sites (Indian River Lagoon, Nearshore Reef and Trident Submarine Base), possibly due to these habitats' different ecological and physical characteristics.
A significant difference (P < 0.001) was observed among the origin of the studied individuals, wherein the highest BCI was found in green turtles from the fishery (N = 180; 1.46 ± 0.20) and intentional capture groups (N = 245; 1.47 ± 0.16), suggesting that turtles found stranded or afloat were debilitated. Rossi et al. (Reference Rossi, Sánchez-Sarmiento, Santos, Prioste, Mott, Grisi Filho, Matushima, Soler-Tovar and Navas-Suárez2015) also detected higher BCI in the intentional capture/bycatch group (N = 187; 1.32 ± 0.17) in Brazilian feeding areas. We considered that the cutaneous tumours negatively impact the affected individuals' vision and swimming abilities, increasing their chances of stranding. According to Herbst (Reference Herbst1994), reports based only on strandings may overrate the prevalence of FP. However, Baptistotte (Reference Baptistotte2007) found a higher prevalence in intentionally captured vs stranded green turtles, which was also observed in studies carried out in Espírito Santo state in 2010 (unpublished data). Comparisons of FP frequency between stranded and intentionally captured turtles in a densely populated and anthropized Brazilian region revealed significant differences: higher tumour proportion in individuals captured in the industrial discharge area (Jesus et al., Reference Jesus, Costa, Mendonça and Zandonade2004; Baptistotte, Reference Baptistotte, Hargrove, Work, Brunson, Foley and Balazs2016).
Our findings demonstrate that a calculated body condition index is more informative than subjective corporal condition, since it avoids inter-observer variation and possible subjective mistakes during visual assessment. We observed that the SBC was useful to classify poor body condition but was not appropriate to indicate the difference between regular and good body condition; therefore, BCI is a helpful additional parameter to evaluate green turtle health. Numerous and/or large tumours can affect the body condition index and interfere with our results but unfortunately we could not estimate the mass of each FP tumour, and our statistical analysis did not consider this influence on BCI calculations.
In summary, FP-affected green turtles did not present lower BCI values than FP-free individuals, and there was no difference among green turtles according to FPSSWA, in contrast with our initial hypothesis based on the pathogenesis of FP. Captured green turtles had higher BCI values than other groups, suggesting that individuals found stranded or afloat were probably debilitated.
Acknowledgements
We thank Projeto TAMAR, the Instituto Chico Mendes de Conservação da Biodiversidade (Chico Mendes Institute for Biodiversity Conservation), the Comissão de Ética no Uso de Animais (Ethics Committee in the use of animals) of the Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, Brazil.
Financial support
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2010/01781-8, 2011/04565-7, 2012/14319-6) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).







