Introduction
Intestinal atresia (IA), a gastrointestinal malformation and a common cause of neonatal intestinal obstruction, is caused by a segmental defect of the intestine, which disrupts the luminal continuity of the intestinal tube during development with an incidence between 0.57 and 6.6 per 10,000 live births. Intestinal atresia was first described in 1684,Reference Grosfeld, O’Neill, Coran and Fonkalsrud1 but the etiology for IA is still poorly understood.
In 1900, a Viennese anatomist, Julius Tandler, proposed a hypothesis on the origins of IA based on his studies of normal duodenal development that the endoderm of the duodenum proximal to the “umbilical loop” underwent a remarkable epithelial thickening to form a proliferative endodermal plug, and the duodenum became a solid core with no lumen at day 42 of development. Later, the duodenum underwent recanalization between 6–7th week of gestation, by forming multiple vacuoles in the epithelial string, which later coalesce into a single lumen between 8–12th week of gestation. Tandler further postulated that failure of local coalescence of vacuoles in the intestine during embryonic period could lead to IA.Reference Tandler2 This “local residual vacuoles” theory not only can explain why the occlusion can occur anywhere along the intestinal tract, but also the restoration of the intestine luminal continuity after surgical removal of the septum and an end-to-end anastomosis of the intestine proximal and distal to the septum. However, surgeons started to challenge the “local residual vacuoles” theory in the etiology of IA, since studies indicated that rat intestines do not undergo this recanalization developmental event,Reference Cheng and Tam3 and duodenal atresia can be generated in mice by mutating either the gene for fibroblast growth factor receptor 2IIIb (Fgfr2IIIb) or its ligand Fgf10.Reference Kanard, Fairbanks and De Langhe4,Reference Fairbanks, Kanard and Del Moral5 Mouse duodenum, like rats, does not form an endodermal plug, it would indicate that this developmental event is not required for duodenal atresia formation. In 1955, Surgeons J.H. Louw and Christiaan Barnard hypothesized that a mechanical compression of the arterial blood supply to the intestine known as “vascular insult theory” was the major etiology of intestinal atresia of the jejunum and ileum.Reference Louw and Barnard6–Reference Barnard8 Taken all these suggest that abnormal embryonic events contribute to IA.Reference Morris, Kennedy and Cochran9 However, there is no temporal and topology study detailing the evolution, morphology and structure of the vacuoles in human embryonic guts, and no report on the identification of the “local residual vacuoles” in IA specimen.
To study the vacuoles and gut recanalization during human embryo development, and to address if residue vacuoles could be identified in IA gut, we performed histological and histochemical study on 6–10 gestation weeks human embryos/fetuses and on the resected septal regions of the IA specimen. In conclusion, our data provide compelling evidence supporting the theory of “local residue vacuole” in the pathogenesis of IA.
Materials and methods
Embryo and fetal tissues
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). Aborted human embryos/fetuses (6–10 weeks of gestation: 3 of crown-rump length (CRL) 8.5–9 mm, approximately gestation 6–7 weeks; 3 of CRL 22–28 mm, approximately gestation 9 weeks); 2 of CRL 31–36 mm, approximately gestation 10 weeks) were paraffin-embedded. Serial sagittal sections (5 µm in thickness) were prepared. Hematoxylin and eosin (H&E) stained sections were scanned using high quality digital scanner (3D HISTECH, Hungary). The use of human tissues for the study has been approved by the Medical Ethics Committee of Wuxi School of Medicine, Jiangnan University.
Patients and septum specimen collection
All seven patients were diagnosed as type I intestinal atresia (IA) based on observation during surgery. The intestine segment with the septum was removed surgically followed with an end-to-end anastomosis at the Department of Surgery of Capital Institute of Pediatrics affiliated Children Hospital (Beijing, China) and Hebei Medical University affiliated 2nd Hospital between September 2017 and March 2019. The normal intestinal wall tissues around the incision of the same patient were harvested and used as control. Patients did not receive any other treatment prior to the surgery. The use of human intestine tissues for the study was approved by the Medical Ethics Committee of Capital Institute of Pediatrics affiliated Children Hospital (Beijing, China) and Hebei Medical University affiliated 2nd Hospital. Written informed consents were obtained from patients’ parents.
Histology analysis
Both the septum and the normal intestinal wall tissue were fixed in 10% formalin (24 h at 4°C), before being dehydrated in alcohol, cleared in xylene and embedded in paraffin. Sections (4 μm thick) were prepared and mounted onto TESPA-coated microscopic glass. Sections were dewaxed, rehydrated for H&E staining (Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 10 min followed by differentiation in a hydrochloride acid-alcohol mixture, and counterstaining in eosin solution for 3 min. Sections were dehydrated in ethanol, cleared in xylene and mounted in neutral balsam before examination under light microscope (Olympus BX40).
Masson trichrome staining
Masson trichrome staining was performed following our previous reports.Reference Zhang, Liu, Wang, Peng and Wong10–Reference Liu, Gao, Du, Zhao and Wong12 In brief, the rehydrated sections were stained in preheated Bouin’s solution at 56°C for 15 min, followed by washing in tap water to remove extra dye. Sections were incubated in working Weigert’s iron hematoxylin solution for 5 min followed by rinsing in water. Sections were further stained in Biebrich scarlet-acid Fucshin solution, then in phosphotungstic/phosphomolybdic acid solution and finally in Aniline Blue solution (5 min each), followed by 2 min staining in acetic acid (1%) and rinsing in tap water. Sections were dehydrated in alcohol, cleared in xylene and mounted in neutral balsam. Sections were examined under light microscope (Olympus BX40).
PAS staining
PAS stain kit (Baso Diagnostics Inc., Zhuhai, China) was used to stain the sections following manufacturer’s protocol. Sections were counterstained with hematoxylin for 2 min, washed, dehydrated and sealed with a neutral resin for microscopic observation.
3D reconstruction of embryonic intestine
Photoshop CS6 (Adobe Systems, America) was used to process all images to uniform images with same size and saved as TIFF format. The 2D images were imported into multi-layer 3D software Amira 6.0.1 (Fei, Thermo Fisher, U.S.A.) for automatic registration between adjacent images, followed by manual rotation and fine adjustment. All processed images were imported into software Mimics 21.0 (Materialise Mimics, Belgium). Manual edition of intestinal wall, mucosa and vacuoles after creation of a new threshold, in which different structures were assigned and marked with different colors. The iterations were set, images were exported and saved in STL format.
Results
Temporal and topology study vacuoles in the developing embryonic and fetal gut during intestinal recanalization
To follow the development of the vacuoles and intestine recanalization of human, we performed histology of human embryos and fetuses of 6–10 weeks of gestation. The intestine of embryos of 6–7th week of gestation has not developed a lumen, and the intestine was filled with cells and vacuoles (Fig. 1a). The vacuoles were covered with a monolayer of epithelial cell (arrowheads; Fig. 1b). Histology analysis of fetuses of 9th week of gestation showed a marked morphological difference as compared with embryos of 6–7th week of gestation (Fig. 2a), in that a narrow continuous gut lumen was now identifiable (compare Figs. 1 with 2a), suggesting that the embryonic gut was at the early stage of intestine recanalization. The outer longitudinal (arrows) and the inner circular (arrowheads) muscle layers, the mucosal epithelium (unfilled arrowheads) was clearly demarcated (Fig. 2a, 2b). The vacuoles were close to the periphery of the developing intestine and tightly packed, in that some vacuoles appeared merging with the intestine wall (asterisk; Fig. 2a, 2b). Histology analysis of fetuses of 10th week of gestation (Fig. 2b) and at the later stage of intestine recanalization showed that the intestine has developed further resulting in a big and prominent luminal space, and only few vacuoles were found merging with the developing mucosal epithelium (asterisk; Fig. 2b).
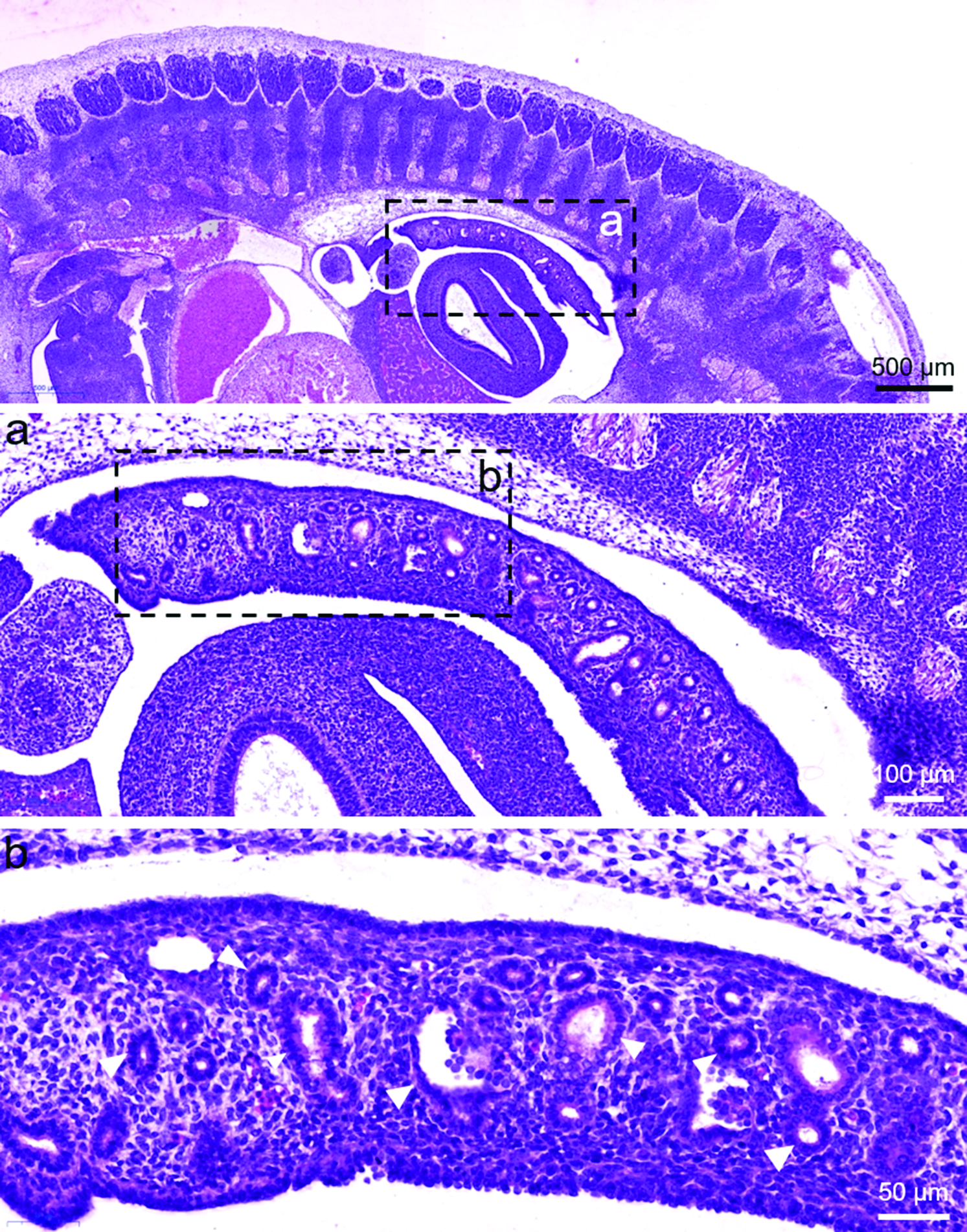
Fig. 1. Histology study of 6–7-week-old human embryos. Sagittal sections of human embryos of CRL 8.5–9 mm (approximately gestation 6–7 weeks) were stained with H&E. The regions highlighted were magnified as shown. The vacuoles (arrowheads) were surrounded by a monolayer of epithelial cell and with an empty cavity inside. No obvious gut lumen was seen.

Fig. 2. Histology study of 9-week-old human embryos. (A) Sagittal sections of human fetuses of CRL 22–28 mm (approximately gestation 9 weeks) were stained with H&E. Regions highlighted were magnified as shown. A narrow gut lumen could be seen in the developing intestine. Numerous vacuoles were localized at the centre of the gut. Vacuoles (asterisk) adjacent to the intestine wall appeared merging with the developing mucosal epithelium (unfilled arrowheads). The outer longitudinal (arrows) and the inner circular muscle (arrowheads) layers were developed in the intestine wall. (B) Sagittal sections of human fetuses of CRL 31–36 mm (approximately gestation 10 weeks) were stained with H&E. Regions highlighted were magnified as shown. The intestine has developed a big and prominent luminal space, and fewer vacuoles were observed. Vacuoles (asterisk) were merging with the developing mucosal epithelium. The outer longitudinal and the inner circular muscle layers were developed in the intestine wall.
To reveal the topology of the vacuoles in the intestinal canal, 3D reconstruction of the embryonic intestine in embryos of 9 weeks of gestation was conducted. The reconstructed 3D image of the embryonic intestine showed the center of the occlusion gut was filled with vacuoles of different sizes. In addition, vacuoles that were close to the developing intestine mucosa appeared merging with the mucosa (Fig. 3). Our 2D histology and 3D topology studies of embryonic intestine revealed that (i) vacuoles were abundant at the centre of the occlusion intestine, and some vacuoles were merging with the developing mucosa at the early stage of intestine recanalization; (ii) few vacuoles were present and were merging with the developing intestinal mucosa at the late stage of intestine recanalization with a prominent intestinal lumen.

Fig. 3. 3D modeling of gut of CRL 22–28 mm embryo. The superimposed 2D images (A) and the pseudo-colors 3D image (B) were shown to indicate various structures: yellow, vacuoles; red, mucosa; brown, intestine canal.
Identification of “residue vacuoles” in the septum of intestinal atresia patients
Seven type I IA patients (postnatal 1–3 days old; jejunal atresia (four males, one female); jejunoileal atresia (one male, one female)) were recruited for the study. Septal tissue was located after the intestinal tract was retracted through a 2 cm small incision onto the abdomen, and a longitudinal incision was then made to expose the septum (Fig. 4). The intestine segment with the septum was removed surgically (longitudinal incision and transverse suture) followed with an end-to-end anastomosis. A longitudinal sectioning along the septum revealed the entire longitudinal plain of the septum at the atretic region (Fig. 5). Histology staining indicated that for the septum with a central hole, the mucosal layer (arrows; Fig. 5a) covered the distal and the proximal sides of the septum and the hole at the center of the septum (arrowhead; Fig. 5a). For the complete separation septum (i.e. septum without a central hole) (Fig. 5b), the mucosal layers cover the distal and the proximal sides of the septum. In addition, loose submucosa and fragmental smooth muscle (unfilled arrowheads) were located in the septum, with no observable serosal layer.

Fig. 4. Pre-operation image and operation photos of IA gut. Image to indicate intestinal obstruction caused by septum in IA patient, and operation images to show the septum tissue.

Fig. 5. Histology study of the septum. Sagittal sections of the septum were stained with H&E. Schematic diagrams to depict the view of the longitudinal section of septum with a central hole (A) and without a central hole (B). Regions highlighted were magnified as shown. Mucosa (arrows) covered both the proximal and distal sides, and at the hole (arrowhead) of the septum with a central hole (A). Mucosa covered both the proximal and distal sides of the septum without a central hole (B). Fragmented smooth muscle fibers in the septum were indicated with unfilled arrowhead.
Intestinal villi were long, orderly arranged and tightly packed in the normal part of the intestine, whereas, the villi were short and loosely packed in the septal regions (Fig. 6). To examine the development of the intestine epithelium, we performed PAS staining for the goblet cells (GC). In the normal intestine part, GC were abundant at the epithelium, in particular, at the basal part of the villi. In contrast, GC were much less abundant in the intestine epithelium of the septal part (Fig. 6).

Fig. 6. Histology and PAS staining of the normal region and the atretic region of IA gut. Section of normal intestine and the septum of IA patient’s gut were processed for H&E and PAS staining.
To identify vacuole in the septum, we performed histology staining of sagittal sections spanning the entire width of the septum. Residual vacuoles were identifiable in the septum of two patients with jejunal atresia. The isolated vacuole was located in the loose submucosal layer of the septum, and with a single layer of cuboidal epithelium or columnar epithelium (Fig. 7). H&E staining of serial sections indicated that the epithelium of the vacuole was well separated from the epithelium of the intestine, and there were no glandular structures located at the vacuole. Masson staining revealed the vacuole epithelium was necrotic with fibrotic tissue at the mesenchyme around the vacuole (Fig. 7).
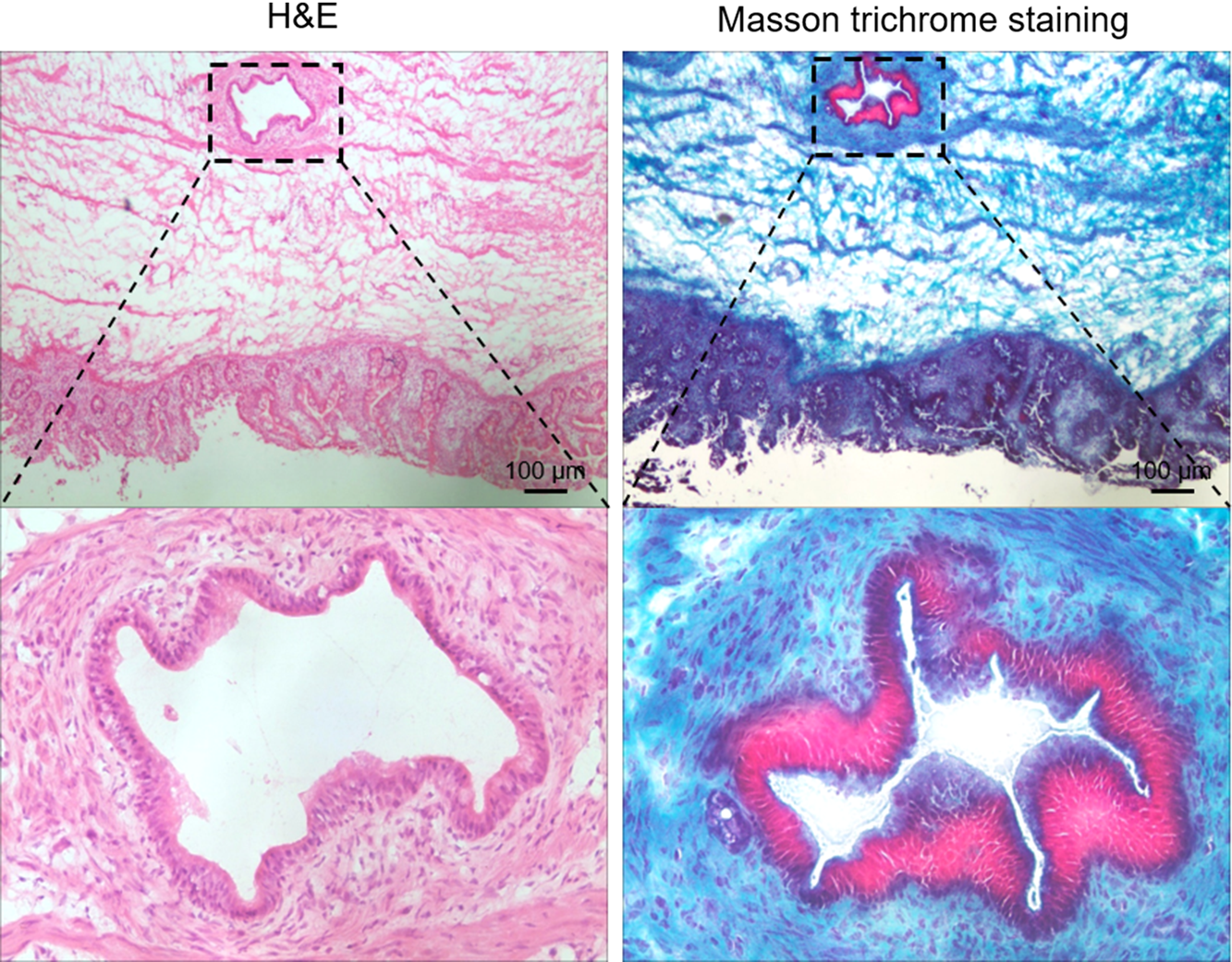
Fig. 7. Histology and Masson staining of septum of IA patient. Sections of the septum of IA patient were processed for H&E and Masson staining. Regions highlighted were magnified as shown.
Discussion
Our temporal and topology studies of human embryonic guts show for the first time the evolution history of the vacuoles during the lumen formation of human intestine, and provide compelling evidence for the “gut recanalization” theory proposed by Julius Tandler.Reference Tandler2 Furthermore, the identification of residual vacuoles in the septum of the atretic region of IA patient, suggests that a failure of intestine recanalization in embryos could be a leading cause of IA.
The human fetal intestine is derived from the endoderm. During the first 3–4 weeks of gestation, a simple tube lined by undifferentiated mesenchyme cells is formed, which can be divided into foregut, midgut and hindgut. At the end of the 3rd week, the buccopharyngeal membrane at the cephalic end of the foregut, ruptures and a physical connection between the amniotic cavity and the primitive intestine is established. During gestation week-7, the cloacal membrane at the posterior end of the hindgut, ruptures, and the continuity between the amniotic cavity, the intestinal tract, and the urogenital tract is completed. After its initial formation, the primitive intestinal tube increases rapidly in length and diameter resulting in a temporary umbilical herniation between gestational week 6 and 12. This period of rapid growth represents the first developmental stage of proliferation and morphogenesis of the intestine. Between gestational week 8 and 10, columnar cells (2–4 cell layers thick) develop and line the entire length of the small intestine. Our data show that at 6–7th week of gestation, the embryonic gut has no lumen, filled with mesenchyme cells and vacuoles. Later at approximately 9th week of gestation, the embryonic gut develops a narrow lumen, and the gut is filled with vacuoles of different sizes, in that some vacuoles are merging with the developing embryonic gut wall. At the later stage (approximately 10th week of gestation), a prominent lumen has been developed, vacuoles have nearly disappeared with only few vacuoles found merging with the intestine wall. The fusion of vacuoles with the developing intestine wall associates with the disappearance of vacuoles and the gut lumen formation between gestation week-6 and week-10. Gut is composed of endoderm and mesoderm,Reference Rodriguez and Downs13–Reference Bourret, Chauvet, de Santa Barbara and Faure15 in which mucosal epithelium arises from endoderm,Reference Micchelli16 while submucosal and muscular layers arise from mesoderm.Reference Bely17 The vacuoles are covered with a monolayer of epithelial cell, indicating that vacuoles are derived from endoderm sharing the same embryonic origin as the intestine mucosal epithelium. Since cells of the same embryonic origin aggregate and cluster together,Reference Kimes, Liu, Neil Hayes and Marron18–Reference Agnew, Green, Brown, Simpson and Binder21 we propose that during the gut lumen formation, epithelial cells of the vacuoles fuse with the developing intestine mucosal epithelium, while the mesenchymal cells aggregate and differentiate into blood vessels, muscularis in submucosal layer and muscles of intestinal wall. Taken all these together suggest that the fusion of vacuoles with the developing intestine mucosal epithelium is a critical event for gut recanalization in embryonic gut development.
We showed that at the septum, the mucosal epithelium was developed with lamina propria and basement membrane, but the submucosa and the longitudinal smooth muscle layers were not properly developed, suggesting that the septal tissues of IA intestine may be a sequel of a defective intestine development in embryos. The vacuoles in the loose submucous layer of the septum were covered by a single layer of epithelium without glandular structure, and were surrounded with fibrous tissue. Hence, the vacuoles in the IA septum could represent a remnant of vacuoles of incomplete vacuole-intestine mucosal epithelium fusion event during embryonic gut recanalization. Our recent finding has revealed that the development and recanalization of vacuoles in the duodenum occurs later than the jejunum and ileum, which may be involved in the more frequent development of atresia/stenosis of the duodenum compared to more distal gastrointestinal tract.Reference Liu, Song and Hao22 However, vacuoles are not identified in all the IA patients in this study, which suggest that other causes such as “vascular accident” cannot be ruled out from the etiology of IA.
Conclusion
In conclusion, fusion of vacuoles with the developing intestine mucosal epithelium and the development of the gut mesenchyme are important events for gut recanalization in embryonic gut development, and perturbation of these developmental events could lead to intestinal atresia.
Acknowledgements
The authors would like to thank all the parents and patients who participated in this study. This work is supported by the Public Service Development and Reform Pilot Project of Beijing Medical Research Institute, BMR2019-11.










