INTRODUCTION
Cryptocaryon irritans Brown, 1951 is a facultatively parasitic prostome ciliate that is capable of infecting a wide range of marine teleosts causing ‘cryptocaryoniasis’. This disease can do great harm to the aquaculture industry and outbreaks have resulted in significant economic losses (Colorni and Burgess, Reference Colorni and Burgess1997; Dickerson, Reference Dickerson, Woo and Leatherland2006). Many chemotherapeutic and physical methods have been used to control cryptocaryoniasis with various degrees of efficacy (Pironet and Jones, Reference Pironet and Jones2000; Hirazawa et al. Reference Hirazawa, Oshima, Hara, Mitsuboshi and Hata2001, Reference Hirazawa, Goto and Shirasu2003; Rigos et al. Reference Rigos, Pavlidis and Divanach2001, Reference Rigos, Karagouni, Kyriazis, Athanasiou, Grigorakis, Kotou and Katharios2013; ). In order to better understand the disease at the molecular level and to develop better methods of biotherapy, transcriptome and proteome analyses of C. irritans have been carried out (Lokanathan et al. Reference Lokanathan, Mohd-Adnan, Wan and Nathan2010; Mai et al. Reference Mai, Li, Li, Li, Huang, Mo and Li2015). Potential antigens for vaccine development have also been identified in C. irritans (Hatanaka et al. Reference Hatanaka, Umeda, Yamashita and Hirazawa2007; Huang et al. Reference Huang, Sun, Guo, Zheng, Xu, Yuan and Liu2012; Lin et al. Reference Lin, Yang, Huang, Ni, Fu, Guo, Wang and Huang2013). In addition, researches in immunology have resulted in the isolation of several effective substances from fishes (e.g. an antiparasitic protein from Siganus oramin and an antimicrobial peptide from Pseudosciaena crocea) that are lethal to C. irritans (Burgess and Matthews, Reference Burgess and Matthews1995; Yambot and Song, Reference Yambot and Song2006; Bai et al. Reference Bai, Xie, Zhu, Dan and Li2008; Wang et al. Reference Wang, Xie and Li2010; Li et al. Reference Li, Dan and Li2013; Niu et al. Reference Niu, Jin, Xu, Qiao, Wu, Mao, Su and Wang2013; Yin et al. Reference Yin, Gong, Ke and Li2015 Reference Yin, Sun, Tang, Dan and Li b ). Nevertheless, more vulnerable features during the life cycle and some crucial life history processes of C. irritans still need to be exploited in order to render preventions and treatments more effective (Colorni and Burgess, Reference Colorni and Burgess1997; Huang et al. Reference Huang, Xu, Guo, Lin, Ye, Yuan, Sun and Ni2013).
Numerous studies have been carried out on the life history and general morphology of C. irritans (Cheung et al. Reference Cheung, Nigrelli and Ruggieri1981; Colorni and Diamant, Reference Colorni and Diamant1993; Matthews et al. Reference Matthews, Matthews and Burgess1993; Diggles, Reference Diggles1997; Huang et al. Reference Huang, Ma and Li2005; Li et al. Reference Li, Huang, Ma and Xie2006; Ma et al. Reference Ma, Li, Xie and Huang2006). However, certain morphological characteristics at different stages of its life cycle have yet to be revealed in detail, e.g. the circumoral dikinetid cilium–palp triplets, the adoral brosse, the membranous folds and the contractile vacuole. Furthermore, detailed morphological evidence is also needed in order to improve understanding of the systematics of C. irritans and its evolutionary relationships within the class Prostomatea (Diggles and Adlard, Reference Diggles and Adlard1995; Colorni and Burgess, Reference Colorni and Burgess1997; Wright and Colorni, Reference Wright and Colorni2002; Lynn, Reference Lynn2008).
In this study, the morphology of cells at different stages in the life cycle of C. irritans, i.e. trophont, protomont, tomont and theront, was investigated using light microscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Detailed structures of various organelles and their changes at different life stages and possible functions of C. irritans were discussed (Colorni and Burgess, Reference Colorni and Burgess1997; Dickerson, Reference Dickerson, Woo and Leatherland2006).
MATERIALS AND METHODS
Culture and collection of C. irritans
Cells of C. irritans were isolated from a naturally infected host fish, Larimichthys crocea, from an aquaculture farm, in Ningde, Fufa Fisheries Co., Ltd., Fujian Province in China. Propagation was conducted using a method modified from Yin et al. (Reference Yin, Gong, Ke and Li2015a ) using P. crocea as the host. Cells at each stage of the life cycle of C. irritans were collected as follows. Trophonts were scraped from the skin or gill of host. Protomonts naturally shed from the host were collected using a glass pipette. Tomonts were brushed off and collected from the bottom of the aquarium containing infected L. crocea. Large numbers of theronts were obtained by incubating collected tomonts at 25 °C in well-oxygenated fresh sea water.
Observation of living cells and silver staining
Living cells were isolated and observed in vivo using differential interference contrast microscopy. Chatton–Lwoff silver nitrate staining (Corliss, Reference Corliss1953; Wilbert and Song, Reference Wilbert and Song2008) was used to reveal the infraciliature and argyrome of theronts. Theronts were fixed in 7:7:5 mixture of 3% K2Cr2O7, 1% CrO3 and 2% OsO4, and then preserved in Da Fano (1 g Co(NO3)2·6H2O, 1 g NaCl and 10 ml formalin in 90 ml distilled water) solution for 1 h. A small drop of concentrated cells and a little warm (35–45 °C) saline gelatin were mixed on a glass slide, then they were spread out for a 100–300 µm thick layer with the excess fluid drawn off. The glass slide was then placed on ice cubes and soak with 3% silver nitrate solution. The specimen was developed under ultraviolet light, and the usual dehydrating series were run up and into xylene and mounted in neutral synthetic resin.
SEM and TEM
Sample preparation for SEM followed Gu and Ni (Reference Gu and Ni1993). Trophonts, protomonts and tomonts were fixed in 2·5% glutaraldehyde at 4 °C for 24 h. Theronts were fixed in a 4:1 mixture of saturated solution of HgCl2 and 1% OsO4 at 4 °C for 10 min. In order to remove the cilia from the theronts, cells were fixed in 4% OsO4. All fixation solutions were diluted in 0·1 m sodium cacodylate buffer (pH 7·2). Fixed cells were washed with buffer, dehydrated in a graded ethanol series, critical-point dried, placed on aluminium stubs and sputter coated with platinum. To observe cells within tomonts, tomonts adhered to stub were lacerated by a blade and the cyst wall was stripped off using a needle. Prepared samples were observed in Hitachi S-4800 SEM.
Sample preparation for TEM followed Gu et al. (Reference Gu, Chen, Ni and Zhang2002). Trophonts, protomonts and tomonts were fixed in 3% glutaraldehyde at 4 °C for 24 h. Theronts were fixed in a 1:1 mixture of 2·5% glutaraldehyde and 2% OsO4 at 4 °C for 30 min. After washing with buffer, samples were post-fixed in 1% OSO4 at 4 °C for 2 h. All fixation solutions were diluted in 0·1 m sodium cacodylate buffer (pH 7·2). Fixed cells were washed with buffer, dehydrated in a graded acetone series, embedded with Epon 812 and polymerized at 37 °C for 16 h, 45 °C for 24 h and 60 °C for 48 h. Ultrathin sections were cut with a diamond knife and then stained with uranyl acetate and lead citrate. The sections were examined in Hitachi HT-7700 TEM.
RESULTS
Trophont and protomont
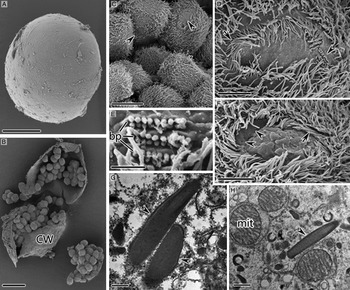
Trophonts of C. irritans isolated from the fin or gill of the host L. crocea were invariably found near the small bones. They were extremely flexible and displayed a range of different body shapes (Fig. 1A). They sank to the bottom of the aquarium rapidly after exiting from host fishes. The free-swimming protomont stage lasted for an extremely short period of time, generally <2 h. Trophonts scraped from host fish tissues and naturally shed protomonts were invariably ellipsoidal. Their surfaces were covered by numerous tumula protuberances, and each cell had 60–80 rows of 7–8 µm long somatic cilia (Fig. 1B). Generally, the region of each somatic kinety near the cytostome had three or four pairs of dikinetids, each bearing two cilia, the remainder of the kinety being composed of monokinetids (Fig. 1D). In a small minority of cells, the entire somatic kineties were composed of monokinetids (Fig. 1E).
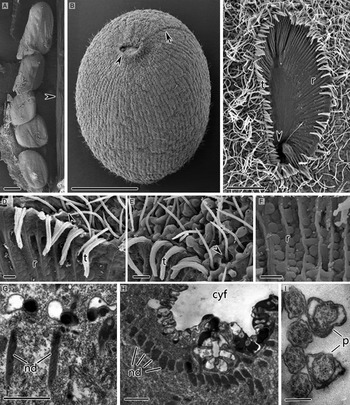
Fig. 1. SEM images (A–F) and TEM images (G–I) of trophonts (A, C–I) and protomonts (B) of Cryptocaryon irritans. (A) Differently shaped trophonts in one cavity of gill tissue, arrowhead marks a tiny bone of the gill. (B) Holistic view of protomont, showing cytostome (arrowhead) and aboral membranous folds (arrow). (C) Holistic view of cytostome, arrowhead depicts the entrance of the cytopharynx. (D) Partial view of cytostome, showing three or four pairs of dikinetids with double cilia at the anterior end of somatic kineties (arrowhead). (E) Three or four monokinetids at the anterior end of somatic kineties in a minority of cells (arrowhead). (F) Ridges in the cytopharyngeal depression and inter-ridge spherical protuberances. (G, H) The nematodesmata. (I) Transverse section of the circumoral triplets. cyf, cytopharynx funnel; nd, nematodesmata; p, oral palps; r, ridges in the cytopharyngeal depression; t, circumoral cilium–palp triplets. Scale bars 100 µm (A, B), 10 µm (C), 1 µm (D–H) and 0·2 µm (I).
Cytostome and cytopharynx
In trophonts and protomonts, the cytostome was located at the anterior end of the cell and was 50–70 µm long and 25–35 µm wide (Fig. 1C). The cytopharynx was slanting funnel shape and extended from the cytostome deep into the interior of the cell (Fig. 1C). Radial ridges in the cytopharyngeal depression converged into bundles; the ridges were thick and well developed in their superficial 10–20 µm region, becoming delicate and smooth deeper within the cell (Fig. 1C, D and F). Numerous spherical protuberances, 0·2–1 µm in diameter, were distributed between the ridges (Fig. 1F). The cytostome was surrounded by 100–120 dikinetids forming the circumoral ciliary row (Fig. 1C–E). Each dikinetid bore two cilia and was associated with an adjacent oral palp, forming a cilium–palp triplet; each triplet corresponded to one cytopharyngeal ridge (Fig. 1D). The nematodesmata below the circumoral dikinetids were single compact microtubular bundles (Fig. 1G and H). The oral palps, in which no toxicysts were observed, had a three-layer membrane structure encircling the electron-lucent cytoplasm; the outer two layers were closely juxtaposed and widely separated from the internal one (Fig. 1I).
Membranous folds and adoral brosse
Three or four wall-like membranous folds of different lengths were located near to, but not directly connected with, the cytostome (Fig. 2A–C). These folds extended longitudinally backwards and occupied about 1/3 body length (Figs 1B and 2A). Cilia aligned individually in rows between the membranous folds, and these ciliary rows lay parallel to the somatic kineties closest to them (Fig. 2A and B). Three segments of the adoral brosse, recognizable by the clavate cilia, could be seen among the dense cilia at the frontal end of the membranous folds (Fig. 2D).

Fig. 2. SEM (A–D, F) and TEM (E, G, I) images of trophonts of Cryptocaryon irritans. (A, B) Membranous folds on the aboral surface. (C) The membranous folds are near, but do not directly connect with, the cytostome. (D) Three segments of adoral brosse at the frontal end of membranous folds (arrowheads). (E) Longitudinal section of trophont contractile vacuole. (F) Contractile vacuole pore openings (arrowheads). (G) Contractile vacuole pore, arrowheads mark the microtubules in the contractile vacuole pore perisome and main vacuole wall; arrow depicts the microtubules connecting the contractile vacuole pore perisome to the main vacuole wall. (H) Peripheral spongiome and mitochondria outside the spongiome of the contractile vacuole in the trophont. CV, contractile vacuole; cvp, contractile vacuole pore; MF, membranous folds on the aboral surface; mit, mitochondria; p, oral palps; r, ridges in the cytopharyngeal depression; sp, peripheral spongiome; t, circumoral cilium–palp triplets. Scale bars 10 µm (A) and 2 µm (B–H).
Contractile vacuole
Trophonts and protomonts had multiple contractile vacuoles, each located beneath the pellicle and connected to the external environment via a contractile vacuole pore (Fig. 2E). The contractile vacuole pores had ring-like openings on the surface of cells when viewed by SEM (Fig. 2F). Each was cylindrical or cupuliform and subtended by a slanting, spirally aligned single layer of microtubules inside the perisome (Fig. 2G). The contractile vacuole was surrounded by thick, spongy membranous system, the so-called spongiome, which was surrounded by numerous mitochondria (Fig. 2E and H). The wall of contractile vacuole was thicker near the pellicle and thinner on the opposite side (Fig. 2E). A single layer of radially distributed microtubules was associated with the pellicle side of the vacuole wall; microtubules were also present connecting the vacuole wall to the contractile vacuole pore perisome (Fig. 2E and G).
Tomont
Following the short free-swimming stage, the protomonts adhered to surfaces at the bottom of the aquarium and began to secrete cyst wall materials slowly forming the cyst wall (Fig. 3A). In most cases, a typical palintomic cell division in tomonts began in 1–2 days after encystation and was completed within 2–3 days. This progress could produce up to 200–400 individual small cells, which would develop into the theront (Fig. 3B). Cilia existed throughout the whole division process in tomonts and beat actively when observed in vivo (Fig. 3C). An apparently oral primordium could be seen including a circular area without cilia on the surface of tomites following cytokinesis during telophase of cell division (Fig. 3C). The peripheral somatic kineties were distributed radially from the circumoral dikinetid ciliary row that surrounded the glabrous circular area, and were separated from this row by a short gap (Fig. 3D). The circumoral dikinetid row was not fully closed during the early stage of its development, but rather there was a gap outside of which the three segments of the adoral brosse primordium were aligned at an angle (Fig. 3D). Each segment of the brosse contained 9–12 pairs of dikinetids (Fig. 3E). The circumoral ciliary row became fully closed around the cytostome following further development. The inner circular glabrous area sank down starting from the periphery, and the ridge-shaped rudiment of cytopharyngeal depression began to form (Fig. 3F). Toxicyst precursors appeared as electron-dense cigar-shaped structures in the cytoplasm of the diving tomonts and were located near the oral primordium in the tomites at late-stage development of tomonts (Fig. 3G and H).

Fig. 3. SEM (A–F) and TEM (G, H) images of tomonts of Cryptocaryon irritans. (A) Holistic view of tomont. (B) A dissected tomont with dividing cells inside. (C) Tomites at anaphase of cell division, arrowheads indicate the oral primordium of each tomite. (D) Oral primordium of early development stage, arrow depicts the not fully closed circumoral dikinetid ciliary row, and arrowhead marks adoral brosse primordium. (E) Adoral brosse primordium. (F) Relatively developed oral primordium, arrow indicates the fully closed circumoral ciliary row, and arrowheads refer to the ridge-shaped rudiment of cytopharyngeal depression forming from the periphery of the sunken circular area. (G, H) Toxicyst precursors (arrowheads). bp, brosse primordium; CW, cyst wall; mit, mitochondria. Scale bars 100 µm (A, B), 5 µm (D, F), 1 µm (C, E), 0·5 µm (H) and 0·2 µm (G).
Theront
Theronts were 60–80 µm × 20–30 µm and ellipsoidal with length–width ratio of 3:1–2:1 (Fig. 4A–C). The number of somatic kineties of theronts was equal to that of trophonts and protomonts. The dense cilia of theronts beat synergistically when swimming and formed spiral oscillatory waves on the body surface, giving the general outline of the theronts a screw-like appearance (Fig. 4B). Theronts swam fast while rotating about their main axis, towards a random direction. Theronts always performed rotational drilling action when they encountered large foreign material or when invaded host tissues.
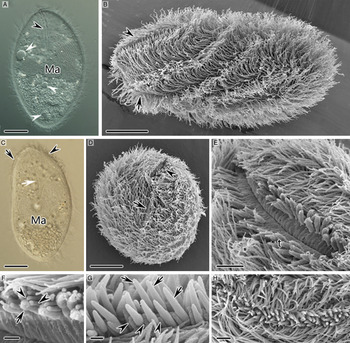
Fig. 4. Cryptocaryon irritans in vivo (A, C) and viewed by SEM (B, D–H). (A–C) Holistic views of theronts, arrowheads and arrows mark the cytostome and the membranous folds on the aboral surface respectively, white arrowheads indicate the contractile vacuoles and white arrow depicts the horn-shaped cytopharynx. (D) Apical view of theront, arrowhead and arrow mark the cytostome and the membranous folds respectively. (E) Holistic view of cytostome. (F) Circumoral cilium–palp triplets of typical cell, arrow indicates the slightly longer palps and arrowheads indicate the dikinetid cilia. (G) Circumoral cilium–palp triplets in some cells, arrows indicate the palps with cigar-shaped extensions and arrowheads mark the dikinetid cilia. (H) Holistic view of cytostome with densely clustered palps. Ma, macronucleus; t, circumoral cilium–palp triplets. Scale bars 10 µm (A–D), 2 µm (E, H) and 0·5 µm (F, G).
Cytostome and cytopharynx
A slit-like cytostome and three longitudinal membranous folds were located respectively at two sides of the anterior end of theronts (Fig. 4B–D). The length of the cytostome was 1/3–1/4 of the cell length (Fig. 4B and E). When viewed by SEM, the cytopharynx usually appeared as a shallow ‘V’-shaped groove with flat, inconspicuous ridges on the wall (Fig. 4E). Circumoral cilium–palp triplets aligned longitudinally along both sides of the slit-like cytostome; the oral palps were slightly longer than the cilia of the dikinetids (Fig. 4E and F). In some cells, the palps were densely clustered with 1·5–2 µm long cigar-shaped extensions (Fig. 4G and H). The cytopharynx was curved in a horn shape and extended to the mid-body region, close to the aboral pellicle when observed in vivo and in TEM (Figs 4C and 5A). The membranous wall between the ridges of the cytopharynx was conspicuously folded into cytoplasm, forming continuous vesicular structures at its cytoplasm side end (Fig. 5B). The nematodesmata, which arose from an electron-dense plate found underlying the circumoral dikinetids were each composed of single compact bundle of several dozen microtubules arranged in rows (Fig. 5C–F). These surrounded the cytopharynx side by side forming a horn-shaped microtubular sheath that encased the cytopharynx (Fig. 5A, C–F). The oral palps also contained microtubules (Fig. 5H). Toxicysts could be found in the oral palps and in the cytoplasm surrounding the oral region (Fig. 5G and H). The toxicysts were cigar-shaped with an electron-dense apex, an electron-lucent core, and some longitudinal lines in the basal portion (Fig. 5I).

Fig. 5. TEM images of theronts of Cryptocaryon irritans. (A) Longitudinal section of cytopharynx, arrowheads indicate the nematodesmata underlying the circumoral dikinetids. (B) Highly folded membranous wall of cytopharynx. (C–E) The nematodesmata, (C) is partially enlarged detail of area marked by white rectangle in (A), arrowheads indicate the electron-dense plate that the nematodesmata originated from. (F) Transverse section of the cytopharynx, note the single compact nematodesmata composed of dozens of microtubules arranged in rows, arrowheads indicate the circumoral dikinetids. (G, H) Longitudinal section of the circumoral cilium–palp triplets and the oral region near them, arrowheads mark the toxicysts in oral palps and in cytoplasm of surrounding oral region, arrows indicate the microtubules contained in the palps. (I) Longitudinal section of a toxicyst. C, cytopharynx; Ma, macronucleus; nd, nematodesmata. Scale bars 2 µm (A, G), 0·5 µm (B–D, F, H) and 0·2 µm (E, I).
Membranous folds and adoral brosse
There were three longitudinal wall-like membranous folds of differing lengths on the aboral surface. They started from the proximal anterior end of the cell and extended backwards to 1/3–1/2 of the cell length. Their rear part might fragment into short segments (Fig. 6A and F). These folds were not revealed by silver nitrate staining (Fig. 6D, E and G). Cilia aligned individually in rows between the membranous folds and had the same pattern as that of the somatic kineties on the left and right sides (Fig. 6A, D, E and G). From TEM observations of transverse sections, these folds were revealed to be pellicular protuberances containing cytoplasm, and were supported by conspicuous microtubules (Fig. 6B and C). The adoral brosse was composed of three small kinetofragments bearing clavate cilia (Fig. 6D–G). These kinetofragments were probably located in pellicular depressions at the anterior end of the membranous folds (Fig. 6E and F), since the kinetofragments could only be observed beneath the level of somatic kineties (Fig. 6D and E).

Fig. 6. Theronts of Cryptocaryon irritans following preparation by SEM (A, F, I), silver nitrate staining (D, E and G) and TEM (B, C, H, J, K). (A) Membranous folds on the aboral surface. (B) Transverse section of membranous folds. (C) Partially enlarged detail of area marked by rectangle in (B), showing the microtubules supporting the base of membranous folds (arrowheads). (D) Three kinetofragments of adoral brosse. (E) The area (arrowhead) made by the somatic kineties on the right and left side of the brosse (white arrows) and three somatic kineties after the brosse (black arrows, i.e. the ciliary rows between the membrane folds). (F) The adoral brosse bearing clavate cilia at the frontal end of the membranous folds. (G) Many argyrophilic contractile vacuole pores of theront (arrowheads). (H) Longitudinal section of theront contractile vacuole. (I) Contractile vacuole pore openings (arrowheads). (J) Partially enlarged detail of area marked by rectangle in (H), arrowheads indicate the monolayer of microtubules inside the vacuolar wall. (K) Contractile vacuole pore, arrowhead depicts the microtubules connecting the contractile vacuole pore perisome to the main vacuole wall. B, brosse; CV, contractile vacuole; cvp, contractile vacuole pore; MF, membranous folds on the aboral surface. Scale bars 10 µm (G), 2 µm (A, D, F), 0·5 µm (B, H, I) and 0·2 µm (C, J, K).
Contractile vacuole
Each theront had 13–16 contractile vacuoles that contracted with slow and variable frequencies. The contractile interval for the three posterior vacuoles was about 10–15 s, and for most others could be as slow as 1–2 min (Figs 4A and 6G). The contractile vacuole pores were cylindrical in shape and their external openings could occasionally be observed among the dense cilia by SEM (Fig. 6H, I and K). The ultrastructure of the theront contractile vacuole was not identical to that of the trophont and protomont. There was no membranous spongiome surrounding the main vacuole (Fig. 6H). The vacuole wall on the pellicle side was relatively thick and dome-like, with a monolayer microtubules located within, but thin and membranous on the opposite side (Fig. 6H and J). There was also a slanting spirally aligned single layer of microtubules inside the contractile vacuole pore perisome, and additional microtubules connecting the vacuolar wall to the contractile vacuole pore perisome (Fig. 6H and K).
DISCUSSION
Circumoral dikinetid cilium–palp triplets and toxicysts within the palps
Colorni and Diamant (Reference Colorni and Diamant1993) reported that in trophonts and protomonts of C. irritans, the cytostome was surrounded by ciliary triplets with tips fused. Diggles (Reference Diggles1997) discovered that these triplets were clung by dikinetid cilia and oral palps. Our study confirmed the latter finding, which revealed that C. irritans shared common structure with many other free-living prostome ciliates (Bardele, Reference Bardele1999; Lynn, Reference Lynn2008). Moreover, we discovered that cilium–palp triplets also existed in the theront stage and were aligned on both sides of the slit-like cytostome. In this study, the nematodesmata underlying the circumoral dikinetids were found to be single bundles of microtubules. However, C. irritans had traditionally been assigned to the order Prorodontida, but prorodontids were characterized by having paired triangular to trapezoid-shaped bundles of nematodesmata (Huttenlauch, Reference Huttenlauch1987; Hiller, Reference Hiller1991; Bardele, Reference Bardele1999; Wright and Colorni, Reference Wright and Colorni2002). Many prostome ciliates contained toxicysts in their oral palps, which were associated with their predatory lifestyle (Huttenlauch, Reference Huttenlauch1987; Bardele, Reference Bardele1999; Lynn, Reference Lynn2008). Li et al. (Reference Li, Huang, Ma and Xie2006) reported the presence of toxicysts in C. irritans. It was worth noting that in our study there were no toxicysts in the trophonts and protomonts, although they appeared at later stage of tomonts and in the palps of theronts. The cigar-shaped membrane-bound vesicles reported by Matthews et al. (Reference Matthews, Matthews and Burgess1993) in late stages of cell division of tomonts might also be toxicyst precursors. Thus, it was very likely that the toxicysts of C. irritans form and matured in the cytoplasm of late-stage tomonts and might be involved in invasion of host-fish tissue by theronts. Diggles (Reference Diggles1997) suggested that the circumoral triplets in C. irritans corresponded one-to-one to both the somatic kineties and the ridges of the cytopharynx. However, we found that the triplets only corresponded one-to-one to the ridges and conspicuously outnumbered the somatic kineties. Judging from the ciliary pattern of multiple ciliates in class Prostomatea, there was no such one-to-one correspondence pattern between circumoral dikinetids and surrounding somatic kineties (Foissner et al. Reference Foissner, Berger and Kohmann1994).
Adoral brosse
Li et al. (Reference Li, Huang, Ma and Xie2006) reported the presence of a brosse-like structure near the cytostome in trophonts and theronts of C. irritans, consisting of three membranelles each with seven or eight dikinetids. In this study, we found the adoral brosse was composed of three kinetofragments each with 9–12 dikinetids. The adoral brosse was widely present in prostome ciliates but varied in both its morphology and location among different species. It had two basic forms: three or more-rowed dikinetids, and irregularly clustered monokinetids. It might be located either inside or outside the circumoral ciliary row of an apical cytostome or at the aboral side of a subapical cytostome (Lynn and Small, Reference Lynn, Small, Lee, Leedale and Bradbury1985; Lynn, Reference Lynn2008). When composed of dikinetids, the front or right basal bodies usually bore clavate cilia, while the rear or left basal bodies were unciliated (Hiller, Reference Hiller1991, Reference Hiller1993b ; Bardele, Reference Bardele1999; Lynn, Reference Lynn2008). The adoral brosse of C. irritans was located on the centre-right of the aboral surface, outside the circumoral dikinetid ciliary row and was composed of three-rowed dikinetids. The alignment of each dikinetid was orthogonal to the orientation of the brosse. Compared with related families, the morphology of the brosse resembled that of Urotrichidae and Colepidae, but its location was most similar to that of Plagiocampidae. Therefore, the adoral brosse of the family Cryptocaryonidae is unlike that of any other family of the class Prostomatea (Lynn and Small, Reference Lynn, Small, Lee, Leedale and Bradbury1985; Muñoz et al. Reference Muñoz, Téllez and Fernández-Galiano1989; Chen et al. Reference Chen, Wang, Long, Al-Rasheid, Warren and Song2010).
Membranous folds
Diggles (Reference Diggles1997) reported the presence of a post-oral groove in the trophont of C. irritans, and suggested that this might provide a route for transferring to the cytostome food gathered by the beating action of the somatic cilia. Present study found almost identical structure with the previous report in the aspect of morphological features. However, we found that membranous folds were present not only in trophonts and protomonts, but also in theronts. Furthermore, the anterior ends of these folds were adjacent to, but not connect directly with, the cytostome. Therefore, their function of gathering and inducting food to cytostome was highly suspicious. Since the membranous folds and ciliary rows between them were parallel to the somatic kineties, we posited that these folds might be caused by the close proximity of the somatic kineties in this area and high cytoplasmatic elevations of the pellicle between them. A similar arrangement, i.e. with cortical ridges separating the brosse kinetofragments from each other, had been reported in other prostome ciliates (Huttenlauch, Reference Huttenlauch1987; Hiller and Bardele, Reference Hiller and Bardele1988; Hiller, Reference Hiller1991). However, in C. irritans the membranous folds were so long (i.e. 1/3–1/2 of the cell length) and well-developed compared with homologous structures in free-living prostomes that they might be linked to its parasitic lifestyle.
Contractile vacuole
The presence of multiple contractile vacuoles in trophonts, protomonts and theronts of C. irritans reported by Dickerson (Reference Dickerson, Woo and Leatherland2006) was confirmed by our study. Patterson (Reference Patterson1980) summarized several structural types and potential functions of contractile vacuoles in protozoa. The contractile vacuole structure revealed in C. irritans was similar to the type commonly found in many kinetofragminophoran ciliates and simple comparing with the type found in Ichthyophthirius multifiliis and other oligohymenophorean ciliates (Chapman and Kern, Reference Chapman and Kern1983). Ewing and Kocan (Reference Ewing and Kocan1986, Reference Ewing and Kocan1987) found that the contractile vacuoles of the trophonts of I. multifiliis became conspicuous with active pulsation before exiting from host tissue and speculated that this process might swell the space around the cell thus facilitating its escape from the host tissue. This phenomenon was not observed in present study, therefore, whether contractile vacuoles of C. irritans had this function or not remains unclear. Furthermore, contractile vacuoles were not observed in tomonts, and the spongiome was found only in trophonts and protomonts. We thus concluded that the contractile vacuoles might play different roles at different stages in the life cycle. Many free-living prostome ciliates had multiple contractile vacuoles (Leipe, Reference Leipe1989; Hiller, Reference Hiller1993a ), so the number of contractile vacuoles in C. irritans was not necessarily linked to its parasitic lifestyle.
ACKNOWLEDGEMENTS
We thank Ms. Dongmei Xie and Ms. Wanying Liao for their help in sample preparation.
FINANCIAL SUPPORT
This work was funded by the National Natural Science Foundation of China (grant no. 31101932 to F. Y., 31572223 to X. F.) and the Special Scientific Research Funds for Central Non-profit Institutes of China (grant no. 2014M01, East China Sea Fisheries Research Institute; grant no. 2015B05XK01, Chinese Academy of Fishery Sciences, both to F. Y.).