INTRODUCTION
The DIVA Project (latitudinal gradients of biodiversity in the deep sea of the Atlantic Ocean), aims to study biodiversity along an Atlantic latitudinal transect, from the Arctic to the Antarctic in comparable abyssal and bathial depths. In the first expedition of this project, Me 48/1 DIVA 1, on board the RV ‘Meteor’, several Mollusca Solenogastres were collected in the Abyssal Angola Basin at depths between 5100 and 5500 m. Although the abyssal plains account for around 40% of the oceanic bottoms, only nine of the 251 species of Solenogastres described so far are known from depths below 4000 m and of these only two occur at depths below 5000 m (García-Álvarez & Salvini-Plawen, Reference García-Álvarez and Salvini-Plawen2007). Of this small group of species, five belong to the family Simrothiellidae Salvini-Plawen, Reference Salvini-Plawen1978 (Kruppomenia delta Scheltema & Schander, Reference Scheltema and Schander2000; Kruppomenia levis Scheltema & Schander, Reference Scheltema and Schander2000; Spiomenia spiculata Arnofsky, Reference Arnofsky2000; Plawenia schizoradulata (Salvini-Plawen, Reference Salvini-Plawen1978); and Plawenia argentinensis Scheltema & Schander, Reference Scheltema and Schander2000)), two to the family Amphimeniidae Salvini-Plawen, Reference Salvini-Plawen1972 (Pachymenia abyssorum Heath, 1911 and Utralvoherpia abyssalis Salvini-Plawen, Reference Salvini-Plawen1978), one species belonging to the family Acanthomeniidae Thiele, Reference Thiele1913 (Acanthomenia arcuata Scheltema, Reference Scheltema1999) and one species belonging to the family Proneomeniidae Simroth, Reference Simroth and Bronn1893 (Dorymenia profunda Salvini-Plawen, Reference Salvini-Plawen1978). After studying some specimens collected in the expedition Me48/1 DIVA 1, a new genus and two new species of the family Simrothiellidae are described in this article: Spiomenia pusilla sp. nov. and Adoryherpia serrata gen. et sp. nov., which are added to the already known species from abyssal plains.
MATERIALS AND METHODS
The animals were colleted during the expedition Me48/1 DIVA 1 from the Abyssal Angola Basin at depths of between 5125 and 5415 m, using an epibenthic sledge, on a bottom composed of white to light beige muddy sediment with a large amount of globularian foramiferans. The temperature recorded on the bottom was 2.48°C and the salinity 34.8‰ (Kröncke & Türkay, Reference Kröncke and Türkay2003). The specimens were fixed and preserved in 70° ethanol. The animals were photographed and measured and the external anatomy was described. The sclerites were studied by separating small pieces of the mantle. Three specimens of Spiomenia pusilla sp. nov. and two specimens of Adoryherpia serrata gen. et sp. nov. were decalcified in an EDTA solution and the anterior and posterior region of the animals were deposited in paraffin and cut into serial transverse sections of 5 µm. The sections were dyed with Mallory's trichromic stain and the internal anatomy was reconstructed.
DIAGNOSIS (updated after Arnofsky, Reference Arnofsky2000)
Thick cuticle without epidermal papillae. Hollow sclerites with oblique insertion in one layer, some acicular and others with a distal asymmetrical enlargement (captate). Common atriobuccal cavity. Biserial radula of heterodont radular plates with reinforcement (buttress). With a pair of anteroventral radular sacs. Ampulla-shaped ventrolateral foregut glandular organs composed of large glandular cells enclosed in musculature (type C Salvini-Plawen, Reference Salvini-Plawen1978; c.f. type Simrothiella Handl & Todt, Reference Handl and Todt2005). With unpaired genital orifice, copulatory stylets, respiratory folds and dorsoterminal sense organ.
Type species: Spiomenia spiculata Arnofsky, Reference Arnofsky2000
TYPE MATERIAL
Four specimens
Holotype: sectioned and mounted on 5 slides; sclerites on 2 slides. Paratype 1: sectioned and mounted on 6 slides; sclerites on 1 slide. Paratype 3: immature; sectioned and mounted on 6 slides; sclerites on 2 slides. Paratype 4: intact specimen. All from the abyssal Angola basin, station 344 Me48/1 DIVA 1 (17°06′12″S 04°41′42″E–17°07′30″S 04°42′18″E; 5415 m deep).
Depository: Holotype (ZSM Mol 20070742) and paratypes 1–3 are (ZSM Mol 20090074) deposited at the Zoologische Staatssammlung München.
Derivatio nominis
Latin: pusillus, very small. Concerning its small size.
DIAGNOSIS
Slightly elongate and very spiny species, about 2.5 mm long. Cuticle moderately thick, 25–30 µm, without epidermal papillae, bulges or keels. Three types of sclerites: hollow acicular sclerites with distal end asymmetrically enlarged (‘captate’), curved hollow acicular sclerites and blade-shaped scales on both sides of the pedal groove. Atrium without papillae. Pedal groove with one fold that does not extend into the pallial cavity. Biserial radula with about 35 pairs of heterodont plates (50–55 µm broad; lateral end 15 µm high and medial end 7.5 µm high) with a lateral thicker region with three pairs of denticles and a medial thinner region with six smaller median denticles. With paired anteroventral radular sacs and long radular sheath. Ventrolateral foregut glandular organs type C according to Salvini-Plawen (Reference Salvini-Plawen1978) or c.f. type Simrothiella according to Handl & Todt (Reference Handl and Todt2005). Midgut with anterodorsal caecum and without constrictions. Spawning duct unpaired along its whole extension. Unpaired genital orifice. With two pairs of groups of copulatory stylets: a ventral pair, with two stylets and a lateral pair with four stylets, respectively. Pallial cavity divided into a ventral and a dorsal chamber. With two pairs of respiratory folds. With a dorsoterminal sense organ.
DESCRIPTION
Habitus: Specimens 1.5–2.4 mm long and about 0.5 mm thick in the anterior region and 0.4 mm in the posterior region (Figure 1A). Without bulges or keels. Hollow acicular sclerites that project from the cuticle. Pedal pit and fold externally visible. White colour, observed in the specimens fixed and preserved in 70° ethanol.

Fig. 1. Spiomenia pusilla sp. nov. (A) Habitus; (B) sclerites: 1. Captate sclerite. 2. Hollow acicular sclerite. 3. Blade-shaped scale from along the pedal groove; (C, D) anatomical reconstructions from histological sections of the anterior (C) and posterior region (D). Lines 1–4 in C indicate the position of the transverse sections in Figure 2 (A–D). Lines 5–8 in D indicate the position of the transverse sections in Figure 2 (F–I). At, atrium; Ca, dorsal caecum; Cg, cerebral ganglion; Dpa, dorsal pallial cavity; Dso, dorsoterminal sense organ; Go, gonad; Gpd, gonopericadioduct; Lcs, lateral copulatory stylets; Lg, lateral ganglion; He, heart; Mg, midgut; Oe, oesophagus; Pc, pericardium; Pd, pericardioduct; Ph, pharynx; Pp, pedal pit; Ra, radula; Ras, radular sheath; Rf, respiratory fold; Rs, radular sac; Sd, spawning duct; Sr, seminal receptacle; Ts, transverse septum; Vcs, ventrolateral copulatory stylets; Vfo, ventrolateral foregut glandular organs; Vg, ventral glangion; Vpa, ventral pallial cavity.
Mantle: Cuticle moderately thick (25–30 µm) without epidermal papillae. Three types of sclerites with oblique insertion arranged in a single layer (Figure 1B): hollow acicular sclerites with asymmetrically enlarged distal end (‘captate’ according to Arnosfsky, Reference Arnofsky2000), slightly curved, with an inflection point in the proximal end where the curvature reverses (70–300 µm long; 3–10 µm wide); hollow acicular sclerites, slightly curved, with an inflection point in the proximal end (100–400 µm long; 5–10 µm wide); and blade-shaped scales (60–80 µm long; maximum width 10–15 µm) on both sides of the pedal groove and around the atriobuccal cavity.
Pedal groove and pallial cavity: The pedal groove starts with a short and narrow ciliated pedal pit (20 µm long; 50 µm wide; 150 µm high) below the beginning of the pharynx and very close to the atriobuccal cavity (Figure 1C); a large pedal gland on both sides of the pharynx empties into the pit. The pedal groove contains a very small ciliated fold that does not extend into the pallial cavity. The wide pallial cavity opens subterminally. A transverse septum divides its anterior region into two chambers: a dorsal one, into which the anus opens, and a ventral one, where the unpaired spawning duct opens (Figures 1D, 2I). There are two pairs of respiratory folds (Figures 1D, 2I): an anterior one, in the dorsal chamber of the pallial cavity at its dorsal wall and one in the posterior half of the cavity at its lateral walls at the end portion/region of the transverse septum. Two paired groups of copulatory stylets lead out through the ventral region of the pallial cavity (see below and Figures 1D, 2G–H).

Fig. 2. Spiomenia pusilla sp. nov. Sections through different regions of the body. (A) Mouth and ganglia; (B, C) radula and ventrolateral foregut glandular organs (corresponding to lines 2 and 3 in Figure 1C); (D) radula teeth; (E) drawing of the radular teeth; (F) seminal receptacles; (G, H) spawning duct and copulatory stylets; (I) pallial cavity and respiratory folds. A–D correspond to lines 1–4 in Figure 1C; F–I correspond to lines 5–8 in Figure 1D. Cg cerebral ganglion; Dpa, dorsal pallial cavity; Glm, glandular mass; Gpd gonopericardioduct; Lcs, lateral copulatory stylets; Lg, lateral ganglion; Mg, midgut; Mo mouth; Mu, musculature; Oe, oesophagus; Pc, pericardium; Pd, pericardioduct; Ph, pharynx; Ra, radula; Re, rectum; Rf, respiratory fold; Rs, radular sac; Sd, spawning duct; Sr, seminal receptacle; Vcs, ventrolateral copulatory stylets; Vfo, ventrolateral foregut glandular organs; Vpa, ventral pallial cavity; Vs, ventral senus.
Digestive system: The narrow mouth is on the dorsoposterior surface of the common atriobuccal cavity. It leads to an internally folded pharynx, which diameter decreases towards the posterior region. The radular apparatus is composed of two short anteroventral radular sacs (50 µm long) bearing 4–6 radular plates (Figures 1C, 2B), a biserial radula and a long radular sheath (100 µm) with developing plates. The radula consists of 33–35 pairs of heterodont plates (50–55 µm broad; lateral end about 15 µm high and medial end 7.5 µm high), which are thicker at their lateral half and are fixed to the lateral wall of the pharynx (Figures 1C, 2C–E). Taking the terms introduced by Arnofsky (Reference Arnofsky2000) (see above Spiomenia genus diagnosis) into account, the anteriormost and posteriormost magins of the thicker region of the plates could be described as a buttress each. On each buttress there is a row of three denticles opposite to the other buttress' row, so that every denticle forms a pair with its opposite, being observed three pairs of denticles: a lateral pair of 4 µm high denticles, an intermediate pair of denticles, which are 3.5 µm wide on their base and 7.5 µm high and a more median pair of denticles, which are 3 µm wide on their base and 6 µm high, placed in the middle of the buttress. The thinner region of each plate bears a row of 6 regularly arranged smaller denticles, whose size decreases towards the median end of the plate (Figure 2D, E).
The ventrolateral foregut glandular organs consist of ampulla-shaped glandular organs enclosed in musculature (type C according to Salvini-Plawen, Reference Salvini-Plawen1978; c.f. type Simrothiella according to Handl & Todt, Reference Handl and Todt2005) (Figures 1C, 2B, C); they open into the pharynx at the beginning of the anterior radular region. Part of the ventrolateral foregut glandular organs and radular apparatus (except for the radular sheath region) are covered by musculature and connective tissue. There are no vacuolized supporting cells of the radula bolster. The pharynx opens dorsally into a short oesophagus (Figures 1C, 2B, C) that curves anterior–dorsally to open into the midgut anterior to the radular apparatus. The midgut has an anterodorsal caecum situated above the pharynx (Figure 1C) and lacks constrictions. Posteriorly, it leads to a narrow ciliated rectum (Figure 1D) that opens through the anus into the dorsal chamber of the pallial cavity.
Nervous system and sense organs: The unpaired cerebral ganglion (80 µm long; 200 µm wide; 200 µm high) has a trapezoid transverse section and is situated above the beginning of the pharynx (Figures 1C, 2A). The ventral ganglia (130 µm long; 40 µm wide; 60 µm high) are located on both sides of the pedal pit (Figure 1C). Buccal ganglia could not be distinguished. The small ciliated atrium or atrial sense organ is situated in the anterior region of the common atriobuccal cavity and lacks atrial papillae (Figure 1C). Its anterior region is divided by a very short longitudinal fold. A dorsoterminal sense organ is present in the mediodorsal region of the pallial cavity (Figure 1D).
Reproductive system: The gonads bear developing ova/oocytes (15 µm diameter) in their anterior part at the lateromedial walls and spermatozoids in their posterior region. They are confluent with a pair of short and narrow gonopericardioducts, ciliated at the dorsal and lateromedial walls (Figures 1D, 2F); these open into the anterodorsal region of the pericardium. Inside the pericardium (130 µm long; 225 µm wide; 125 µm high) there is a heart made up of two almost fused small auricles and a muscular tubular ventricle (Figures 1D, 2G). Spermatozoids were also observed inside the pericardium. Two narrow pericardioducts (Figures 1D, 2G) start from the ventroposterior region of the pericardium and open into the anterior region of the unpaired spawning duct. Here a pair of large bilobulated seminal receptacles open (Figures 1D, 2F); they are full of spermatozoids and extend towards the anterior region of the body. The spawning duct is unpaired along its whole extension; in its middle region it is lined by glandular and highly vacuolized cells. On both sides of this middle region, it is fused with a large mass of glandular cells, which discharge their secretion into this spawning duct zone (Figure 2H). The spawning duct opens through an unpaired narrow genital orifice into the ventral chamber of the pallial cavity. There are two pairs of copulatory stylet groups, a ventrolateral and a lateral pair (Figures 1D, 2G–I). Each ventrolateral group consists of two large copulatory stylets, one above the other and each within its own sheath. The ventral stylet is hook-shaped and extends about 300 µm from the pallial cavity into the animal's body. The dorsal stylet is rod-shaped, only half the length of the ventral one into the animal's body and also thinner. Both stylets are thicker in their anterior part and their sheaths are associated with strong musculature (protractors). Both sheaths open in the ventral region of the pallial cavity. The lateral stylet groups consist of four stylets each, which lie close to the lateral body wall. One of the stylets extends about 220 µm into the animal's body; the other stylets are only half as long or shorter into the animal's body and also thinner. All stylets have a thin muscular sheath and their distal tips project through the cuticle on both sides lateral to the pallial cavity.
DISCUSSION
Spiomenia pusilla sp. nov. belongs to the order Cavibelonia Salvini-Plawen, Reference Salvini-Plawen1978 because of the presence of hollow acicular sclerites on the mantle and to the family Simrothiellidae Salvini-Plawen, Reference Salvini-Plawen1978, and because of the biserial radula and the ampulla-shaped ventrolateral foregut glandular organs, which are composed of large glandular cells wrapped within musculature (type C according to Salvini-Plawen, Reference Salvini-Plawen1978, c.f. type Simrothiella according to Handl & Todt, Reference Handl and Todt2005). The presence of hollow acicular sclerites with a distal asymmetrical enlargement (‘captate’ according to Arnosfsky, Reference Arnofsky2000) and of a reinforcement (buttress) in the radula plates classify the species within the genus Spiomenia (Arnofsky, Reference Arnofsky2000; Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003; García-Álvarez & Salvini-Plawen, Reference García-Álvarez and Salvini-Plawen2007).
Three species are known so far of the genus Spiomenia (García-Álvarez & Salvini-Plawen, Reference García-Álvarez and Salvini-Plawen2007), to which S. pusilla sp. nov. shows several differences (Table 1). Concerning Spiomenia spiculata Arnofsky, Reference Arnofsky2000, S. pusilla sp. nov. differs because of its radular plates, which are smaller in size and have a lower number of denticles than the plates of S. spiculata; the buttress of the plates extend over the entire lateral region of the plates in S. pusilla sp. nov., while in S. spiculata the buttress lies more medially. In relation to the denticles and the radular plate size, S. pusilla sp. nov. has a lower number of denticles than S. spiculata in shorter plates. On the other hand, the gonopericardioducts are paired along their whole extension in S. pusilla sp. nov., but in S. spiculata the posterior region of the gonopericardioducts are fused. Furthermore, the seminal receptacles of S. pusilla sp. nov. open into the spawning duct, whereas they open into the pericardioducts in S. spiculata. In addition, S. pusilla sp. nov. has two pairs of copulatory stylets and two chambers in the pallial cavity whereas S. spiculata has only one pair of stylets and one chamber in the pallial cavity (Arnofsky, Reference Arnofsky2000).
Table 1. Differences in the radular apparatus of the species of the genus Spiomenia.

The description of Spiomenia praematura Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003, was based on not yet fully developed specimens and the specimens of Spiomenia phaseolosa Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003, were immature without their reproductive system. Spiomenia pusilla sp. nov. differs from S. praematura because it has smaller radular plates with less denticles per plate and none of the denticles are placed laterally to the buttress. Spiomenia pusilla sp. nov. also has a higher number of radular rows and it lacks vacuolized radula supporting cells. In addition, in S. pusilla sp. nov. the atrium lacks papillae, there are two pairs of groups of copulatory stylets and two chambers in the pallial cavity, whereas S. praematura has one pair of groups of copulatory stylets and three chambers in the pallial cavity, as well as prominent suprapallial glands (Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003). For the immature S. phaseolosa, the basic character to differentiate the species from S. pusilla sp. nov. is the structure of the radular apparatus. Spiomenia pusilla sp. nov. differs due to the radular plates are half the size of the plates of S. phaseolosa and in that there are more denticles per plate. In addition, S. phaseolosa possesses numerous distinct, partly branched atrial papillae lacking in S. pusilla sp. nov. (Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003).
The new data that are added by the description of S. pusilla sp. nov. again raise the question about the separation of the genera Plawenia (defined by Scheltema & Schander, Reference Scheltema and Schander2000) and Spiomenia. Although there is still a need for further information, especially concerning the soft anatomy of Plawenia species, to unify both taxa the genera are undoubtedly remarkably similar. Therefore, Todt & Salvini-Plawen (Reference Todt and Salvini-Plawen2003) and Salvini-Plawen (Reference Salvini-Plawen2004) have already discussed the scarcity of significant taxonomic characters for the differentiation of the genera (Table 2). Arnorfsky (Reference Arnofsky2000) named the hollow acicular sclerites of the type ‘captate’, as well as the presence of one to several denticles lateral to the radular plate buttress as the two autopomorphic characters of Spiomenia. Todt & Salvini-Plawen (Reference Todt and Salvini-Plawen2003) pointed out that the presence of sclerites of the type ‘captate’ may be a secondary character for the differentiation at genus level, whereas Salvini-Plawen (Reference Salvini-Plawen2004) considers that both characters appear to be relevant only at the specific rather than generic level. Due to the radulae of S. pusilla sp. nov. and of all Plawenia species (Scheltema & Schander, Reference Scheltema and Schander2000) lack the denticles lateral to buttress of the radular plate, these additional data indicate that both genera are similar in general terms. Therefore, the presence of lateral denticles to the buttress in Spiomenia should not be at least considered as a generic character differentiating between Spiomenia and Plawenia.
Table 2. Differences among the genera of the family Simrothiellidae (expanded from Salvini-Plawen, Reference Salvini-Plawen2004).

+, present; −, absent; ?, unknown; *, original authors' descriptions (see Nierstrasz, Reference Nierstrasz1902; Salvini-Plawen, Reference Salvini-Plawen1978).
DIAGNOSIS
Thin cuticle with hollow acicular sclerites in intercrossing layers. Common atriobuccal cavity. Homodont biserial radula. With paired anterolateral radular sacs. Ventrolateral foregut glandular organs of ampulla-shaped composed of large glandular cells surrounded by a muscular layer (type C Salvini-Plawen, Reference Salvini-Plawen1978; c.f. type Simrothiella, Handl & Todt, Reference Handl and Todt2005). With seminal receptacles. Unpaired genital orifice. Without copulatory stylets. With respiratory folds. Without dorsoterminal sense organ.
Type species: Adoryherpia serrata gen. et sp. nov. Abyssal Angola Basin (South Atlantic Ocean), 5125–5144 m.
TYPE MATERIAL
Two specimens
Holotype: sectioned and mounted on 4 slides; sclerites on 5 slides. Paratype 1: sectioned and mounted on 3 slides. Both from Abyssal Angola Basin, station 318 Me48/1 DIVA 1 (22°20′00″S 03°18′18″E–22°20′12″S 03°18′24″E; water deep: 5125–5144 m).
DEPOSITORY
Holotype (ZSM Mol 20080880) and paratype 1 (ZSM Mol 20080878) are deposited at the Zoologische Staatssammlung München.
Derivato nominis
Latin: serra, serrated tool for cutting; Latin: -atus, deprived of. Concerning the serrated shape of the radular plates.
DIAGNOSIS
Elongated animals about 1.4 mm long. Thin cuticle without epidermal papillae, bulges or keels. With hollow acicular sclerites intercrossing in 2–3 layers. Common atriobuccal cavity. Pedal groove with one fold that does extend into the pallial cavity. Homodont biserial radula with plates about 25 µm broad × 1 µm high with approximately 26 denticles 1 µm high. With pair anteroventral radular sacs. With short radular sheath. Ventrolateral foregut glandular organs type C according to Salvini-Plawen (Reference Salvini-Plawen1978) or c.f. type Simrothiella according to Handl & Todt (Reference Handl and Todt2005). Midgut without constrictions. With one pair of seminal receptacles and seminal vesicles. Spawning duct unpaired along its whole extension. Unpaired genital orifice. Without copulatory stylets. With respiratory folds. Without dorsoterminal sense organ.
DESCRIPTION
Habitus: Specimens about 1.45 mm long, 0.35 mm thick in the anterior region and 0.32 mm in the posterior region. Circular in cross-section. Without bugel or keels. With sclerites projecting from the cuticle. Sclerites are longer in the posterior region of the body, in the dorsal area of the pallial cavity opening. Easily visible pedal groove. White colour, observed after fixation and preservation in 70° ethanol (Figure 3A).
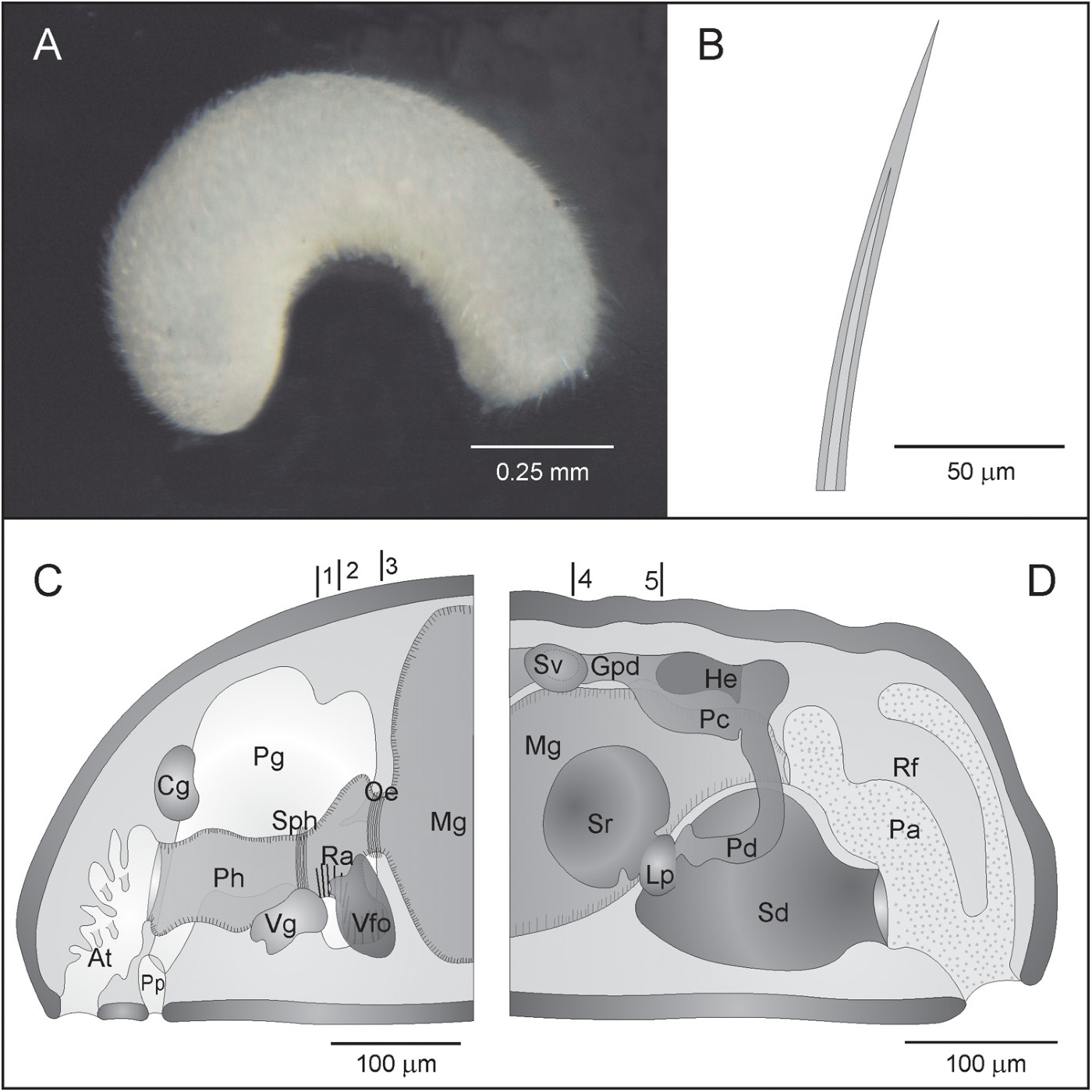
Fig. 3. Adoryherpia serrata gen et sp. nov. (A) Habitus; (B) distal part of a hollow acicular sclerite; (C, D) anatomical reconstructions from histological sections of the anterior (C) and posterior region (D). Lines 1–3 in C indicate the position of the transverse sections in Figure 4 (A–D). Lines 4 and 5 in D indicate the position of the transverse sections in Figure 4 (E–F). At, atrium; Cg, cerebral ganglion; Gpd, gonopericadioduct; He, heart; Lp, lateral pouch; Mg, midgut; Oe, oesophagus; Pa, pallial cavity; Pc, pericardium; Pd, pericardioduct; Pg, pedal gland; Ph, pharynx; Pp, pedal pit; Ra, radula; Rf, respiratory fold; Sd, spawning duct; Sph, sphincter; Sr, seminal receptacle; Sv, seminal vesicle; Vfo, ventrolateral foregut glandular organs; Vg, ventral ganglion.
Mantle: Thin cuticle (15–20 µm) without epidermal papillae; sclerites intercrossingly arranged in 2–3 layers. The sclerites were in a poor condition and only distal pieces (140 µm long) of hollow acicular sclerites (Figure 3B) could be observed.
Pedal groove and pallial cavity: The pedal groove begins in the small pedal pit (20 µm long; 110 µm wide; 50 µm high) situated below the anterior pharynx region. Two very large pedal glands empty into the pedal pit and fill most of the visceral cavity in the anterior region of the specimens (Figures 3C, 4A). The pedal groove bears a small fold that extends into the pallial cavity.

Fig. 4. Adoryherpia serrata gen et sp. nov. Sections through different regions of the body. (A) Radular sacs and pedal gland; (B) pharynx and radular teeth; (C) drawing of the radular teeth; (D) ventrolateral foregut glandular organs; (E) seminal receptacles and seminal vesicles; (F) joining between spawning duct, lateral pouch and seminal receptacle. A and B correspond to lines 1–3 in Figure 3C; D–F correspond to lines 4 and 5 in Figure 3D. Gpd gonopericardioduct; He heart; Lp, lateral pouch; Mg, midgut; Mu, musculature; Oe, oesophagus; Pc, pericardium; Pd, pericardioduct; Pg, pedal gland; Ph, pharynx; Ra, radula; Ras, radular sheath; Rs, radular sac; Sc, support cell; Sd, spawning duct; Sr, seminal receptacle; Sv, seminal vesicle; Vfo, ventrolateral foregut glandular organs; Vg, ventral ganglion.
The pallial cavity (125 µm long; 110 µm wide; 180 µm high) opens subterminally and is lined by a non-ciliated epithelium. It has a dorsoanterior pouch, where the anus opens. The spawning duct opens unpairedly into the ventroanterior region. A pair of respiratory folds are attached to the dorsal wall (Figure 3D).
Digestive system: The mouth is in the dorsoposterior region of the common atriobuccal cavity and leads into the pharynx (175 µm long; 40 µm wide; 60 µm high) presenting slightly folded internal walls and a thin layer of circular musculature. The posterior region of the pharynx has a muscular sphincter, behind which the radular apparatus is situated. The radular apparatus (50 µm long) consists of a pair of short lateral radular sacs (15 µm long) (Figure 4A), an homodont biserial radula with plates 25 µm broad × 1 µm high with about 26 denticles that are 1 µm high (Figure 4B, C) and a very short radular sheath. There is a pair of vacuolized bolster cells that support the radula (Figure 4B). The ventrolateral foregut glandular organs, which are ampulla-shaped, consist of glandular cells enclosed in musculature (type C according to Salvini-Plawen, Reference Salvini-Plawen1978; c.f. type Simrothiella according to Handl & Todt, Reference Handl and Todt2005) (Figures 3C, 4D). These organs open into the pharynx at the middle part of the radula region. The pharynx continues along a short oesophagus surrounded by musculature (Figures 3C, 4D) that does not form a sphincter. The oesophagus opens frontally into the midgut, which lacks constrictions and a caecum (Figure 4E). The posterior region of the midgut narrows and forms a rectum that opens through the anus into the dorsoanterior pouch of the pallial cavity (Figure 3D).
Nervous system and sense organs: The cerebral ganglion is dorsal to the anterior region of the pharynx and is surrounded by the pedal glands. The ventral ganglia are on both sides of the middle region of the pharynx (Figures 3C, 4A). Buccal ganglia could not been distinguished. The atrium or atrial sensitive organ is situated in the anterior region of the common atriobuccal cavity and bears numerous simple papillae (Figure 3C). There is no dorsoterminal sense organ.
Reproductive system: The pair of gonads is dorsal to the midgut and comprises small cells in the anterior region, which could be precursor cells of gametes. In the posterior region of the gonads some oocytes were observed (20 µm diameter) at the medial walls of the gonads. Some spermatozoids were also observed in this region, situated in the remaining space. The gonads are confluent with a pair of short and narrow gonopericardioducts. A pair of small, terminal gonadial pouches (situated laterally to the posterior region of the gonopericardioducts) represents seminal vesicles full of spermatozoids (Figure 4E). The gonopericardioducts open into the pericardium, where a short tubular heart can be observed. The wide pericardium extends laterally on both sides of the rectum (Figure 4F). A pair of pericardioducts originates from the posteroventral region of the pericardium and extends anteriorly where they open into two small lateral pouches of the spawning duct; the latter is unpaired along its whole extension (Figure 3D, 4F). Two large globular seminal receptacles (75 µm long; 75 µm diameter) also open into the lateral pouches of the spawning duct. No spermatozoids were observed within the receptacles (Figure 4E, F). The throughout fused spawning duct is lined by a glandular epithelium that has cells stained in different shades (most probably subsequent states of differentiation). The spawning duct opens into the ventroanterior region of the pallial cavity through a unpaired genital orifice (Figure 3D). There are neither copulatory stylets nor (pre)pallial spicules.
DISCUSSION
The new species Adoryherpia serrata belongs to the order Cavibelonia Salvini-Plawen, Reference Salvini-Plawen1978, because the studied specimens bear acicular hollow sclerites, and to the family Simrothiellidae Salvini-Plawen, Reference Salvini-Plawen1978, because of the biserial radula, the pair of radular sacs and the ventrolateral foregut glandular organs of type C (Salvini-Plawen, Reference Salvini-Plawen1978). The family Simrothiellidae comprises to date 13 genera (García-Álvarez & Salvini-Plawen, Reference García-Álvarez and Salvini-Plawen2007; Salvini-Plawen, Reference Salvini-Plawen2008), from which Adoryherpia gen. nov. shows several differences, as presented in Table 2.
Adoryherpia gen. nov. differs from Simrothiella Pilsbry, Reference Pilsbry and Sharp1898, Birasoherpia Salvini-Plawen, Reference Salvini-Plawen1978, Helicoradomenia Scheltema & Kuzirian, Reference Scheltema and Kuzirian1991, Plawenia Scheltema & Schander, Reference Scheltema and Schander2000, Spiomenia Arnofsky, Reference Arnofsky2000, Sensilloherpia Salvini-Plawen, Reference Salvini-Plawen2008 and Dictyaloherpia Salvini-Plawen, Reference Salvini-Plawen2008, because it lacks heterodont radular plates, copulatory stylets and a dorsoterminal sense organ. In addition, the new genus differs from Simrothiella as it lacks constrictions in the midgut (Pilsbry, Reference Pilsbry and Sharp1898) and from Birasoherpia as it lacks the tubular glands that open into the atriobuccal cavity (Scheltema & Kuzirian, Reference Scheltema and Kuzirian1991; Salvini-Plawen, Reference Salvini-Plawen1978); it differs from Helicoradomenia, Sensilloherpia and Dictyaloherpia as it does not have solid sclerites or any kind of anteriodorsal sensory organ or dorsofrontal sensory pit (see also Salvini-Plawen, Reference Salvini-Plawen2008); and it differs from Spiomenia because it does not have hollow acicular sclerites type ‘captate’ and sclerites in one layer (Arnofsky, Reference Arnofsky2000).
Adoryherpia gen. nov. differs from Cyclomenia Nierstrasz, Reference Nierstrasz1902, and Aploradoherpia Salvini-Plawen, Reference Salvini-Plawen2004, because it has a pair of radular sacs and lacks a dorsoterminal sense organ. Furthermore, it differs from Cyclomenia as it has a common atriobuccal cavity, seminal receptacles and lacks copulatory stylets (Nierstrasz, Reference Nierstrasz1902).
Adoryherpia gen. nov. is different from Biserramenia Salvini-Plawen, Reference Salvini-Plawen1968, by the fact that it has a common atriobuccal cavity, paired radular sacs and respiratory folds (Salvini-Plawen, Reference Salvini-Plawen1968).
Regarding the radula, the radular sacs, the common atriobuccal cavity and the seminal receptacles, the new genus Adoryherpia is most similar to Kruppomenia Nierstrasz, Reference Nierstrasz and Lo1903; from this genus, however, it differs by the lack of copulatory stylets and dorsoterminal sense organ (Nierstrasz, Reference Nierstrasz and Lo1903; Scheltema & Schander, Reference Scheltema and Schander2000; Todt & Salvini-Plawen, Reference Todt and Salvini-Plawen2003).
ACKNOWLEDGEMENTS
The cruises M48/1 (DIVA-1) and M63/2 (DIVA-2) were financed by the German Science Foundation (DFG). We are indebted to Dr M. Türkay, Professor Wägele and Professor Martínez Arbizu for inviting one of us (Professor V. Urgorri) to participate in this cruise. We also thank Professor Luitfried Salvini-Plawen, for his help in improving this work. This publication is a contribution to the Census of Marine Life project Census of Abyssal Marine Life (CeDAMAr), under the FPU Programme (MEC, Spanish government) and is also part of the research projects: ‘DIVA-Artabria I’ (Xunta de Galicia Regional Government—PGIDT01PXI20008PR) and ‘DIVA-Artabria II’ (MEC, Spanish Government CTM-2004-00740; Dirección Xeral de I+D+I (R & D+i Directorate General), Xunta de Galicia—PGIDIT07PXIB200120PR).








