Introduction
Due to the use of diverse electronic equipment, electromagnetic fields (EMF) have become almost omnipresent in modern societies. In recent years, a variety of unspecific health complaints has been reported by patients alleged to be caused by exposure to EMF. These complaints encompass somatic (e.g. skin or gastrointestinal disturbances) and neurasthenic (e.g. fatigue, concentration difficulties, sleep disturbances) symptoms (Levallois, Reference Levallois2002). Especially radiation from mobile phones and their base stations are frequently thought to cause these complaints and are suspected to be harmful. In contrast, a considerable body of epidemiological (Feychting et al. Reference Feychting, Ahlbom and Kheifets2005) as well as experimental studies (Rubin et al. Reference Rubin, Das and Wessely2005) have not been able to establish a clear causal relationship between these symptoms and the exposure. The use of the term ‘electromagnetic hypersensitivity’ for this syndrome is widespread despite its lacking nosological substantiation. The importance of this syndrome is reflected by its considerable prevalence in westsern communities, which has been estimated at up to 3% (Hillert et al. Reference Hillert, Berglind, Arnetz and Bellander2002; Levallois et al. Reference Levallois, Neutra, Lee and Hristova2002) with a high rate of disablement among affected patients (Stenberg et al. Reference Stenberg, Bergdahl, Edvardsson, Eriksson, Linden and Widman2002). Hence, better understanding of the pathophysiology of this syndrome should help to identify the underlying processes.
From a clinical point of view, many features of electromagnetic hypersensitive patients resemble and overlap with chronic fatigue or other syndromes of environmental intolerances such as ‘multiple chemical sensitivity’ or ‘sick building syndrome’ (Barsky & Borus, Reference Barsky and Borus1999). Symptoms are unspecific, fluctuating and no clear trigger can be found. Higher dysfunctional cognitive processes such as anticipation and mis-attribution seem to play important roles in symptom generation and maintenance in these diseases (Harlacher & Schahn, Reference Harlacher, Schahn and Kals1998; Barsky & Borus, Reference Barsky and Borus1999). In a number of other psychiatric diseases such as major depression or somatoform disorders, specific cognitive correlates have already been identified and were successfully incorporated in respective psychotherapy models for these disorders. Based on these findings, the first intervention studies using cognitive behavioural therapy were able to show clinical improvement in patients with electromagnetic hypersensitivity (Hillert et al. Reference Hillert, Kolmodin, Dolling and Arnetz1998). Detecting the neurobiological correlates of cognitive disturbances linked to electromagnetic hypersensitivity may improve therapeutic interventions.
On a neurobiological level, the first evidence for an alteration of cortical functioning as one potential neurobiological correlate of symptom manifestation in these patients has been found recently by measuring cortical excitability using transcranial magnetic stimulation (TMS; Landgrebe et al. Reference Landgrebe, Hauser, Langguth, Frick, Hajak and Eichhammer2007). These data point to deficiencies in adaptive abilities due to alterations of glutamatergic neurotransmission via N-methyl-d-aspartic acid (NMDA) receptors. In close accordance with these findings, recent work has emphasized high vulnerability against environmental stressors, especially affecting the autonomous nervous system in patients with electromagnetic hypersensitivity (Lyskov et al. Reference Lyskov, Sandstrom and Hansson2001; Sandstrom et al. Reference Sandstrom, Lyskov, Hornsten, Hansson, Wiklund, Rask, Klucharev, Stenberg and Bjerle2003).
Taken together, the pathophysiology of electromagnetic hypersensitivity seems to be much more complex than a simple somatic reaction to exposure to EMF. Instead, it appears that symptom generation might result from a complex interplay of intra-individual factors (e.g. behavioural traits, cognitive strategies, vulnerability of the nervous system function, genetic background) and environmental factors (e.g. stress, EMF exposure). However, the extent to which these factors are involved in the pathogenesis of electromagnetic hypersensitivity remains largely unknown. Therefore, this study combines for the first time the assessment of individual cognitive strategies, the ability to perceive EMF, the level of complaints, as well as the neurobiological characterization with TMS in order to achieve further insights into the complex pathophysiology of electromagnetic hypersensitivity.
Method
Study design and population sample
This study used a case-control design comparing subjects claiming to be electromagnetic hypersensitive with a sample of age- and gender-matched controls who were living in the same close vicinity or working at the same workplace in a comparable position (1:2 matching if the patient was working, 1:1 if not working). Matching location of private domicile and workplace should minimize potential influences of environmental physical and social stressors.
Inclusion criteria for electromagnetic hypersensitive patients were: (1) a symptom load of at least 19 points on the ‘Regensburger EMF-complaint list’ (Frick et al. Reference Frick, Mayer, Hauser, Binder, Rosner and Eichhammer2006) which corresponds to the 4-weeks complaint level of the upper one-third in the general population; (2) attribution of the health symptoms experienced to named electromagnetic emission sources (e.g. mobile phone base stations, hotspots, etc); (3) aged 18–75 years. Exclusion criteria encompassed all obstacles for TMS measurements (e.g. cranial metal implants, cardiac pacemakers, etc.). No further exclusion criteria were used. Subjects with concomitant psychiatric or internal diseases were not excluded from the study except in the case of an unstable medical condition.
Patients were recruited by newspaper announcements or informative events at various public locations such as public health offices, university buildings, etc. Altogether, a total of 135 patients were interested in participating, of which 101 patients were eligible according to the above-mentioned criteria. Twelve subjects withdrew from the study, when informed about the measurement procedures. Thus, 89 cases and 107 controls (living place 65 subjects, workplace 42 subjects) were enrolled into the study. Patients and controls stemmed from small-sized Bavarian cities (Regensburg, Weiden, Straubing, Neumarkt, Landshut, Kempten) and Austrian cities (Feldkirchen, Klagenfurt). Due to mostly technical reasons (e.g. patient living without comparable neighbours or lacking a colleague of the same gender and age), no regular matching pattern could be realized and two employed and six unemployed patients remained without a control subject. Therefore the statistical approach treated cases and controls as independent samples, which means a more conservative approach to detect differences between the two groups. As age and gender were not exactly balanced, these two variables were introduced as statistical covariates for most of the analyses.
Before starting the study, sample-size calculations (n Query 3.0 software; Statistical Solutions, Saugus, MA, USA) using data from a pilot study (Landgrebe et al. Reference Landgrebe, Hauser, Langguth, Frick, Hajak and Eichhammer2007) had revealed that to detect a difference of 9 points on the major study endpoint (discriminative ability) with a power of 90% and restricting type I error risk to 5% required the enrolment of two study groups of 90 subjects each.
Assessment of sociodemographic data, medical history and EMF-specific cognitive strategies
Sociodemographic data and medical history of all study participants were collected using a structured interview. Sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI; Buysse et al. Reference Buysse, Reynolds, Monk, Berman and Kupfer1989). In order to distinguish electromagnetic hypersensitivity from somatoform disorders, the German standardized interview Screening For Somatoform Disorders (SOMS; Rief et al. Reference Rief, Hillert and Heuser1997) was applied. Major depression and anxiety disorders were assessed using the short-form of the World Health Organization Composite International Diagnostic Interview (CIDI-SF; Nelson et al. Reference Nelson, Kessler and Mroczek2001). Qualitative interviews with electromagnetic hypersensitive patients from an earlier pilot study (Frick et al. Reference Frick, Meyer, Hauser and Eichhammer2004) on subjects' self-experience as ‘electromagnetic hypersensitives’ were used to construct a 42-item questionnaire assessing cognitive aspects of their health status. Items among others covered aspects of rumination, tendency to externalize potential causes of bodily sensations, symptom catastrophizing, distrust in orthodox medicine, stabilizing self-esteem from the symptoms experienced, perceived vulnerability, and intolerance of bodily complaints.
Determination of perception thresholds and cortical excitability by TMS
Individual perception thresholds were determined according to Frick et al. (Reference Frick, Kharraz, Hauser, Wiegand, Rehm, Kovatsits and Eichhammer2005). In order to enrol patients and controls from a larger regional background, transportable TMS equipment was used (MagPro magnetic stimulator X100 including MagOption; Medtronic, Copenhagen, Denmark). The perception experiment was conducted with both the test person and the rater, who gave all instructions and was kept blind with respect to the stimulus protocol throughout the experiment. The stimulating physician used two optically identical stimulation coils (real: MCF-B65; sham: MCF-P-B65) placed over the left dorsolateral prefrontal cortex, stood behind the test person and therefore could not be seen by the test person. The rater increased stimulating intensities in steps of 3%, ranging from 0% to 57% of the maximum stimulator output (~1.8 T). Test persons were not informed about the increasing pulse intensities, but knew that each pulse had a 50% probability of representing a real magnetic stimulus or to be only an acoustic click without an accompanying magnetic pulse. After each applied stimulus the participants were asked whether they felt any kind of sensation. After two consecutive positive responses the lower value was recorded as the perception threshold of this series and the stimulation condition was changed without informing the test person on the altered mode of stimulation. In the case of no sensory perception during the whole series of 19 pulses with the same coil, a right-censored threshold value of 57% was recorded. Four series of real and sham stimulation were applied in individually randomized ABAB versus BABA design. All test persons (except one from the electromagnetic hypersensitive group who withdrew his informed consent after the structured interview before beginning the perception experiment) completed the whole perception experiment.
Following the perception experiment, parameters of cortical excitability [i.e. active and resting motor thresholds (RMT), intra-cortical inhibition (ICI) and intra-cortical facilitation (ICF) and cortical silent period] were determined according to Rossini et al. (Reference Rossini, Barker, Berardelli, Caramia, Caruso, Cracco, Dimitrijevic, Hallett, Katayama and Lucking1994). In brief, motor-evoked potentials (MEP) were measured from the right abductor digiti minimi muscle (ADM) using surface electrodes in a belly-tendon-montage connected to an EMG (filters: 20 Hz to 3 kHz; Keypoint, Medtronic, Copenhagen, Denmark). MEP amplitudes were measured peak to peak. To assess muscle relaxation, 50 ms of prestimulus EMG were recorded. With a slightly suprathreshold stimulus intensity, the optimal position for eliciting maximal amplitude MEP was determined and marked to ensure constant coil placement throughout the experiment. Reducing the stimulus intensity in steps of 1%, we defined the RMT as the lowest intensity at which at least five of 10 consecutive MEP were ⩾50 μV in amplitude while the investigated muscle was at rest. Audio-visual electromyographic feedback was provided to control for muscle relaxation. Active motor threshold was determined as the lowest stimulation intensity that evoked an MEP ⩾250 μV in at least five of 10 consecutive trials during voluntary abduction of the ADM muscle.
ICI and ICF were measured using the paired-pulse TMS protocol (Kujirai et al. Reference Kujirai, Caramia, Rothwell, Day, Thompson, Ferbert, Wroe, Asselman and Marsden1993; Ziemann et al. Reference Ziemann, Rothwell and Ridding1996). The intensity of the first (conditioning) stimulus was set at 80% of RMT. The second (test) stimulus was delivered at an intensity that produced MEP of ~1 mV in the resting ADM. Interstimulus intervals of 2 and 15 ms were tested, each interval at least 10 times. The interval between sweeps was 4 s. The effect of conditioning stimuli on MEP amplitude at each interstimulus interval was determined as the ratio of the average amplitude of the conditioned MEP to the average amplitude of the unconditioned test MEP (cMEP:MEP) for each 10-trial block. MEP were digitally recorded and analysed with the software vision analyser (Brain Products GmbH, Gilching, Germany). The cortical silent period was recorded according to Moll et al. (Reference Moll, Heinrich and Rothenberger2001) using a stimulation intensity of 150% RMT.
The study protocol was approved by the local ethics committee. Written informed consent was obtained from every subject prior to study enrolment.
Statistical analysis
The major study endpoint for the perception experiment was the ability of the subjects to discriminate between a real magnetic stimulation and a sham condition. This was measured by subtracting the recorded threshold of the real magnetic pulse condition from the threshold of the sham condition in the original series 1 and 2 and in the repeating conditions 3 and 4. Higher values of the δ variables indicate a better competence of differentiating between both conditions. Using right-censored thresholds (e.g. in the sham condition, when a subject expressed no sensation throughout the whole series) to calculate the signal–noise distance gives a lower limit for the ability of the respective subject to differentiate between the two conditions. The procedure chosen thus is conservative for detecting group differences.
The statistical analysis was a priori defined as an analysis of covariance (ANCOVA) with two between-subjects factors: grouping factor 1 represents the differences between electromagnetic hypersensitive subjects and their controls; grouping factor 2 represents the randomization scheme ABAB or BABA for applying the real and sham coil (two levels). Female gender (0/1) and age were introduced as linear covariates. A repeated-measurement factor (signal–noise distance in the first two series versus the last two series) controls for potential learning effects throughout the experiment: did subjects profit during series 3 and 4 from their experiences during the first two series? The statistical test for the between-subjects factor ‘electromagnetic hypersensitives versus controls’ was considered the confirmatory test of the experiment. All analyses of variance were performed using sas procedure GLM (SAS Institute Inc., Cary, NC, USA).
Differences between electromagnetic hypersensitive subjects and controls with respect to dysfunctional cognitions could have been assessed using a series of t tests with group membership as the classifying variable and each of the 37 items as a separate dependent variable. Beside from problems due to inflation of type I error risk, this approach would also not contribute to restrict the interpretation of potential cognitive differences to the most important aspects, because inter-correlations of items are not adjusted for. Therefore we chose a multivariate logistic regression approach with group membership as the dichotomously measured ‘dependent’ variable and items of the questionnaire as potential predictor variables. Logistic regression was favoured over discriminant analysis (which offers an alternative) because of fewer statistical assumptions and (in our case) a better misclassification behaviour of the approach.
Group differences in health status variables with heavily skewed distribution (e.g. sick days) were tested using the non-parametric Mann–Whitney U test. Comparison of rates in cross-tabulations was accomplished using χ2 tests or, in case of cells with expected frequencies below value 5, using Fisher's exact test.
Results
Sociodemographic description of study groups
Details of sociodemographic characteristics are shown in Table 1. The study groups did not differ with respect to age and education. Due to the matching rule of this study (one control from the surroundings of the private domicile, irrespective of the control's employment situation, and one additional and necessarily employed control from the working situation, if the index person was employed his/herself), clearly the proportion of employed controls was higher than that of electromagnetic hypersensitives.
Table 1. Sociodemographic data and psychiatric co-morbidity of EHS and controls

EHS, Electromagnetic hypersensitive patients; n.s., non-significant; PSQI, Pittsburgh Sleep Quality Index; EMF, electromagnetic fields.
Values are given as mean (standard deviation) or proportion.
No significant differences were found in body mass index and smoking behaviour. Perceived health status, sick days and doctoral visits during the last year, as well as subjective sleep quality measured by the PSQI, were all less favourably reported by the electromagnetic hypersensitive group. The EMF-specific health complaint score was about three times higher in the group of electromagnetic hypersensitive patients, and psychiatric co-morbidity could be shown to be more prevalent in the same group with regard to major depression, generalized anxiety disorder and somatoform disorder. Concomitant internal medical conditions were rare and comparable in both groups (five subjects in the electromagnetic hypersensitive group and three subjects in the control group). Taken together, data indicate poorer health conditions in the group of electromagnetic hypersensitive patients as compared with controls.
Assessment of cognitive strategies
Five items of the questionnaire on cognitions assess potential advantages drawn from the self-characterizationas being ‘electromagnetic hypersensitive’. For the obvious reason that the ‘non-electromagnetic hypersensitives’ could not answer those items, no comparisons could be made for this subscale. Thus in a first step the remaining 37 items were explored by univariate t tests for differences between electromagnetic hypersensitives and controls. All items covering aspects of perceived vulnerability, of rumination with health complaints, and distrust in orthodox medicine were found to differ between the two groups. None of the items assessing the tendency to externalize potential causes of bodily complaints or symptom catastrophizing, and only one item of 14 covering a broad range of cognitions related to stabilizing one's self-esteem from being electromagnetic hypersensitive could be shown to differ between the two groups (not shown).
In a second step of analysis, a stepwise logistic regression procedure was performed on the group membership exploring all 37 items and additionally the time (in min) that it had taken the respondents to fill out the screening instrument (Regensburg EMF-complaint list). Table 2 summarizes the six items that significantly predicted group membership in a parsimonious statistical model with a sensitivity of 84% and a specificity of 70% at the function value=0.5 of the logistic regression function variable.
Table 2. Cognitions separating ‘electromagnetic hypersensitives’ from controlsFootnote a

EMF, Electromagnetic fields.
a Together with the time to complete the questionnaire, the shown six items (variables) from a 37-item questionnaire give the most parsimonious statistical model to separate ‘electromagnetic hypersensitives’ from controls.
b The probability modelled is that for membership in the control group.
c All items were coded from 1 (=disagree) to 4 (=strongly agree).
Determination of perception thresholds
After the structured interview, one electromagnetic hypersensitive patient withdrew his informed consent. Therefore, only 88 patients underwent TMS measurements. Table 3 summarizes the results of the perception experiment of all four series by group and experimental condition. Fig. 1 illustrates the proportion of subjects (pooled over four series) not experiencing a sensation throughout the experiment as a function of the increasing pulse intensity (i.e. as a function of waiting time in the case of sham exposure). As censored data occurred, a Kaplan–Meier estimate of the survivor function was chosen. As can be seen, only 40% of the electromagnetic hypersensitive group, but more than 60% of the controls felt consistently no sensation throughout the complete sham series of 19 clicks. The median of the perception threshold under transcranial stimulation is comparable between both groups (21% v. 24% of the maximum stimulator output).

Fig. 1. Sensory perception as a function of pulse intensities. With increasing ordinal number of pulses given, fewer subjects remain who had no sensation. In the case of transcranial magnetic stimulation (- - -), order numbers of pulses correspond to an increase of 3% of the maximum power of the magnetic stimulator. Sham (–––) pulses order numbers were projected to the same scale. All four sequential series determining the perception threshold were pooled. (a) Electromagnetic hypersensitives (four series pooled) (n=88). (b) Controls (four series pooled) (n=107).
Table 3. Measured perception thresholdsFootnote a by group and experimental condition

EHS, Electromagnetic hypersensitive patients.
Values are given as mean (standard deviation).
a Perception threshold given as % maximum stimulator output.
Electromagnetic hypersensitive patients displayed a diminished ability [F(1, 186)=6.77, p=0.01] to discriminate the two conditions as compared with their controls [meanseries 1+2=10.5 (s.d.=20.5) v. 13.6 (s.d.=21.2), meanseries 3+4=10.8 (s.d.=21.3) v. 17.0 (s.d. 18.3)]. Discriminative ability was also significantly influenced by age (F=9.25, p=0.0027), gender (F=7.45, p=0.0070) and sequence (ABAB versus BABA) of the four series (F=13.95, p=0.0002). If the perception experiment started with a series of sham magnetic stimuli, discriminating sham and magnetic pulses was easier for all test persons. Age exerted a negative impact on discriminative ability: the older, the less accurately could subjects discriminate the two experimental conditions. But this effect was partly compensated in the group of electromagnetic hypersensitives (F for interaction=5.18, p=0.024). Here, older subjects were not worse in discriminating than younger test persons. There were no significant learning effects or interactions of the learning condition with any of the between-subjects variables.
Cortical excitability
Parameters of cortical excitability were measured subsequently to the perception experiment and are depicted in Table 4. Resting and active motor thresholds (both F values for group differences <1, n.s.) as well as the cortical silent period (F=2.62, p=0.1075) did not differ significantly between study groups even after adjusting for age and gender. However, women (F=4.82, p=0.0294) and older volunteers (F=4.36, p=0.0381) displayed higher active thresholds in both study groups as compared with men and younger volunteers, respectively.
Table 4. Parameters of cortical excitability of EHS and controls
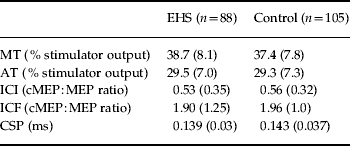
EHS, Electromagnetic hypersensitive patients; MT, resting motor threshold; AT, active motor threshold; ICI, intra-cortical inhibition; cMEP, conditioned motor evoked potential; MEP, unconditioned motor evoked potential; ICF, intra-cortical facilitation; CSP, cortical silent period.
Values are given as mean (standard deviation).
With respect to ICI and ICF, results of the ANCOVA model were not straightforward, because group differences, age and the intra-individual inhibition–facilitation gradient interacted in a complex manner. There were small but significant differences between study groups (main effect) with less inhibition and more facilitation for controls (ratios below and above 1 are slightly higher in the control group: F=4.92, p=0.0278, see Table 4). A powerful main effect could be found for age in the form of decreasing inhibition and increasing facilitation in older age groups (see Fig. 2). The intra-individual gradients of inhibition–facilitation as a function of the interstimulus interval were dependent on subjects' ages (F for interaction=5.09, p=0.0253) and group membership (F for interaction=4.39, p=0.0374). Age and group as two significant between-subjects main effects also had a two-way interaction (F=4.22, p=0.0414). Finally, a three-way interaction of gradient×group×age proved significant (F=4.14, p=0.0433). Gender had neither a direct nor an indirect impact on this situation. In order to interpret this complex interplay, Fig. 2 visualizes the results with age given in three equally sized classes (<45 years, 45–54 years and ⩾55 years).

Fig. 2. Cortical excitability of electromagnetic hypersensitive patients (EHS) and controls depicted in equally sized age classes (young, <45 years; middle, 45–54 years; old, ⩾55 years). Intra-cortical inhibition (ICI) results from an interstimulus interval of 2 ms; intra-cortical facilitation (ICF) results from an interstimulus interval of 15 ms. Cortical excitability is expressed as the ratio of conditioned motor-evoked potentials to unconditioned motor-evoked potentials (cMEP:MEP). Age effects on ICI and ICF are shown (- - -). Bracketing indicates significant interaction effects of age and group: ICF is reduced in young and middle-aged EHS and increased in old EHS compared with controls.
Discussion
This study examined a large sample of electromagnetic hypersensitive patients on their individual ability to perceive EMF along with their individual symptom load and possible disposing factors on a cognitive and neurobiological level. Results of a pilot study could be replicated and extended. It could be shown that electromagnetic hypersensitive patients (1) exhibit specific dysfunctional cognitive strategies, (2) do have a lower ability to discriminate real from sham magnetic stimuli as compared with controls, and (3) show alterations in their cortical excitability.
Psychiatric co-morbidity and health status
Major depression, generalized anxiety disorder, and somatoform disorder have been observed significantly more often among electromagnetic hypersensitive patients than controls according to the used screening instruments (CIDI-SF, SOMS). This fact has also been demonstrated in other samples of electromagnetic hypersensitive patients (Bergdahl & Bergdahl, Reference Bergdahl and Bergdahl2001) as well as in other functional somatic syndromes such as multiple chemical sensitivity (Bornschein et al. Reference Bornschein, Hausteiner, Zilker and Forstl2002). Although the electromagnetic hypersensitive patients show many characteristics of a somatoform disorder [e.g. chronic disease, many fluctuating symptoms not explained by a physical illness, increased rumination according to the International Classification of Diseases (ICD)-10], interestingly only about 10% fulfilled the criteria of a somatoform disorder according to the SOMS. This fact illustrates the difficulty of standardized diagnosis of these atypical somatoform disorders using operational screening instruments. However, the found neurobiological alterations (see below) can at present not improve differential diagnosis of these diseases.
The significantly worse health status and the higher rate of sick days and doctoral visits during the last year point to the high morbidity of electromagnetic hypersensitives. Furthermore, the high prevalence of electromagnetic hypersensitivity along with increased utilization of the health system underlines the economic impact of this syndrome. Compared with other somatoform disorders, recognizing and effectively treating these patients (e.g. with early interventions with cognitive behavioural therapy) might help to reduce these costs and improve their health status (Hiller et al. Reference Hiller, Fichter and Rief2003; Bleichhardt et al. Reference Bleichhardt, Timmer and Rief2004).
Dysfunctional cognitions
The structured interview including questionnaires to assess the individual health status and specific beliefs regarding danger and health impact of EMF revealed differences in cognitions between electromagnetic hypersensitive patients and controls. A number of items from the subscale on ‘stabilization of self-esteem’ contributed significantly to the prediction of group membership. The items describe the feeling of being special because of EMF, and therefore serve to stabilize self-esteem. In addition, corresponding to the findings on somatoform disorders, items covering vulnerability and intolerance against physical symptoms differed between the two groups. This can be explained by ‘somatosensory amplification’ (Barsky & Borus, Reference Barsky and Borus1999) which may play a pivotal role in symptom generation in electromagnetic hypersensitive patients. According to this pathophysiological concept, the increased awareness of any kind of somatic disturbances may lead to further attention to physiological somatic reactions and increased self-observance. As a consequence, this leads to a hyper-arousal resulting in further enhancement of these physiological reactions, which has been observed with various methods in electromagnetic hypersensitive patients (Lyskov et al. Reference Lyskov, Sandstrom and Hansson2001; Sandstrom et al. Reference Sandstrom, Lyskov, Hornsten, Hansson, Wiklund, Rask, Klucharev, Stenberg and Bjerle2003). This vicious cycle may finally lead to an impairment of the patient to separate internal perceptions from external stimuli. One may assume that this is one of the potential reasons for the decreased performance of the electromagnetic hypersensitive patients in our perception experiment. As a consequence, cognitive behavioural therapeutic approaches aiming at interfering with these processes should result in both improving health status and better performance in the perception experiment. This fact should be addressed in future studies. Furthermore, the differences reported in our investigation concerning increased rumination, measured by a specific item along with a larger amount of time electromagnetic hypersensitive patients needed to complete the questionnaire, further underline the importance of dysfunctional cognitions for the maintenance of electromagnetic hypersensitivity (Harlacher & Schahn, Reference Harlacher, Schahn and Kals1998), which has also been shown in other functional somatic illnesses (Bailer et al. Reference Bailer, Witthoft, Bayerl and Rist2007). In line with these concepts, especially cognitive behavioural therapeutic approaches appeared to be effective in electromagnetic hypersensitive patients (Hillert et al. Reference Hillert, Kolmodin, Dolling and Arnetz1998; Rubin et al. Reference Rubin, Das and Wessely2006).
Alterations of cortical excitability
In agreement with the findings of the pilot study (Landgrebe et al. Reference Landgrebe, Hauser, Langguth, Frick, Hajak and Eichhammer2007), the paired-pulse experiment again revealed a significant alteration of cortical excitability in electromagnetic hypersensitive patients. In young and middle-aged patients, ICF was significantly reduced compared with controls, thereby confirming our earlier results. All other parameters of cortical excitability as measured by TMS did not differ between both groups. In the elderly patient group, however, ICF was increased compared with controls; this is a new finding that was not observed in the pilot study probably due to a different age range of 18 to 65 years in the former study. Data from other studies yield conflicting results regarding the influence of age on cortical excitability (Peinemann et al. Reference Peinemann, Lehner, Conrad and Siebner2001; Wassermann, Reference Wassermann2002). One potential explanation for these differences may be that the relative amount of ICI and ICF depends on the different physical properties of the used magnetic stimulators (i.e. Medtronic™ versus Magstim™; monophasic versus biphasic pulses; see Kammer et al. Reference Kammer, Beck, Thielscher, Laubis-Herrmann and Topka2001; Peinemann et al. Reference Peinemann, Lehner, Conrad and Siebner2001). Nevertheless, in both our pilot study (using Magstim devices) and the current study (using Medtronic devices), electromagnetic hypersensitive patients differed significantly from healthy controls with respect to ICF.
Until now, the contribution of altered cortical excitability reflected by changes in ICF to symptom generation in people suffering from electromagnetic hypersensitivity is unclear. Possibly, it is just another hint for an increasingly irritable nervous system function in these patients (Lyskov et al. Reference Lyskov, Sandstrom and Hansson2001; Sandstrom et al. Reference Sandstrom, Lyskov, Hornsten, Hansson, Wiklund, Rask, Klucharev, Stenberg and Bjerle2003). On the other side, alterations in ICF may play a more specific role in symptom generation in this syndrome. ICF measured with TMS mainly reflects intra-cortical, NMDA-glutamatergic neurotransmission and was discussed with regard to adaptation abilities of the individual (Liepert et al. Reference Liepert, Schwenkreis, Tegenthoff and Malin1997; Schwenkreis et al. Reference Schwenkreis, Witscher, Janssen, Addo, Dertwinkel, Zenz, Malin and Tegenthoff1999). Based on this theoretical framework, changes in ICF may indicate dysfunctional cortical processes, which may lead to reduced adaptation abilities of these individuals. However, the link between altered neurobiological parameters and dysfunctional cognitive strategies and the health complaints in electromagnetic hypersensitive patients is far from being clear. Furthermore, it remains to be elucidated whether the alterations of cortical excitability reported here represent state or trait characteristics. Intervention studies using cognitive behavioural approaches together with measurement of cortical excitability parameters will be able to answer these questions.
Interestingly, Ferreri et al. (Reference Ferreri, Curcio, Pasqualetti, De Gennaro, Fini and Rossini2006) recently found significant increases in ICF in young healthy controls during and after 45 min exposure to mobile phone radiation, thereby demonstrating that measuring cortical excitability with TMS seems to be a promising approach to assess the impact of EMF exposure on central nervous system function. However, only healthy test persons and no electromagnetic hypersensitive patients were measured in that study. Although Ferreri et al. found the opposite effect as compared with our results (increase of ICF while here we found a decrease in that age group), ICF seems to be a sensitive marker, which is influenced by EMF exposure. The discrepancy with our data is probably due to the differences in the study design with respect to study populations and exposure settings. In the study by Ferreri et al. (Reference Ferreri, Curcio, Pasqualetti, De Gennaro, Fini and Rossini2006), the effect of an acute exposure (mobile phone exposure for 45 min) on cortical excitability was measured with TMS in a healthy, non-electromagnetic hypersensitive population to test the acute effect of EMF exposure on cortical excitability. In contrast, our study compared cortical excitability of electromagnetic hypersensitive patients with healthy controls without acute, short-time exposure to test whether electromagnetic hypersensitivity is associated with alterations in cortical excitability. In both studies, long-term exposure levels to EMF have not been assessed and no evidence exists that electromagnetic hypersensitivity is associated with increased long-term exposure. As pointed out, in our study only electromagnetic hypersensitive patients showed changes in ICF, which argues in favour of a possible genuine neurobiological vulnerability of electromagnetic hypersensitive patients for EMF. Owing to our study design, we cannot exclude that long-term exposure to EMF together with an increased individual vulnerability may lead to symptom formation in these patients. Future studies should therefore focus on the topic of whether electromagnetic hypersensitive patients demonstrate differential changes in cortical excitability during acute mobile phone radiation exposure as compared with controls, thereby extending the findings of Ferreri et al. (Reference Ferreri, Curcio, Pasqualetti, De Gennaro, Fini and Rossini2006). Furthermore, it would be of interest whether other functional somatic diseases such as multichemical sensitivity or other chronic somatoform disorders (e.g. chronic pain) may show changes of cortical excitability similar to changes in our study population. For diagnostic reasons, however, alterations of cortical excitability will be insufficient to distinguish electromagnetic hypersensitivity from other similar conditions, since the pathophysiological relevance of the changes are at present largely unknown. However, corresponding alterations in cortical excitability may further point to common pathophysiological mechanisms of these disease entities and may give further evidence for the ‘single syndrome hypothesis’ (Ciccone & Natelson, Reference Ciccone and Natelson2003). Including also a control group from this disease entity such as multiple chemical sensitivity into this study was not possible, because the focus of this study was to replicate the initial findings of neurobiological alterations in the pilot study.
Taken together, we found in the up-to-date largest sample of electromagnetic hypersensitive patients significant differences on a cognitive (tendency to increased rumination and intolerance against physical symptoms) and neurobiological (altered ICF) level, pointing to a greater genuine individual vulnerability. This fact along with miscellaneous environmental influences may lead to the generation of symptoms in patients with electromagnetic hypersensitivity. Due to the study design it cannot be ruled out that along with a genuine vulnerability, long-term exposure to EMF may promote the exacerbation of electromagnetic hypersensitivity. But other stressors with ubiquitous prevalence in modern societies could serve as triggers as well. This question should be addressed in future studies. Furthermore, TMS has been proven to be a useful tool in characterizing somatoform disorders on a neurobiological level. The relevance of TMS parameters for diagnosing other somatoform disorders should be proven in the future.
Acknowledgements
This study was supported by a grant from the German Federal Ministry for the Environment, Nature Conservation, and Nuclear Safety (UFOPLAN project StSch 4357).
Declaration of Interest
None.