Introduction
Ephestia kuehniella Zeller, 1879 (Lepidoptera: Pyralidae) and Sitotroga cerealella (Oliver, 1789) (Lepidoptera: Gelechiidae) are very important pests of stored grain in subtropical and warm temperate regions of the world (Bushra & Aslam, Reference Bushra and Aslam2014; Tarlack et al., Reference Tarlack, Mehrkhou and Mousavi2015). Despite the economic importance of these insects, they are extensively used in the laboratory production of parasitoids and predators for biological control of a large number of key pests (e.g. Dias-Pini et al., Reference Dias-Pini, DaSilva, Penaflor and Parra2014; St-Onge et al., Reference St-Onge, Cormier, Todorova and Lucas2016).
The increase in the occurrence of insecticide resistant strains of pests and of environmental problems associated with chemical control has shown the way to intensify research into different control methods, such as biological control (Lebedev, Reference Lebedev1970; Debach, Reference Debach1974; Crowder & Jabbour, Reference Crowder and Jabbour2014). In the case of lepidopteran pests that cause damage on the larval stage, the use of egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae) can eliminate the pest before it causes damage (Greenberg et al., Reference Greenberg, Morrison, Nordlund and King1998; Van Lenteren, Reference Van Lenteren, Gurrand and Wratten2000). However, to achieve a large-scale production of these parasitoids it was necessary to create a so-called factitious host on an industrial scale (Daumal et al., Reference Daumal, Voegelé and Brun1975). Factitious hosts vary depending on the specificity of the reared natural enemy and the methodologies that are available at the moment.
For the mass rearing of Trichogramma, the most used hosts are E. kuehniella or S. cerealella (Voegelé et al., Reference Voegelé, Daumal, Brun and Onillon1974; Hassan, Reference Hassan1981; Hansen et al., Reference Hansen, Henrik and Hell2004). The culture of S. cerealella has been developed in order to study the production of a variety of insects (e.g. Weston & Rattlingourd, Reference Weston and Rattlingourd2000; Koleva & Ganeva, Reference Koleva and Ganeva2009; Akter et al., Reference Akter, Jahan and Bhuiyan2013). At the same time, similar studies have been performed with E. kuehniella to produce Chelomus sp. (Hymenoptera: Braconidae) and Trichogramma spp. (e.g. Biliotti & Daumal, Reference Biliotti and Daumal1969; Mansour, Reference Mansour2010; Nadeem, Reference Nadeem2012; Perveen & Sultan, Reference Perveen and Sultan2012).
The intensive production of these two hosts occurs in the grain (S. cerealella) or in the bran (E. kuehniella) by adding fresh eggs to the food source. Some of the most important parameters to obtain high quality hosts are the amount of eggs, food quality, and intraspecific competition (Tavares & Daumal, Reference Tavares and Daumal1983; Nathan et al., Reference Nathan, Kalaivani, Mankin and Murugan2006; Nadeem et al., Reference Nadeem, Hamed and Shafique2011; Nadeem, Reference Nadeem2012; Tavares et al., Reference Tavares, Ribeiro and Oliveira2012 a, Reference Tavares, Ribeiro, Oliveira and Vieira b ).
In recent decades, the massive production of biological control agents has relied on the most modern cultivation techniques and quality control, similar to those employed in industrial units, making use of advanced techniques, and technological equipment and electronics, such as computers with real-time management (Daumal et al., Reference Daumal, Jourdheuil and Tomassone1974; Tavares, Reference Tavares1986; Tavares & Vieira, Reference Tavares and Vieira1992; Silveira, Reference Silveira1997; Manjunath, Reference Manjunath2014).
Considering the technical, abiotic and biotic factors, which are imposed on the hosts in massive production (Tavares et al., Reference Tavares, Anunciada, Oliveira and Vieira1989; Reference Tavares, Ribeiro and Oliveira2012 a, Reference Tavares, Ribeiro, Oliveira and Vieira b ), we set out to investigate the final phase of the biological cycle that begins with adult emergence of S. cerealella and E. kuehniella.
Variations in the quantity or quality of an acceptable diet can have profound effects on insect development (Pashley et al., Reference Pashley, Ardí and Hammond1995; Bentancourt et al., Reference Bentancourt, Scatoni, Gonzalez and Franco2003; Bauerfeind & Fischer, Reference Bauerfeind and Fischer2005). The quantities of protein and amino acids ingested are important for optimal growth and reproduction (Chapman, Reference Chapman1998). Poor nutrition early in life has negative effects on adult body mass, lifespan, secondary trait expression and reproduction in many invertebrates (Bauerfeind & Fischer, Reference Bauerfeind and Fischer2005).
In order to contribute to the best choice of a factitious host for mass production of natural enemies, our objectives in this study was to analyze the effects of food sources on the oviposition period, adult weight and sex ratio of S. cerealella and E. kuehniella and integrate adult emergence and oviposition period into a common model, allowing prediction of egg production along the time period after emergence, thereby supporting a more effective management of factitious host production.
Materials and methods
Essay establishment
We used five transparent plastic boxes (10 × 25 × 9 cm3), with a lid containing two holes for air renewal (5 cm in diameter, covered by copper mesh <0.05 mm) for insect rearing. We used 200 g of food, bran for E. kuehniella and grain for S. cerealella, per box. In the E. kuehniella assay, we introduced bran into a corrugated structure similar to that of bees’ nest (cavities were 2.0 cm deep and 0.5 cm in diameter). This amount of food was considered surplus, exceeding the needs of this group of individuals, as demonstrated in previous studies (Tavares et al., Reference Tavares, Anunciada, Oliveira and Vieira1989; Tavares & Vieira, Reference Tavares and Vieira1992; Tavares et al., Reference Tavares, Ribeiro and Oliveira2012 a, Reference Tavares, Ribeiro, Oliveira and Vieira b ). We placed 200 eggs aged <24 h on top of the food source and reared the insects at 25 ± 0.5°C, 70 ± 5% relative humidity, and a photoperiod of 16/8 (L:D) h, for about 5 days, inside a climatic chamber, until larval hatching. The boxes were placed randomly inside the growth chamber and the respective positions were regularly changed during maintenance. We replicated this entire procedure twice.
Treatment preparation
On the first day after hatching, we prepared two groups of S. cerealella and E. kuehniella larvae that were maintained on the same type of food until the end of the experiment (i.e., adult emergence): barley grain or corn grain for S. cerealella; and barley bran and corn bran for E. kuehniella, the most commonly used in mass rearing of these insects. We used five rearing boxes for each insect × diet combination, and we replicated this entire procedure twice.
Measurement of adult parameters
Immediately after adult emergence, we isolated couples into clear plastic boxes (7 cm in diameter × 3 cm in height) to mate. These boxes contained a black cardboard circle to facilitate egg counting, and couples were moved daily into a new box. We used 30 pairs of newly emerged adults randomly selected from the two diet treatments. In order to minimize changes in the abiotic conditions described above, we performed daily observations and data recording in the same conditions of the respective treatment and always between 8 and 12 a.m. Recorded parameters included: number of day(s) to adult emergence, counted after the first emergence (variability in adult emergence); adult insect sex and mass (Mettler scale Type ME30); the daily number of eggs laid per female, during the first 5 days after emergence.
Statistical analysis and modeling
Modeling approach
To identify possible differences between hosts and food sources, we aimed to model adult emergence and the oviposition period, and to integrate that information into a predictive model of egg production along time. Therefore, we tested probabilistic and survival models, instead of using traditional statistical tools (e.g. analysis of variance, ANOVA) only.
Modeling of adult emergence
A preliminary analysis, using the Kolmogorov–Smirnov test, showed that time to adult emergence did not follow normal, Poisson or uniform distributions. Thus, time to adult emergence was modeled with the Gompertz sigmoid function (Gompertz, Reference Gompertz1825; Moreira et al., Reference Moreira, Martins, Silva and Moura2012). This allows modeling the occurrence of events as a function of time, such as seed germination or survival rates, by using their accumulated relative frequency. We calculated Gompertz curves, modeling accumulated adult emergence (%) as a function of time (days), for each combination of species/diet and by sex, using the nonlinear regression functions of SPSS 21.0 (SPSS Inc., Chicago, IL), with the equation:
where y is the accumulated emergence, t is time and ε is model error. The parameters of the model are interpreted as follows: a + c, the model asymptote (in this case, the maximum accumulated emergence); b, the slope of the growing part of the curve (larger b meaning a more synchronous emergence); and m, the inflexion point of the model, indicating if adult emergence, as a whole, needs more or less time. Based on the calculated models we also estimated T50 (i.e., the time taken for emergence percentages to reach 50%). We compared parameters m and b among models by using a t-test specific for regression coefficients (Zar, Reference Zar1996), and adjusted the error rate (α = 0.05) based on the number of tests used to compare species/diets and sexes. To account for replicate and box variability, all the replicates were included, individually, in the dataset used for modeling. Therefore, model predictions incorporated the variation between replicates.
Modeling of oviposition period
We also modeled the mean number of eggs laid per female, as a function of time, by using the regression functions of SPSS. We tested several model families, namely linear, logarithmic, exponential, and polynomial; and we selected the best model based on the determination coefficient and on the significance of the regression and of the respective parameters.
Model integration
In order to predict egg production along time we integrated the adult emergence model and the oviposition model for each combination of insect × diet. This allowed predicting total egg production along time. Finally, as an example, we simulated a scenario for a high and stable egg production during a period of about 30 days, for each of the combinations host × food source
Statistical testing
Besides estimating model parameters and calculating model adjustments, we also applied ANOVA and a Tukey test to compare the total number of eggs laid per female. For the three insect × diet combinations, sex ratios (% of females) were compared by using contingency table analysis (Zar, Reference Zar1996), applied to the bulk of the data. Adults’ weights were transformed by √(x + 0.5) and the comparison of the three insect × diet combinations were performed using a two way nested ANOVA, followed by Tukey test.
Results
Modeling of adult emergence
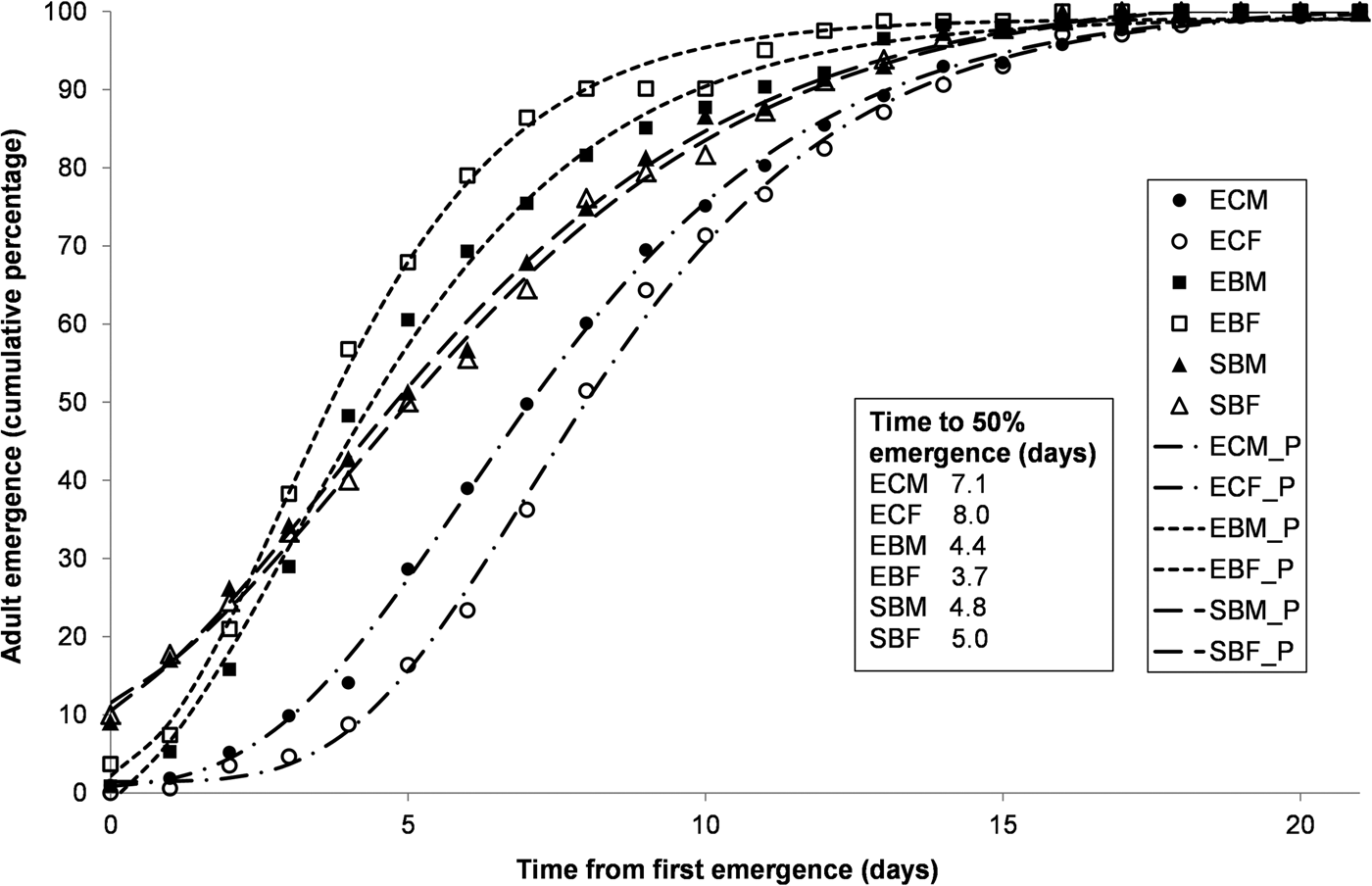
Larvae of S. cerealella in corn grain did not develop up to the pupal stage. In general, for all of the three combinations of species/diet that reached the pupal stage, and both for males and females, adult accumulated emergence showed a close adjustment to the Gompertz model (fig. 1). For those three species/diet combinations, 100% emergence was obtained in a period extending for about 20 days. Regarding E. kuehniella, emergence was faster for barley bran than for corn bran as shown by the shape of the curves, but also by the values of T50, which were much lower for both males and females grown on barley bran. Regarding S. cerealella grown on barley grain, the results were intermediate.
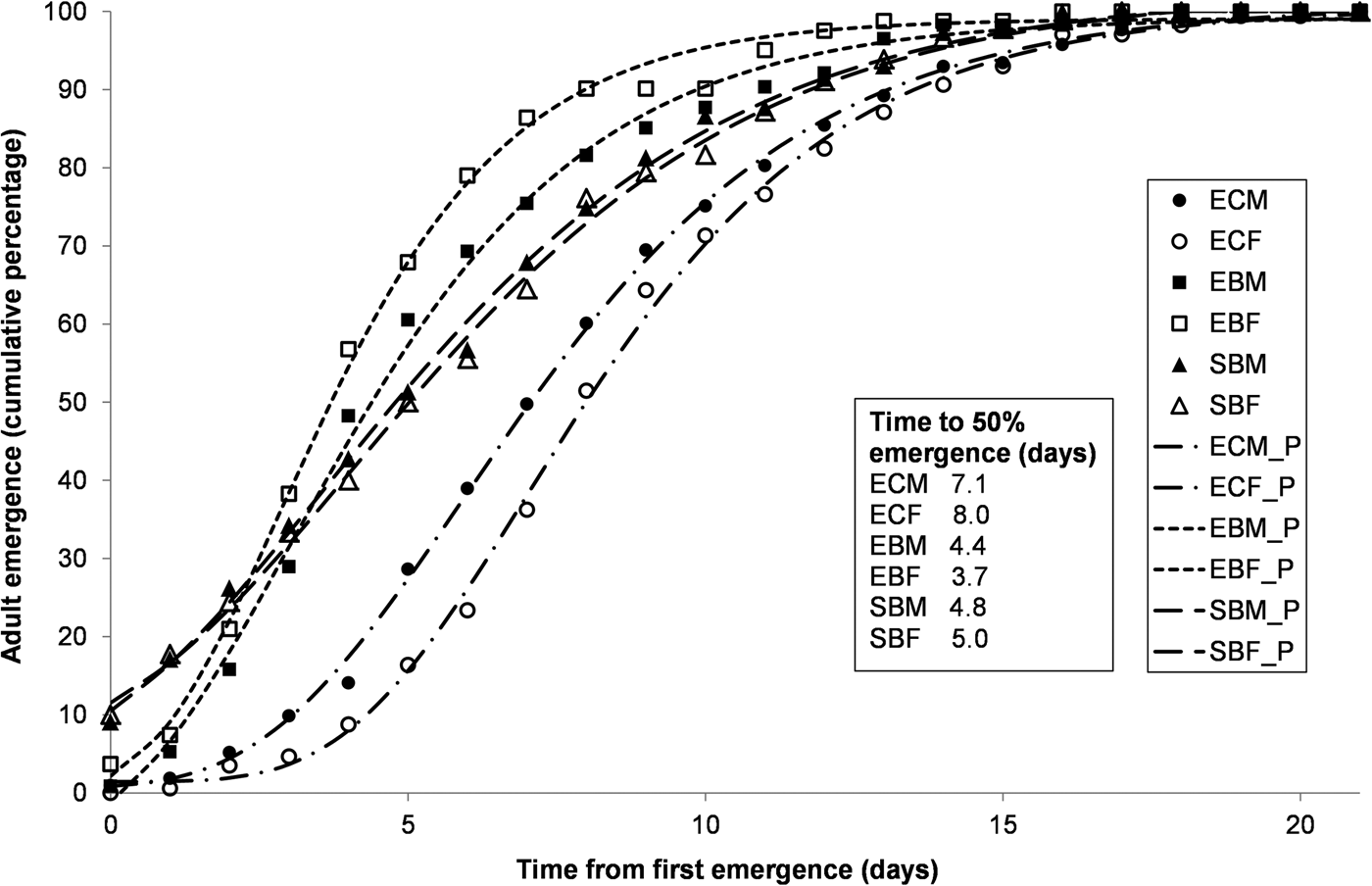
Fig. 1. Accumulated emergence curves for adults of Ephestia kuehniella produced on corn bran (EC) or on barley bran (EB) and of Sitotroga cerealella produced on barley grain (SB) during 21 days. The analysis was performed separately for males (M) and females (F). Observed (points) and predicted values (lines estimated by Gompertz model), and T50 (time for 50% emergence in days). All models with an R2 above 0.9.
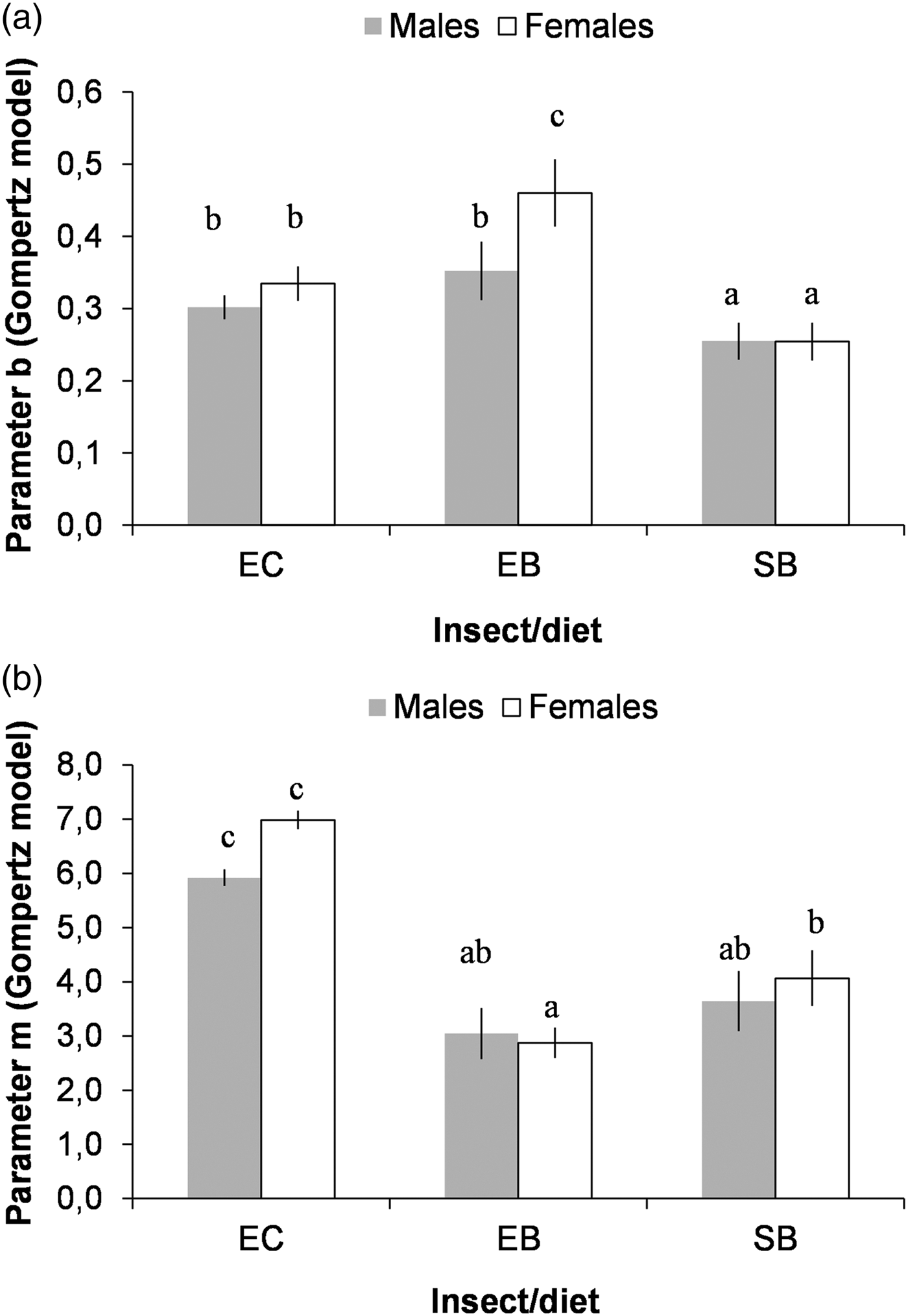
Considering the values of the parameters of the Gompertz model, we found that: (i) adult emergence was more synchronous (larger values of b) in E. kuehniella in corn and barley bran than for S. cerealella in barley grain (fig. 2a), and this was more pronounced for the females of E. kuehniella in barley bran; and (ii) adult emergence extended for a significantly longer period of time for E. kuehniella in corn bran (larger values of m, fig. 2b).
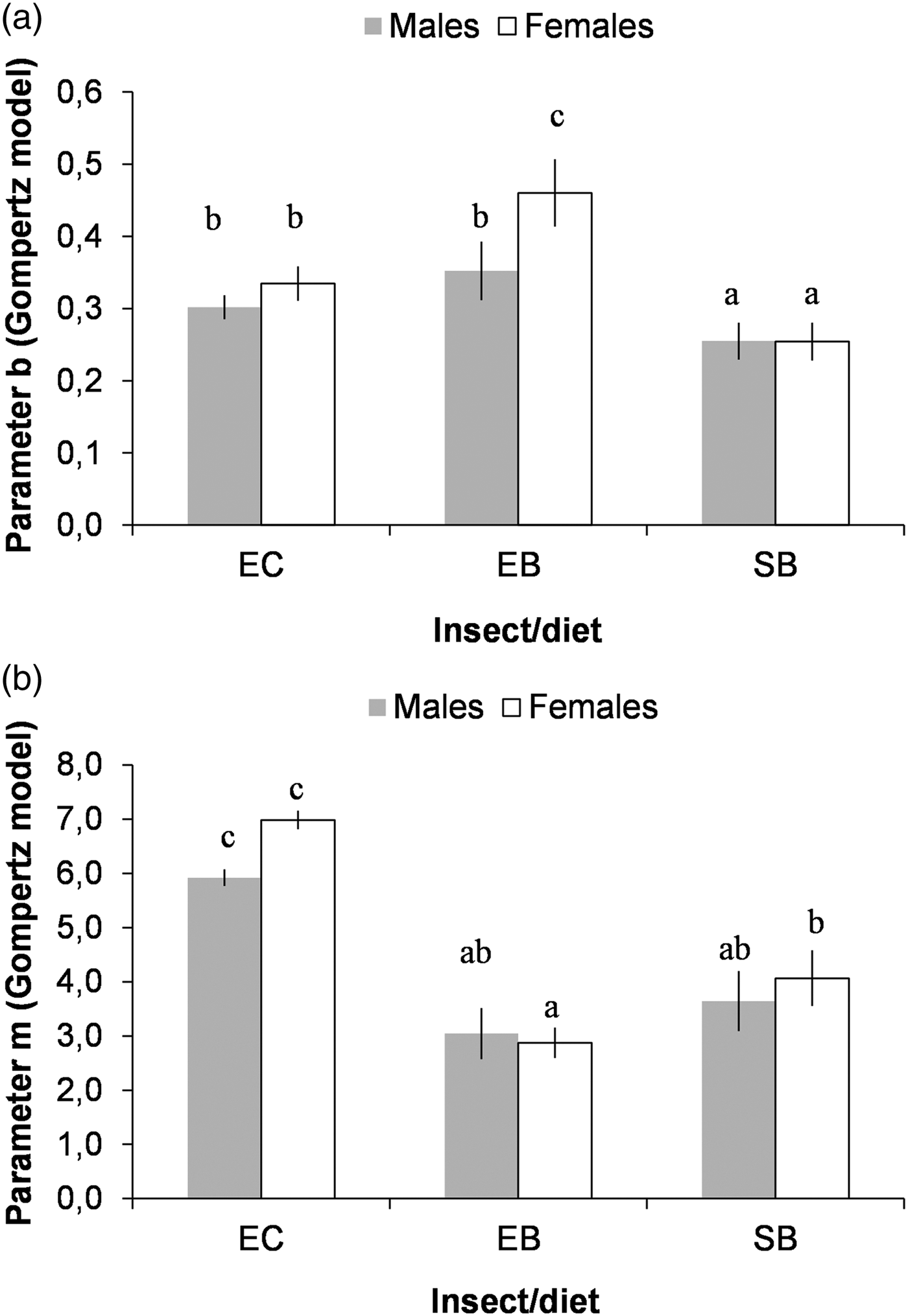
Fig. 2. Comparison of the estimated parameters b (a), and m (b), of the Gompertz model, for the three combinations of species/diet and by sex: Ephestia kuehniella produced on corn bran (EC) or on barley bran (EB) and Sitotroga cerealella produced on barley grain (SB). Means followed by different letters are significantly different (T-test for comparison of regression coefficients: P < 0.05).
Analysis of adult parameters
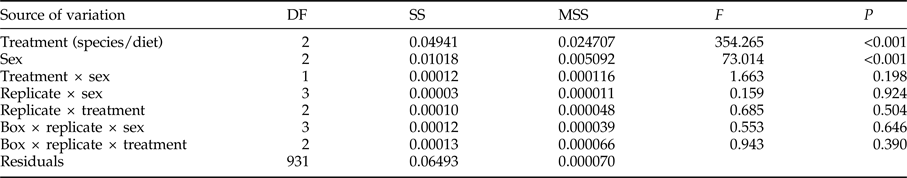
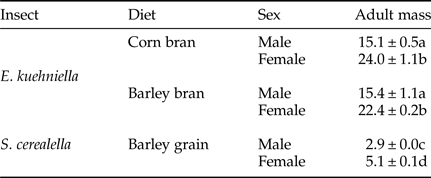
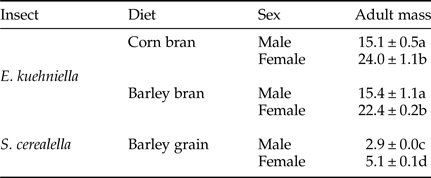
For adult weight, significant differences were found between species/diet combinations and between sexes, without significant interactions or significant differences between replicates and/or boxes (table 1). Adult weight was significantly lower for S. cerealella than for E. kuehniella, and in all cases females showed significantly larger weight than males (table 2).
Table 1. A two way (Treatment × Sex) nested ANOVA examining the effect of three combinations of species/diet and of sex on the adult weight of E. kuehniella and S. cerealella reared on three diets. A nested ANOVA was applied to identify possible differences among boxes and essay replicates.
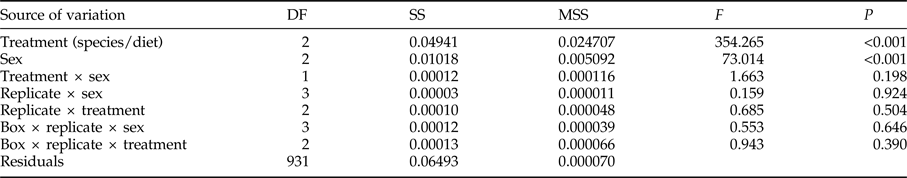
Degrees of freedom (DF), sum of squares (SS), mean sum of squares (MSS), test statistic (F) and significance (P).
Table 2. Mean (± SEM) of adults’ weight (mg) for males and females, of E. kuehniella and S. cerealella reared on three diets, corn bran, barley bran and barley grain, respectively. Means followed by different letters are significantly different (Tukey test applied after ANOVA: P < 0.05).
Sex ratio (% of females) was not significantly different among the three combinations of species/diet (χ2 = 3.2; df = 2; P = 0.199), with a higher proportion of males in both species (table 3). The mean number of eggs produced by female was significantly larger in E. kuehniella than in S. cerealella (F = 344.0; df = 2, 87; P < 0.001; table 3).
Table 3. Sex ratio (% of females), and number of eggs per female for E. kuehniella and S. cerealella reared on three diets, corn bran, barley bran and barley grain, respectively. Sex ratio - No significant differences were found (2 test); Eggs/female - Means followed by different letters are significantly different (Tukey test applied after ANOVA: P < 0.05).

Modeling of oviposition period
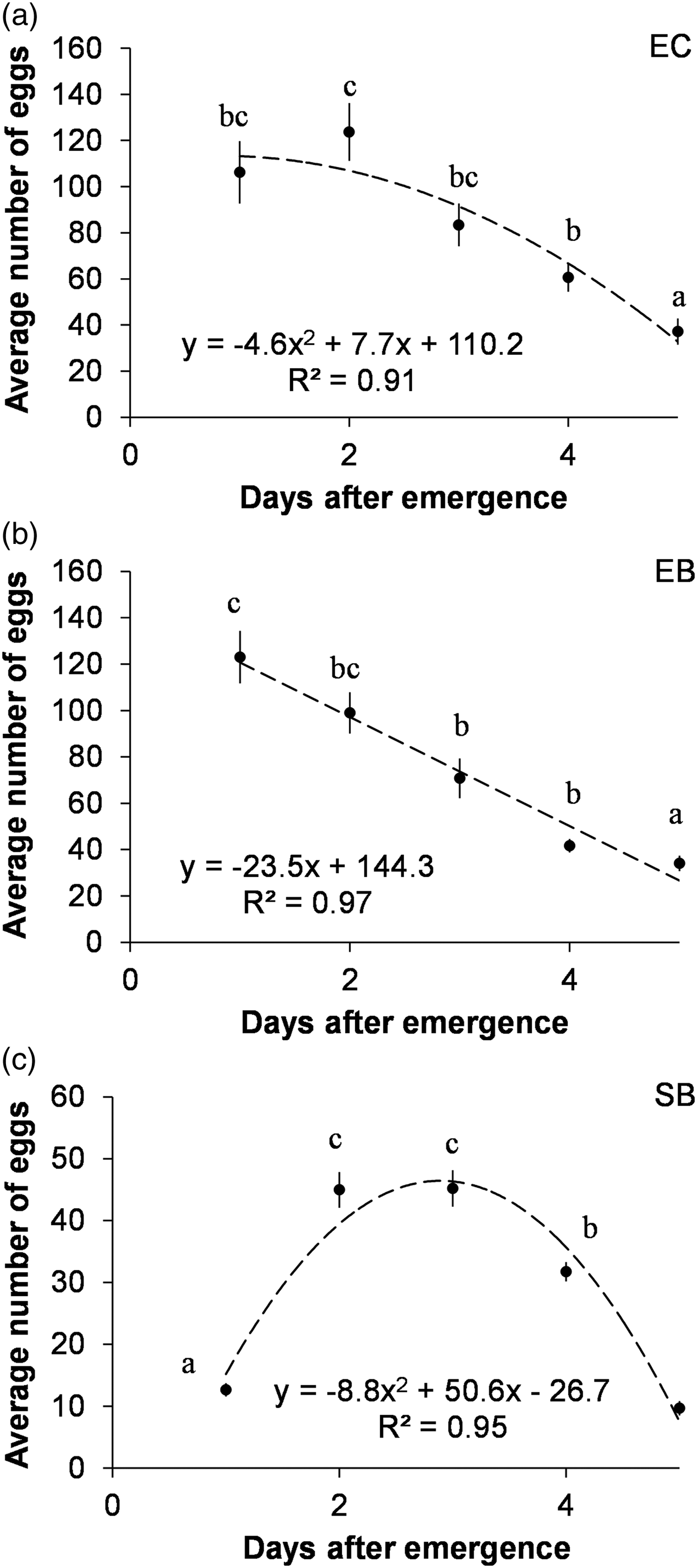
For E. kuehniella, the number of eggs laid after emergence generally decreased with time, although following a slightly different model in the two diets, a polynomial model for corn bran and a linear model for barley bran (fig. 3). Regarding S. cerealella, the best model was considerably different, a polynomial showing a first increase in oviposition followed by a later decline (fig. 3).

Fig. 3. Temporal variation of the mean number of eggs laid per female, after emergence, for three combinations of insect species/diet. Means followed by different letters are significantly different between days (Tukey test applied after ANOVA: P < 0.05).
Model integration
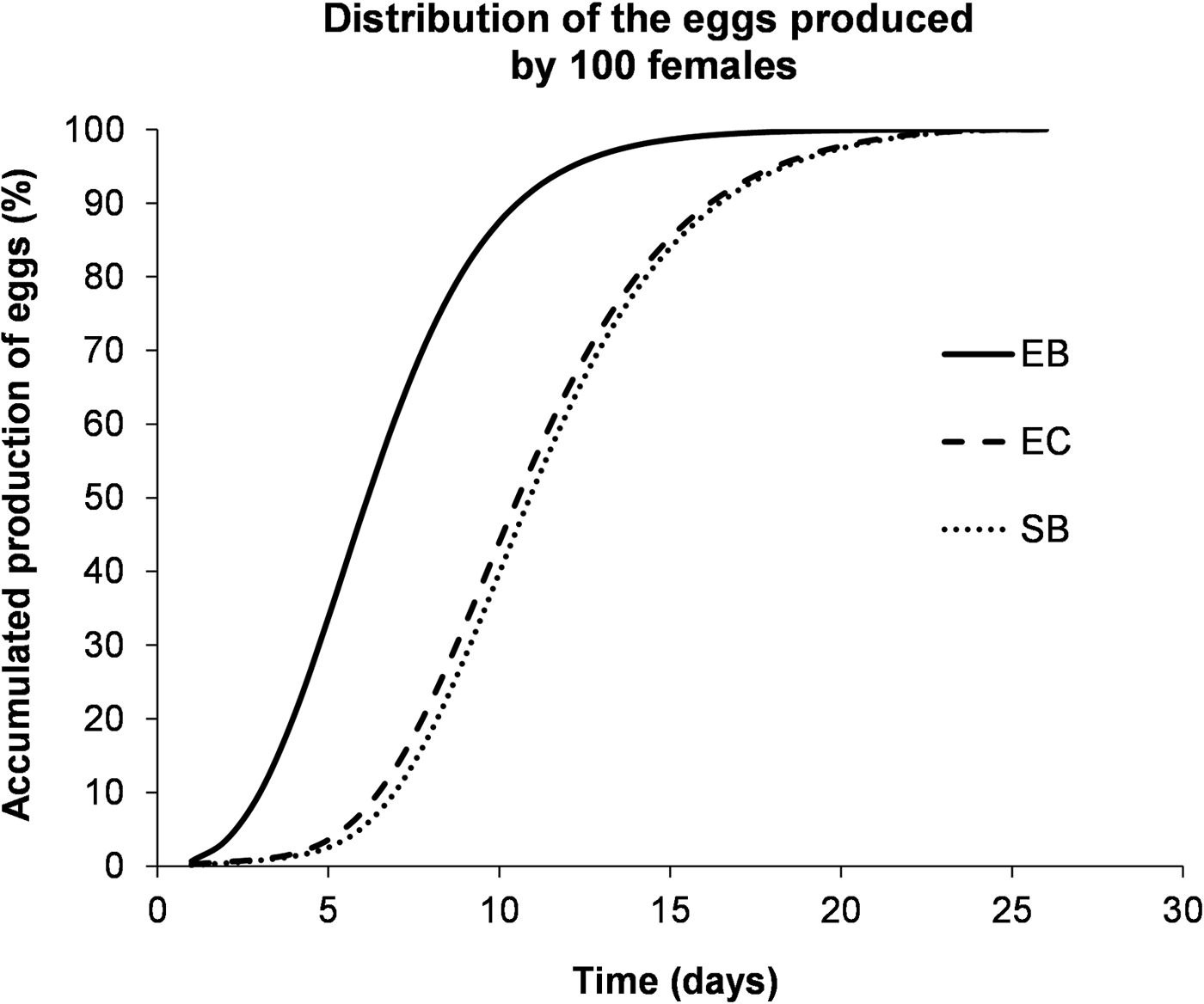
By integrating the models for adult emergence and oviposition period, we found that E. kuehniella reared with barley bran originated the earliest and highest peak of egg production, about 6 days after adult emergence, reaching 90% of oviposition in the first 10 days (fig. 4). In contrast, both E. kuehniella reared in corn bran and S. cerealella reared in barley grain needed more than 10 days to reach a production peak and more than 15 days to reach 90% of oviposition, with S. cerealella showing a much lower production (fig. 4).

Fig. 4. Accumulated production of eggs (%) for 100 females as a function of time (days after the emergence of the first adult), based on the integration of adult emergence and female oviposition models.
Based on the models described in fig. 4, it is possible to simulate massive egg production searching for a management strategy that can be applied according to research or application needs. In fig. 5 we simulated the best strategy for a high and stable production of factitious host eggs for a period of 20 days, for example, to support the establishment of an essay with entomophagous insects. It is clear that accumulated egg production would be much larger in E. kuehniella than in S. cerealella. Also, E. kuehniella showed a faster and more concentrated production in time on barley than on corn, as expected due to the faster adult emergence rate on that food source. Other similar predictions would be possible, depending on the desired egg production both in terms of quantity and duration.
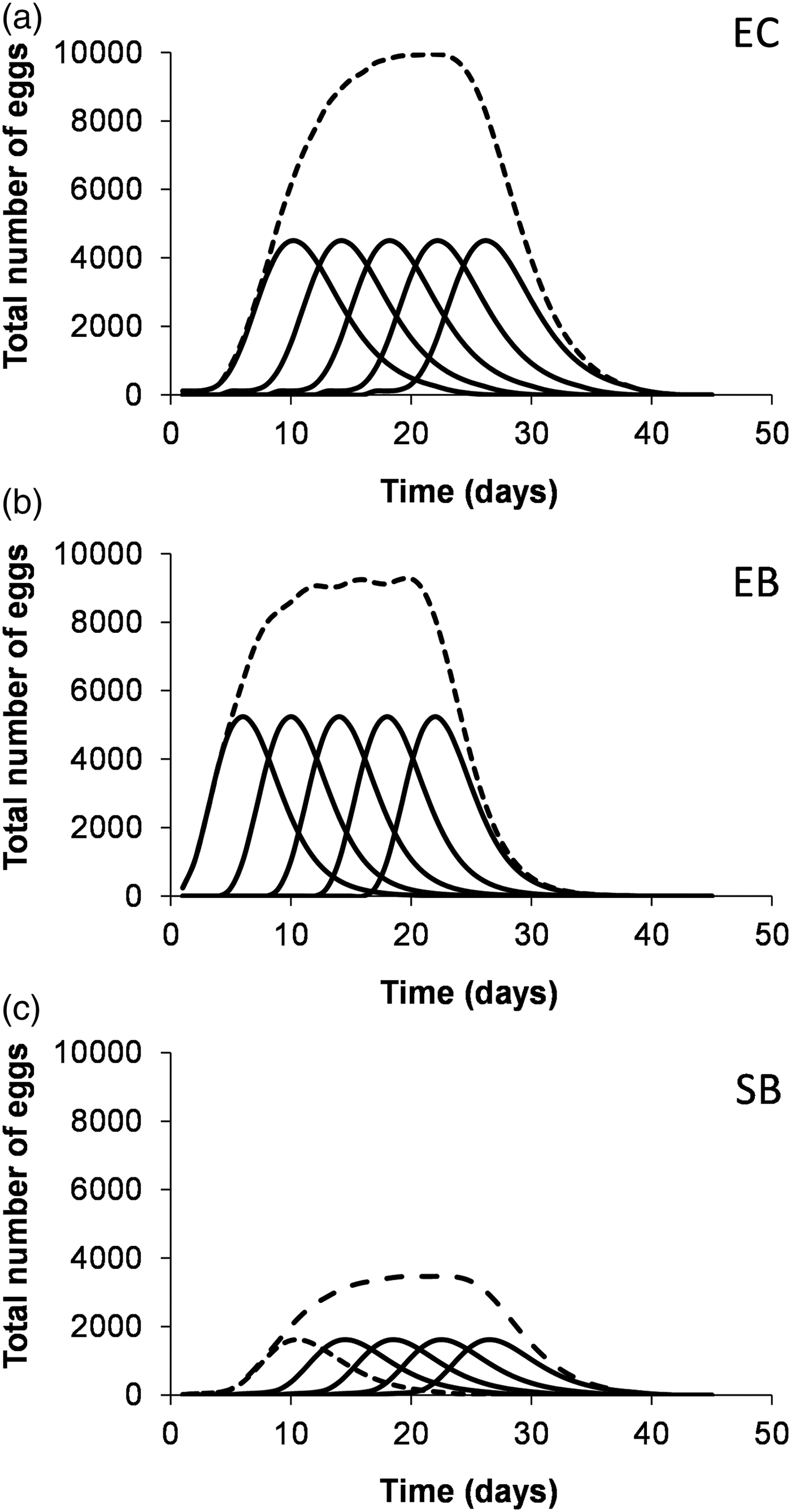
Fig. 5. This figure illustrates the simulation of a high and stable egg production, between days 10 and 30, by initiating the culture of five insect batches at five days’ intervals. Estimated egg production for 5 × 100 females of Ephestia kuehniella reared with corn bran (EC), barley bran (EB) and Sitotroga cerealella reared with barley grain (SB), as a function of time (days after the emergence of the first adult), based on the integration of adult emergence and female oviposition models. Full line: egg production per batch; Broken line: total egg production.
Discussion
The two factitious hosts E kuehniella and S. cerealella are very important pests of stored products but are also widely used to rear parasitoids and predators. The present study was carried out to investigate the effects of two natural diets on the emergence period, oviposition period, adult mass and sex ratio. We also analyzed the possibility of integrating adult emergence and oviposition curves, in order to predict egg production along time. Under the abiotic conditions adopted for this study, the time to reach approximately 100% of adults’ emergence was less than 3 weeks and faster for E. kuehniella in barley grain, in agreement with previous observations (Daumal et al., Reference Daumal, Jourdheuil and Tomassone1974; Tavares & Vieira, Reference Tavares and Vieira1992). This research showed that food source had a clear effect on the adult emergence curve of E. kuehniella, leading to a significant effect on the estimated time for 50% of adult emergence.
Moreover, the use of survival analysis tools such as the Gompertz model (e.g. Hougaard, Reference Hougaard2000; Damos & Soulopoulou, Reference Damos and Soulopoulou2015), showed to be fully applicable to the modeling of insect emergence in mass production situations. Using relatively straightforward modeling devices, it was possible to integrate adult emergence and oviposition. Therefore, our modeling approach, not only allowed to point out the most meaningful biological differences between the two hosts, but will also allow to predict egg production in different scenarios, thereby supporting mass production management, as shown in fig. 5. Our modeling approach has the advantage of including parameters with biological meaning, such as the time to 50% adult emergence or the oviposition curve, while keeping a straightforward statistical tractability (Ratkowsky & Reddy, Reference Ratkowsky and Reddy2017). This type of simulation can be performed using a common spreadsheet, or more specific and open software such as R (R Core Team, 2014). Although the obtained models have resulted directly from experimental data and therefore have taken into account the average biological parameters of the factitious hosts, slight adjustments might be required in the transition to egg production in more massive conditions, particularly if other aspects such as cost-effectiveness are also to be considered (Pascacio-Villafán et al., Reference Pascacio-Villafán, Birke, Williams and Aluja2017). Thus, the models should be further validated using the previously accumulated expertise in the management of mass rearing facilities (Tavares et al., Reference Tavares, Anunciada, Oliveira and Vieira1989, Reference Tavares, Ribeiro and Oliveira2012 a, Reference Tavares, Ribeiro, Oliveira and Vieira b ).
In our study the effect of food type was clearly visible on the shape of the oviposition curves. Variation in the quantity or quality of an acceptable diet can have profound effects on insect physiology, development and fecundity (Raccaud-Shoeller, Reference Raccaud-Shoeller1980; Chapman, Reference Chapman1998; Triplehorn & Johnson, Reference Triplehorn and Johnson2005; Bushra & Aslam, Reference Bushra and Aslam2014; Tarlack et al., Reference Tarlack, Mehrkhou and Mousavi2015), which in turn has direct implications on the biological cycle, particularly on adult emergence and oviposition rhythm (Nadeem et al., Reference Nadeem, Hamed and Shafique2011; Nadeem, Reference Nadeem2012; Tavares et al., Reference Tavares, Ribeiro and Oliveira2012a , Reference Tavares, Ribeiro, Oliveira and Vieira b ).
Marked differences were also found between the two tested factitious hosts, namely a more synchronous emergence in the adults of E. kuehniella, when compared with S. cerealella, but also distinct models regarding the oviposition period. Both moths show ovaries of the polytrophic meroistic type, but with notable differences in the evolution of oogenesis, as demonstrated by different authors (e.g. in E. kuehniella: Baroughi-Bonab, Reference Baroughi-Bonab1965; Xu et al., Reference Xu, Wang and He2008; Xu, Reference Xu2010; Tarlack et al., Reference Tarlack, Mehrkhou and Mousavi2015, and in S. cerealella: Stockel, Reference Stockel1973; Prakash et al., Reference Prakash, Chhillar and Kashyap2004). E. kuehniella females show a high number of oocysts, already formed at the time of emergence. In contrast, in other species such as S. cerealella, oocyte maturation happens only 1 day after female emergence. The oocytes pass through the ovarioles, maturing along the way. Consequently, the spatial sequence in the ovariole reflects the temporal sequence of oocyte maturation (Triplehorn & Johnson, Reference Triplehorn and Johnson2005).
Therefore, the biological and behavioral differences between these Lepidoptera require adjustments in the production, which will affect various biological parameters, namely the duration of the life cycle, the heterogeneity of emergences, the weight of the adults and the respective oviposition over time. Knowledge of such differences is important for planning mass production of insects in biofactories in order to combine factitious host availability and biological agents production (Tavares & Vieira, Reference Tavares and Vieira1992; Silveira, Reference Silveira1997; Manjunath, Reference Manjunath2014).
Concerning the host's choice for the production of biological control agents, such decision will depend on specialized studies, which among others take into account the levels of trophic relationships, the price in the cereal market, hand labor and energy, the existence of the species in the natural ecosystem, and equipment and methodologies applied to culture (Van Lenteren, Reference Van Lenteren2011; Gaffar, et al., Reference Gaffar, El-Naggar, Hendi and Mikhail2013).
We demonstrated that relatively simple modeling devices with biological meaning could integrate both adult emergence and oviposition and predict egg production under different scenarios. Clearly, this is important when an efficient means of producing eggs of the factitious hosts is needed for the rearing of egg parasitoids, such as Trichogramma spp., to maximize the number of high quality biological control agents at the lowest rearing cost,.
Acknowledgements
The authors thank the Department of Biology of the Azores University and the Azores Regional Government for supplying the experimental requirements and for supporting this research.