Introduction
Accurate measures of fitness are crucial for assessing the ecological and evolutionary trajectories of host–parasite interactions. Fitness is generally defined as the average contribution of one genotype or allele to the next or successive generations, compared to other genotypes or alleles (Futuyma, Reference Futuyma1986). Therefore, fitness of parasites is often expressed as their transmission success, or, more formally, the number of secondary infections caused by a primary infection (May and Anderson, Reference May and Anderson1979). However, transmission-based fitness is a composite variable which integrates different underlying parasite traits that individually contribute to transmission success (Antolin, Reference Antolin2008; McCallum et al. Reference McCallum, Fenton, Hudson, Lee, Levick, Norman, Perkins, Viney, Wilson and Lello2017). These traits constitute fitness components that are related to specific phases of infection (e.g. infection success, host resource exploitation efficiency, reproductive output, etc.) and are often under host genotype and/or environmental control (Vale and Little, Reference Vale and Little2009; Wolinska and King, Reference Wolinska and King2009; Van den Wyngaert et al. Reference Van den Wyngaert, Vanholsbeeck, Spaak and Ibelings2014). Unravelling how the host genotype and external environment modulate these individual traits would grant a deeper understanding of the biology of the interaction between antagonists, with regard to the mechanisms of infection, their ecophysiology, and the basis of host–parasite compatibility. Such insights allow for better predictions of disease dynamics in natural settings. This also contributes to the identification of the range of environmental conditions that delineate infection hotspots and/or environmental refuges from infection, which modulate the intensity of parasite-mediated selection on host populations, and thereby regulate host–parasite interactions in the wild (e.g. Kraaijeveld and Godfray, Reference Kraaijeveld and Godfray1999; Lively, Reference Lively1999).
Phytoplankton represent the base of most aquatic food webs and is a major driver of global biogeochemical cycles (Falkowski, Reference Falkowski2012). Phytoplankton can be lethally infected by parasitic fungi belonging to the early diverging phylum Chytridiomycota (i.e. chytrids) (Sommer et al. Reference Sommer, Adrian, De Senerpont Domis, Elser, Gaedke, Ibelings, Jeppesen, Lürling, Molinero and Mooij2012). The best known representative of this group of parasites is the species Batrachochytrium dendrobatidis, which drives massive amphibian population declines worldwide (Vredenburg et al. Reference Vredenburg, Knapp, Tunstall and Briggs2010). Chytrids infecting phytoplankton have been reported in the past (Braun, Reference Braun1856; Canter, Reference Canter1947; Canter and Lund, Reference Canter and Lund1948, Reference Canter and Lund1951), but they are attracting renewed interest as accumulating evidence demonstrates their unexpected diversity and widespread distribution in pelagic marine, brackish and freshwater habitats worldwide (Lefèvre et al. Reference Lefèvre, Roussel, Amblard and Sime-Ngando2008; Lepère et al. Reference Lepère, Domaizon and Debroas2008; Grossart et al. Reference Grossart, Wurzbacher, James and Kagami2016; Hassett and Gradinger, Reference Hassett and Gradinger2016). The lifecycle of chytrids is characterized by a free-swimming stage in the form of flagellated zoospores that are assumed to find suitable hosts in the water column by chemotaxis (Muehlstein et al. Reference Muehlstein, Amon and Leffler1988; Scholz et al. Reference Scholz, Küpper, Vyverman, Ólafsson and Karsten2017). Once encysted on their host, chytrids develop a rhizoid system that penetrates the host cell wall to extract nutrients from it. As infection spreads, zoospores gradually develop into sporangia, epibiotic reproductive structures that release new zoospores upon maturation (Ibelings et al. Reference Ibelings, De Bruin, Kagami, Rijkeboer, Brehm and Donk2004). Although often neglected, chytrid parasitism has potentially profound ecological implications (reviewed in Frenken et al. Reference Frenken, Alacid, Berger, Bourne, Gerphagnon, Grossart, Gsell, Ibelings, Kagami and Agha2017). As lethal parasites, chytrids shape the structure and dynamics of phytoplankton populations and have the potential to delay or suppress algal blooms (Gerphagnon et al. Reference Gerphagnon, Colombet, Latour and Sime-Ngando2017). Furthermore, by imposing strong selection on their hosts, chytrids can drive the evolution of phytoplankton populations (Gsell et al. Reference Gsell, de Senerpont Domis, Verhoeven, Van Donk and Ibelings2013b). From a food web perspective, chytrids constitute a high-quality food source for zooplankton consumers. Therefore, chytrid parasitism can establish alternative trophic links in aquatic food webs and circumvent trophic bottlenecks typically imposed by the dominance of inedible or toxic phytoplankton (Kagami et al. Reference Kagami, von Elert, Ibelings, de Bruin and Van Donk2007; Agha et al. Reference Agha, Saebelfeld, Manthey, Rohrlack and Wolinska2016).
However, chytrid infections, including their underlying mechanisms and modulation by the environment, remain poorly characterized. Previous studies of chytrids infecting phytoplankton have been restricted for the most part to a few chytrid taxa available in culture. For instance, infections by the chytrids Zygorhyzidium planktonicum and Rhizophydium planktonicum, both parasitizing the diatom Asterionella formosa, have been shown to be modulated by abiotic factors such as light, nutrients and temperature (Canter and Jaworski, Reference Canter and Jaworski1981; Bruning and Ringelberg, Reference Bruning and Ringelberg1987; Bruning, Reference Bruning1991a, Reference Bruningb). Moreover, in this diatom-chytrid system, temperature variation was shown to change the ranking of susceptibility among conspecific host genotypes, indicating genotype × environment interactions (Gsell et al. Reference Gsell, de Senerpont Domis, Van Donk and Ibelings2013a). In the present work, we focus on a related host–parasite system consisting of the chytrid Rhizophydium megarrhizum infecting cyanobacteria of the bloom-forming and toxin-producing genus Planktothrix. Previous studies have shown that R. megarrhizum can infect different, but not all, Planktothrix spp. strains (Sønstebø and Rohrlack, Reference Sønstebø and Rohrlack2011). Similarly, low temperatures have been shown to alleviate or even suppress infection, suggesting environmental control of chytridiomycosis (Rohrlack et al. Reference Rohrlack, Haande, Molversmyr and Kyle2015). However, parasite fitness in these studies was expressed as the incidence of infection (measured two days after exposure to the parasite), leaving the question open as to which specific parasite traits are modulated by environmental variation and how. Here, we assess the overall fitness (i.e. transmission-based fitness proxy) of the chytrid parasite R. megarrhizum across temperature and host genotypic variation. Simultaneously, we examine different underlying parasite traits related to ability to infect (i.e. incidence and intensity of infection) and reproductive output (i.e. sizes of sporangia and zoosporic propagules), which jointly contribute to overall fitness. We aim to disentangle the effects of host and external environment on these individual parasite traits and thereby to contribute to a better characterization of the ecophysiology of this host–parasite system and its underlying infection mechanisms.
Materials and methods
Host and parasite strains
The chytrid parasite strain Chy-Kol2008 was isolated in 2008 from Lake Kobotnvatet (Norway) and identified as R. megarrhizum (Sønstebø and Rohrlack, Reference Sønstebø and Rohrlack2011). Two host strains belonging to the filamentous, bloom-forming cyanobacterial genus Planktothrix were used: NIVA-CYA98 (Planktothrix rubescens, isolated from Lake Steinsfjörden (Norway) in 1982) and NIVA-CYA630 (Planktothrix agardhii isolated from Lake Lyseren (Norway) in 2008). Host strains were maintained in Z8 medium as non-axenic batch cultures under 16 °C and 15 μm photons m−2 s−1. The parasite was maintained in culture by transferring zoospore suspensions into uninfected cultures of the host strain NIVA-CYA98, every 2 weeks.
Experimental setup
Before the start of the experiment, host strains were acclimated as semi-continuous cultures for 3 weeks to their respective temperatures (8, 12, 16 and 20 °C). Ten days before the start of the experiment, a culture of the strain NIVA CYA98 was infected with the chytrid parasite and incubated at 16 °C and 20 μm photons m−2 s−1. After 10 days, a purified zoospore suspension was obtained by sequential filtration through sterile 10 and 5 μm nylon meshes and a 3 μm polycarbonate filter. The resulting filtrate was microscopically checked for the absence of host filaments and zoospore density was quantified under a Nikon Ti Eclypse inverted microscope using a Sedgewick Rafter chamber after fixation of a 1 mL aliquot with acid Lugol. The purified zoospore suspension was then used to infect triplicate 100 mL aliquots of the acclimated host strains NIVA-CYA98 and NIVA-CYA630 (host density 2000 filaments mL−1), providing a final zoospore density of 750 mL−1. Optical density at 750 nm of each host strain was correlated to filament concentrations, and then used to obtain the desired initial host densities.
After initial infection, experimental cultures were sampled daily or, for low-temperature treatments, every other day, until incidence of infection in the cultures reached a clear asymptote (i.e. plateau). After gently homogenizing the suspension, 1.5 mL aliquots were collected and fixed in 2% formaldehyde and stored at 4 °C from 2 to 5 weeks until analysis. Samples were used to investigate four different parasite traits: incidence of infection (i.e. proportion of infected filaments in the population), intensity of infection (i.e. mean number of sporangia per infected filament), volume of mature/empty sporangia, and size of zoospores.
Incidence of infection was determined by the proportion of infected hosts after sequentially examining 200 cyanobacterial filaments. To compare the temporal dynamics of the incidence of infection across temperature treatments, a unit of physiological time was employed, namely degree-days. A degree-day is simply the product of 24-h days and temperature. The use of this unit normalizes differences in host and parasite metabolic rates under different temperatures, making it possible to compare parasite fitness along temperature gradients (e.g. Mitchell et al. Reference Mitchell, Rogers, Little and Read2005).
Transmission-based fitness was determined after the formulation of May and Anderson (Reference May and Anderson1983):
where R 0 is the number of infections caused by a single primary infection, β is the parasite transmission rate, N is the density of hosts (kept constant in our experiment), α is the parasite virulence (equal to 1 in this host–parasite system, as every infection is lethal), b is the rate of parasite independent mortality (assumed constant and negligible in our experiment), and ν is the host recovery rate (equal to zero here). Therefore, in our experiment, differences in parasite fitness across genetic and thermal variation could be fully attributed to changes in the transmission rate β, whose values were calculated by logistic regression of the data reflecting the proportion of infected filaments over physiological time. Parasite transmission-based fitness was hence expressed as transmission rate.
In addition, intensity of infection, size of sporangia and size of zoospores were evaluated. Intensity of infection, or the mean number of infections (i.e. encysted zoospores or sporangia) present on single hosts, was determined after examining 200 infected filaments per sample. Mean sporangial volumes were estimated for 20 empty or mature sporangia per sample (distinguishable by a well-developed thickened sporangium wall). Volume was estimated by measuring their two semi-axes under a Nikon Ti Eclypse inverted microscope and the NIS-Element BR 4.5 software, and assimilating them as rotational ellipsoids with the volume
where d 1 and d 2 are the short and the long semi-axes, respectively. In case multiple empty/mature sporangia were present on a single host filament, only the biggest was measured and included in the analyses. In order to evaluate the potential effect of multiple infections on single hosts on sporangial size (due to putatively increased competition for host resources), for every measured empty/mature sporangium, the number of individual infections present on the host was also recorded. Lastly, mean zoospore size was determined from the diameter of 50 measured zoospores in each sample. Intensity of infection, mean sporangial volume and zoospore size were determined from four subsamples per experimental unit (i.e. repeated measures) corresponding to time points where the incidence of infection had plateaued. Thereby, a well-established level of intensity of infection within the population, as well as a sufficient number of fully developed sporangia and released zoospores were ensured.
Data analysis
Fixed effects of temperature and host strain were tested on each of the studied parasite fitness traits: transmission rate, intensity of infection, mean volume of sporangia, and mean size of zoospores. Transmission rate (β) was calculated for each biological replicate by logistic regression of data reflecting changes in incidence of infection over physiological time. Main and interactive effects of host genotype and temperature on transmission rate were evaluated using a linear model. This was followed by a contrast test comparing mean transmission rates within individual host genotypes across temperatures, and between host genotypes within the same temperature (least-squares means test with Holm's P value adjustment).
For intensity of infection, mean sporangial volume, and zoospore sizes, fixed effects of temperature and host strain were examined by fitting linear mixed models. For sporangial volume, data needed to be log-transformed to satisfy distributional assumptions of the residuals. In order to account for repeated measures on biological replicates at four different time points (see the previous section), experimental unit (from which repeated measures were taken) and experimental unit nested within sampling time were tested as possible random effects structures in the respective models. However, regardless of the selected random factor structure, the resulting estimates of variance components were zero, indicating that within-group variability was not sufficient to warrant incorporating random effects in the models. In fact, including random effects consistently resulted in poorer quality for the mixed models, as shown by higher AIC values (Akaike Information Criterion, a relative measure of model quality), compared with their non-mixed counterparts. The exclusion of random factors was further evaluated by fitting mixed and non-mixed models in parallel. In all cases, the results were almost identical and not qualitatively different (data not shown). Therefore, the output of degenerate linear models is reported, i.e. models where random effects were excluded, despite their initial inclusion as imposed by the experimental design (Bates et al. Reference Bates, Kliegl, Vasishth and Baayen2015).
For all models, selection of optimal fixed covariate structures was performed by formulating full models including all fixed terms (temperature, host strain and their interaction term). Full models were then compared with a nested model excluding one of the terms. Individual fixed terms were excluded when such comparisons did not yield significant differences (i.e. including the term did not significantly improve the model). After optimal model selection, the significance of individual fixed terms was determined by Type III F-tests relative to an intercept-only model. In addition, the proportion of variance explained by each fixed term is reported with regard to the full model. This was calculated as the sequential sum of squares of each term, divided by the total sum of squares in the full model. All statistical analyses were performed using Rstudio (v.0.99.903). Linear mixed modeling was performed using the ‘lme4’ package (Bates, Reference Bates2010).
Results
Cultures incubated at 8 °C did not become infected and were hence excluded from the analyses. Incidence of infection increased fastest with physiological time at 20 °C compared with 16 °C and 12 °C, reaching a plateau at around 100 degree-days (Fig. 1A). Transmission rates were accordingly higher at 20 °C, independently of the host strain infected (Fig. 1B, Table 1). At lower temperatures transmission rates dropped for both host strains. Most of the variance in the data was explained by temperature (86.2%, Table 1). Although host strain showed a significant effect, it only explained about 7% of the variance. In fact, transmission rates did not display significant differences between host strains, except at 12 °C (Table 2). A significant host strain × temperature interaction was found, although it displayed little explanatory power (3.1%, Table 1).
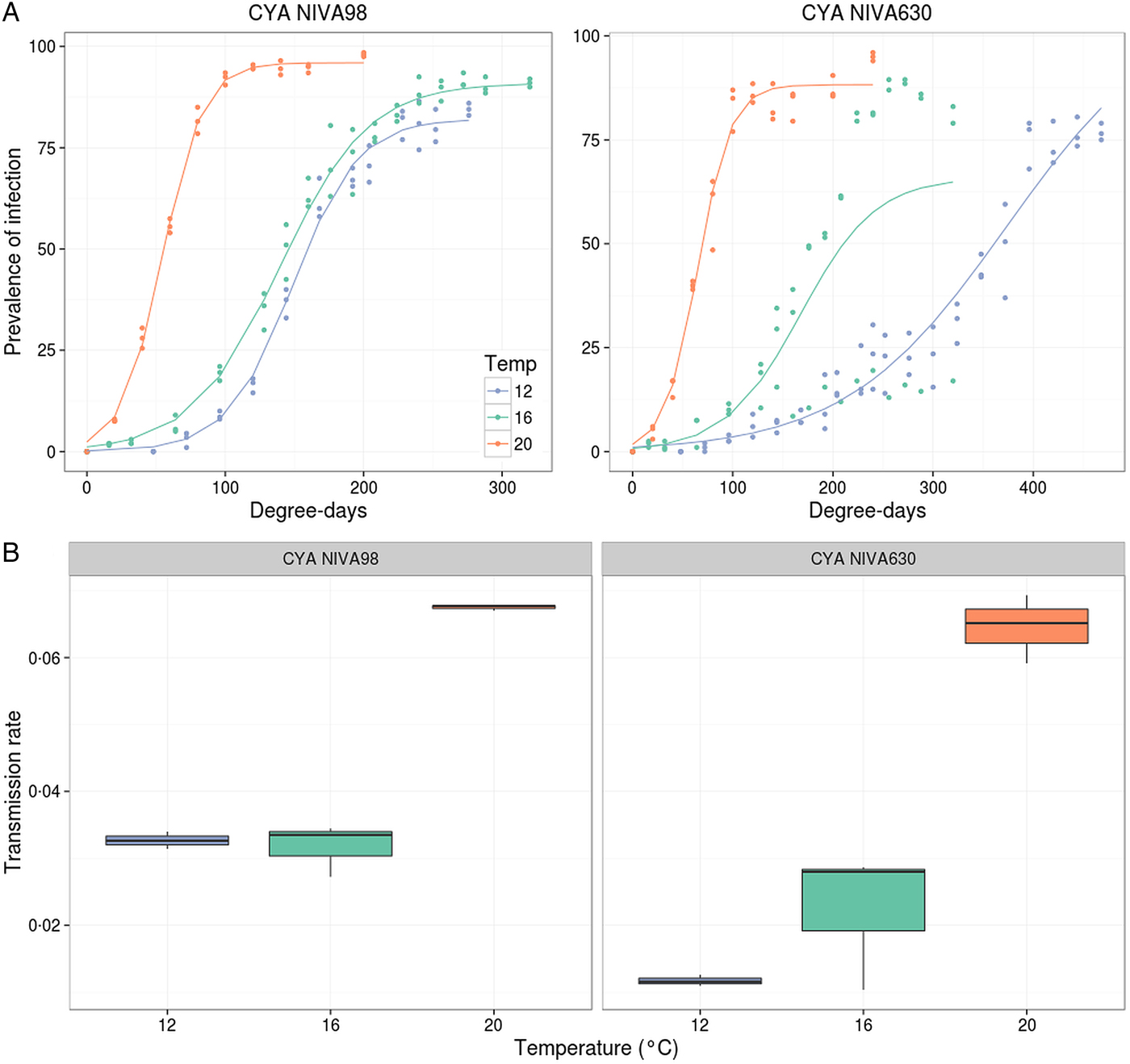
Fig. 1. Change in prevalence of infection with physiological time (A) and estimated transmission rates (B) for different temperatures and host strains. (A) Lines represent logistic fits of data from pooling all three biological replicates. (B) Transmission rates (β) were estimated from logistic regressions for each biological replicate.
Table 1. Linear models for fixed effects of host strain, temperature and their interaction on parasite transmission rates, intensity of infection, sporangial volumes and zoospore sizes

For zoospore sizes, the interaction term was removed in the reduced model as its inclusion did not significantly improve the full model. Variance explained on individual terms stems from sum squares quotients in the full model. d.f.: degrees of freedom; NS: not significant (i.e. term was excluded in the reduced model).
Table 2. Contrast tests for transmission rates

Comparisons are made for infections on the same host strain under different temperatures, and for different host strains under the same temperature. Significant P values are depicted in bold.
Intensity of infection (i.e. mean number of individual infections per infected host) on the NIVA-CYA98 strain was conspicuously higher (4.0 ± 0.03) than on NIVA-CYA630 (2.4 ± 0.04), regardless of temperature (Fig. 2B). Host strain explained over 92% of the variance in the data, whereas temperature did not have a significant effect in the model. A significant host strain × temperature interaction was found, although it explained less than 1.5% of the variance of the data (Table 1).

Fig. 2. Mean intenstity of infection (A), sporangial biovolume (B), and zoospore size (C) (±s.e.) under different host strain and temperature combinations.
Mean sporangial volume was higher on NIVA-CYA98 than on NIVA-CYA630 (ca. 55%). Sporangial volumes decreased with increasing temperature (Fig. 2A). In fact, linear models showed that variation in sporangial volumes was mostly explained by both host strain and temperature (34 and 22%, respectively; Table 1). A host strain × temperature interaction term was maintained in the final model, although its effect on the mean volume of sporangia was slightly below significance levels (P = 0.051; Table 1).
Zoospore sizes decreased with increasing temperature independently of host strain (Fig. 2C). The reduced linear model showed that zoospore sizes were affected mostly by temperature (explaining over 66% of the variance), whereas host strain only accounted for 9% of the variance (Table 1). The interaction term was not significant in the full model (F 2,12 P = 0.186) and was excluded from the final model.
Discussion
Overall chytrid fitness, as defined by transmission rate (May and Anderson, Reference May and Anderson1983), was largely modulated by thermal variation (Fig. 1A, Table 1). The chytrid parasite did not cause infections at 8 °C, supporting the existence of a low-temperature infection refuge in this system and stressing the importance of the external environment as a major modulator of chytrid disease outcome (Gsell et al. Reference Gsell, de Senerpont Domis, Van Donk and Ibelings2013a; Rohrlack et al. Reference Rohrlack, Haande, Molversmyr and Kyle2015). The fact that host strain had only marginal explanatory power on overall transmission-based fitness (<7% of the variance; significant differences among host strains were only found at 12 °C) might initially point toward a limited impact of host genotype on the outcome of infection. This, however, contrasts with earlier reports of chytrid parasites of phytoplankton displaying rather narrow host ranges (Canter and Jaworski, Reference Canter and Jaworski1979; De Bruin et al. Reference De Bruin, Ibelings, Kagami, Mooij and Van Donk2008; Sønstebø and Rohrlack, Reference Sønstebø and Rohrlack2011). While these discrepancies might be initially attributed to the small set of host strains used here, which is unreflective of the natural host genetic diversity, close examination of individual parasite traits did reveal significant effects of host genotype on parasite performance. Intensity of infection (a measure of zoospore encystment success) and size of sporangia and zoospores (jointly acting as proxies of parasite reproductive output) responded to host genotype and thermal variation in different ways, indicating that the interplay of these underlying traits jointly determines overall parasite fitness. The parasite consistently performed better in all investigated individual traits when infecting the strain NIVA CYA98, most likely due to the fact that this strain was used for routine maintenance of the parasite over years and the chytrid strain might hence have adapted to it (De Bruin et al. Reference De Bruin, Ibelings, Kagami, Mooij and Van Donk2008). Still, the use of integrative fitness proxies (in this case transmission rates), masked these otherwise notorious differences among host strains observed when individual parasite traits were investigated. Differential response of individual parasite traits to environmental or host genotype variation are also common in other host–parasite systems (Fels and Kaltz, Reference Fels and Kaltz2006; Vale et al. Reference Vale, Stjernman and Little2008). Altogether, this stresses the important point that, by expressing parasite fitness in terms of transmission success, we integrate (but also overlook) underlying parasite traits that act as components of overall fitness, e.g. infection success, reproductive output, etc., into a single metric. Whereas integrative measures of fitness are often useful, they provide limited insights into the underlying mechanisms of infection. Instead, disentangling how individual parasite traits are affected by both host and external environment grants a deeper understanding of the ecophysiology of the host–parasite interaction.
For instance, in contrast to overall fitness, intensity of infection was shown to be controlled almost exclusively by host genotype, with temperature exerting no significant effect. This supports the notion that chytrid encystment is likely mediated by cell-to-cell contact, with host genotype-dependent cell surface characteristics acting as a first barrier against infection. By analogy with other zoosporic parasites, cell surface features involved in chytrid–host compatibility likely consist of carbohydrates and lectins that are prone to polymorphism across conspecific strains (Hinch and Clarke, Reference Hinch and Clarke1980; Petre and Kamoun, Reference Petre and Kamoun2014). The fact that intensity of infection (i.e. encystment success) was controlled by host genotype variation exclusively, together with observations that the chytrid strain used here is able to encyst on some, but not all conspecific host strains (Sønstebø and Rohrlack, Reference Sønstebø and Rohrlack2011), indicates that cell-to-cell compatibility plays a pivotal role in defining host–parasite specificity in this system. Importantly, observed differences in intensity of infection across susceptible host strains suggest that chytrid–host compatibility at the cell surface level is not simply a binary trait (resistant/susceptible), but encompasses different degrees of affinity among compatible host–parasite genotypes. Host densities in our experiment were kept constant and equal encounter rates can hence be assumed across treatments. Thus, observed changes in intensity of infection across host strains can be solely attributed to dissimilar affinity of the parasite towards their respective cellular surfaces. High cell-to-cell affinity might be a decisive factor for successful encystment and infection, especially under natural conditions where host densities (and hence parasite–host encounters) are typically much lower than those provided in our experiment.
Sporangial sizes also responded differently to host genotype and thermal variation compared to overall transmission-based fitness. Upon encystment, chytrids penetrate their host and secrete proteases to digest and extract nutrients from it. Therefore, final sporangial sizes arguably reflect the efficiency with which the parasite extracts and incorporates host resources into its own biomass, ultimately determining its reproductive output. Exploitation efficiency can be modulated, minimized, or suppressed by cyanobacterial hosts through the production of an array of intracellular oligopeptides with diverse protease inhibiting properties (Welker and von Dohren, Reference Welker and von Dohren2006). Cyanobacterial knockout mutants unable to produce individual oligopeptides showed increased susceptibility against a chytrid parasite, compared with the wild type strain (Rohrlack et al. Reference Rohrlack, Christiansen and Kurmayer2013), suggesting that these secondary metabolites are involved in anti-chytrid defence. Interestingly, the synthesis of these cyanobacterial oligopeptides is genetically determined by the presence or absence of encoding gene clusters, whose distribution across conspecific cyanobacterial strains in natural populations is remarkably patchy. This leads to chemically polymorphic populations with potentially different susceptibility to a given chytrid parasite (Agha and Quesada, Reference Agha and Quesada2014). The host strains used in this experiment are no exception. Despite small phylogenetic distance, they possess different intracellular oligopeptide compositions (Rohrlack et al. Reference Rohrlack, Edvardsen, Skulberg, Halstvedt, Utkilen, Ptacnik and Skulberg2008) that might lead to different susceptibility and thereby elicit differences in final sporangial sizes under identical infection conditions. Beside host-specific defensive traits, sporangial size might arguably be affected by the number of zoospores co-infecting a single host. Co-infecting zoospores engage in competition for host resources and such competition might modulate disease outcome. Indeed, an inverse relationship between the size of mature sporangia and the number of infections present on the host was found (Fig. 3), supporting the idea that increased competition among zoospores leads to reduced sporangial sizes. However, host genotype differences in susceptibility seem to override this effect; in spite of a consistently higher intensity of infection on strain NIVA-CYA98 (implying increased competition for host resources), consistently bigger sporangia were found on this strain (Fig. 2). Moreover, for the same number of infections, systematically bigger sporangia were found on strain NIVA-CYA98 (Fig. 3), further suggesting that host genotype differences in susceptibility override effects derived from parasite intraspecific competition under multiple infections.

Fig. 3. Relation between the number of infections on a single host and the volume of the biggest mature sporangium at the tested temperatures. Data represent 80 sporangial measurements per temperature–host strain combination.
In light of the consistent positive correlations between sporangial size and the numbers of contained zoospores repeatedly reported elsewhere (Bruning, Reference Bruning1991b; Gerphagnon et al. Reference Gerphagnon, Latour, Colombet and Sime-Ngando2013; Van den Wyngaert et al. Reference Van den Wyngaert, Vanholsbeeck, Spaak and Ibelings2014), sporangial size is typically regarded as a proxy of chytrid reproductive output. Therefore, we expected smaller sporangia under higher temperatures to imply a reduction in per capita reproductive output of the parasite. However, this contrasted with overall higher transmission rates observed at higher temperatures. Instead, together with a reduction in sporangial sizes, we also recorded a systematic reduction in zoospore sizes at higher temperatures. Chytrids might be able to compensate for reductions in final sporangial size by producing smaller zoospores, thereby stabilizing per capita reproductive output along temperature gradients. As zoospores rely on internal energy reserves to actively find a suitable host, this strategy might come at the cost of producing propagules with shorter infective lifetimes. However, shorter zoospore lifetimes might not impact parasite transmission when host densities are high, which typically coincides with the summer season and high temperatures. In addition to the production of smaller zoospores, high transmission rates at higher temperatures can also be maintained by faster sporulation times (i.e. the time needed by the parasite to develop a fully mature sporangium after encystment on the host; Van den Wyngaert et al. Reference Van den Wyngaert, Vanholsbeeck, Spaak and Ibelings2014), a fitness component that we could not address with our experimental setup. Despite the obvious need to demonstrate these possibilities, it is appealing to speculate that chytrids are able to exploit such trade-offs between reproductive output and propagule longevity depending on environmental context (e.g. host density, seasonal fluctuations) to maximize their fitness.
All in all, chytrid transmission success is the result of an interplay between individual traits, each differently affected by the host and/or external environments. Successful evasion of host barrier defenses (i.e. encystment success mediated by cell-to-cell compatibility) seems to be driven by the host environment alone (genotype × genotype interactions). In contrast, parasite fitness traits related to the efficiency with which the parasite extracts nutrients from the host appear to be under joint control by both host and external environment, indicating genotype × environment interactions. This exemplifies the importance of addressing underlying fitness traits to better characterize the interaction between host and parasite, including infection mechanisms and the influence of immediate and external environment on the outcome of the disease.
Acknowledgements
The authors would like to thank Mark Phillipo for proofreading the manuscript. Three anonymous reviewers are also acknowledged for useful comments on an earlier version of the manuscript.
Financial support
This work was supported by a postdoctoral grant by the Alexander von Humboldt Foundation granted to RA.








