Introduction
A complex body of literature implicates bacteria in the pathophysiology of chronic rhinosinusitis. Much of this research explores the observation that bacteria in chronic rhinosinusitis patients are found within a biofilm. A biofilm is a loose definition for the natural state in which over 90 per cent of bacteria exist, whereby they form an assemblage of microbial cells encased in a matrix of polysaccharide material.Reference Donlan1 Bacterial biofilms are associated with notoriously difficult to treat clinical infections such as device-associated and chronic wound infections.Reference Vestby, Grønseth, Simm and Nesse2 Despite an incomplete current understanding of the role of biofilms in severe chronic rhinosinusitis, their coexistence has been well documented and their presence is associated with poorer disease outcomes.Reference Głowacki, Tomaszewski, Stre¸k, Tomaszewska, Zgórska-Świerzy and Markiewicz3–Reference Zhang, Linkin, Finkelman, O'Malley, Thaler and Doghramji6
Recent studies show that the extent of microbial dysbiosis in chronic rhinosinusitis is related to mucosal inflammation,Reference Rom, Bassiouni, Eykman, Liu, Paramasivan and Alvarado7 but evidence suggests that antibiotics are an ineffective long-term strategy in managing the disease.Reference Orlandi, Kingdom, Hwang, Smith, Alt and Baroody8,Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma9 A number of non-antibiotic, anti-biofilm specific therapies exist with the aim of combatting the recalcitrant nature of biofilms in chronic rhinosinusitis. While benchtop evidence of the anti-biofilm effect of these intranasal preparations has been established, their clinical effectiveness is poorly understood. A formal review of their effect on the disease burden in chronic rhinosinusitis is lacking.
The aim of this study was to systematically review the literature regarding the efficacy of topical, non-steroid, non-antibiotic anti-biofilm specific therapy in adult patients with chronic rhinosinusitis, as measured by changes in validated, chronic rhinosinusitis specific, patient-reported outcome measures.
Materials and methods
Protocol and registration
Article identification and assessment was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (‘PRISMA-P’). The review protocol was registered prospectively in the Prospero database (registration number: CRD42019131888).
Eligibility criteria
Types of studies
Studies reporting English language, original patient data in peer reviewed journals were included. Review articles, case series or trials involving less than five participants were excluded.
Types of participants
The population of interest was adults with a diagnosis of chronic rhinosinusitis. No restrictions were placed on whether participants had undergone previous sinonasal surgery to allow for inclusion of patients across the spectrum of chronic rhinosinusitis disease severity. Studies were restricted to human, in vivo trials only.
Types of interventions
An ‘anti-biofilm specific therapy’ was defined as any non-steroid, non-antibiotic treatment for which preclinical evidence (in vitro, ex vivo or in vivo animal model data) was available and demonstrating an effect on biofilm structure or function in the context of chronic rhinosinusitis or upper airway inflammatory disorders. Only local therapies were considered.
Type of comparators
Studies evaluating anti-biofilm specific therapies versus either no intervention, placebo, non-antibiofilm therapy or other anti-biofilm specific treatments were included.
Types of outcome measures
The primary outcome was a change in validatedReference Rudmik, Hopkins, Peters, Smith, Schlosser and Soler10 patient-reported outcome measures for adult patients with chronic rhinosinusitis before and after treatment.
Information sources and search methods
Studies were identified by a combination of a systematic search of electronic databases and scanning reference lists of relevant articles. A systematic literature search of eight databases was performed in December 2019 using the full historical range. A combination of Medical Subject Heading (‘MeSH’) terms and keywords (Appendix 1) were used to devise a search strategy.
Study selection
Two authors (AT and JF) undertook the search strategy, reviewed and selected trials and evaluated these against the eligibility criteria. Initial screening was on title review, followed by thorough abstract and full text review. As part of the full text review process, all interventions were cross-referenced with the literature to ensure that there was published in vitro or ex vivo evidence of an anti-biofilm effect in the context of chronic rhinosinusitis or upper airway inflammatory disorders. Any studies evaluating interventions for which this evidence did not exist were excluded. Reference lists of articles identified were examined for additional studies, which if deemed relevant, were themselves subject to title, abstract and full text review. Any disagreement between reviewers was discussed until arbitration was agreed upon.
Quality assessment
The quality of each article was assessed using the Standard Quality Assessment Criteria for Evaluating Papers from a Variety of FieldsReference Kmet, Lee and Cook11 by two authors (AT and JF), with any discrepancies reconciled by discussion. This tool requires the examiner to score 14 aspects of the trial on a 3-point scale, with the total summary score being a conglomeration of the scores for each section normalised to a number on a scale of 0 to 1.
Results
Study selection
The search strategy is summarised in Figure 1. The formal search strategy produced a total of 1007 records, which reduced to 669 after removal of duplicates. Subsequent title and abstract review excluded a further 654 articles yielding 16 studies for eligibility assessment by full text review. A further 17 studies were identified as potentially relevant after reference list review and individual searching. Of these 33 studies, a total of 13 studies met eligibility criteria and were included in the review and are summarised in Table 1.
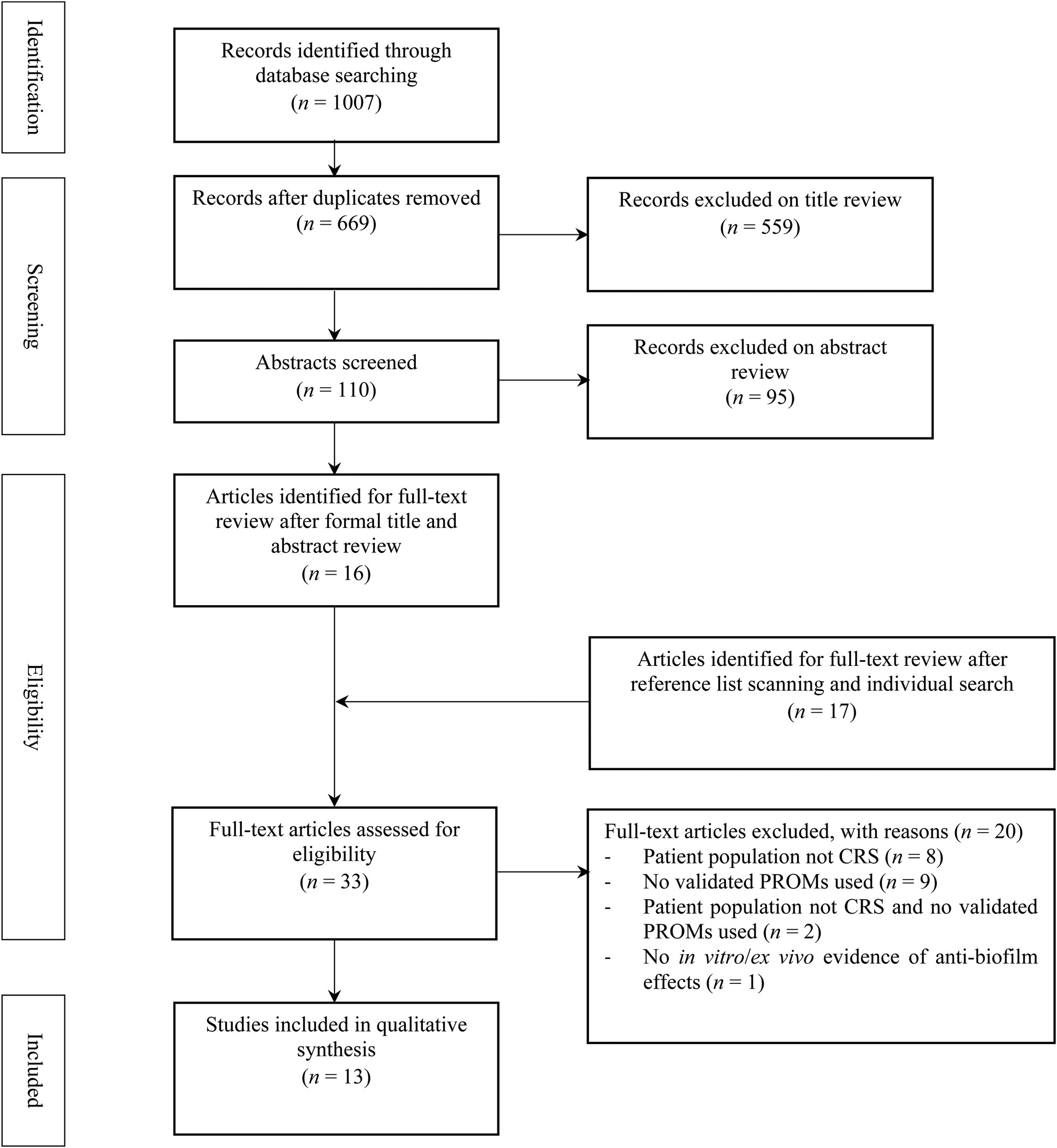
Fig. 1. Flow diagram of study selection. CRS = chronic rhinosinusitis; PROM = patient reported outcome measure
Table 1. Summary of included studies

PROM = patient reported outcome measure; CRS = chronic rhinosinusitis; RCT = randomised controlled trial; SNOT = Sino-Nasal Outcome Test; EPOS = European Position Paper on Rhinosinusitis and Nasal Polyps; ESS = endoscopic sinus surgery; AAO–HNSF = American Academy of Otolaryngology–Head and Neck Surgery; CCPG = Canadian Clinical Practice Guidelines; RSOM = rhinosinusitis outcome measure; RTF = rhinosinusitis task force
Study characteristics
Participants
There was a total of 469 participants, with 236 receiving the intervention regime, 193 receiving the comparator and 40 receiving both. Sample sizes ranged from 9 to 122 participants. A total of 455 (97.0 per cent) of the included participants had undergone sinonasal surgery prior to the use of their anti-biofilm therapy. Of the 13 studies included, a total of 10 identified the chronic rhinosinusitis diagnostic criteria used to evaluate patients for inclusion.
Interventions
The 13 included studies identified 7 different topical anti-biofilm specific therapies. Six were intranasal preparations and one was transcutaneous (pulsed ultrasound). Intranasal therapies were administered by a combination of sinonasal irrigation (9 of 12), nasal spray (2 of 12) and intranasal nebuliser (1 of 12). Durations of treatments ranged from 10 days to 7 weeks, while follow-up periods ranged from 10 days to 3 months. The regimes of the interventions used are displayed in Table 1.
Comparators
A comparator arm was present in 10 of 13 included trials. A total of 8 of 10 trials used saline or water in an identical delivery method as comparator, while 2 of 10 used saline or water plus culture directed oral antibiotics. Three studies had no comparator arm.
Outcomes
Three different validated patient-reported outcome measures were used in the eligible studies. There was a high degree of homogeneity in the outcome measures utilised with the Sino-Nasal Outcome Test (SNOT)-22 being the most common (used in 11 of 13 studies), followed by SNOT-20 (2 of 13) and Rhinosinusitis Outcome Measure-31 (1 of 13). In each of these scoring systems, a negative score change represents an improvement, with the more negative the value, the greater the improvement.
Studies
Of the 13 included studies, 9 were randomised controlled trials (including 1 crossover), 3 were clinical trials and 1 was a pilot study. Six of the 13 studies reported double-blinding in their protocols, 4 were single-blinded (clinician only) and 3 were unblinded trials.
Results of individual treatments
A summary of the outcomes of the included studies is found in Table 2. The proposed anti-biofilm mechanisms for each of the identified treatments as demonstrated in preclinical studies, is summarised in Table 3.
Table 2. Summary of outcomes of included studies

SNOT = Sino-Nasal Outcome Test; RSOM = rhinosinusitis outcome measure
Table 3. Summary of preclinical evidence for anti-biofilm therapies

CRS = chronic rhinosinusitis; S aureus = Staphylococcus aureus; P aeruginosa = Pseudomonas aeruginosa; S epidermidis = Staphylococcus epidermidis
Colloidal silver
Colloidal silver is a widely used colloid consisting of microscopic silver particles suspended in solution. Intranasal colloidal silver was evaluated in two studies which included a total of 44 patients.
Scott et al. Reference Scott, Krishnan, Rotenberg and Sowerby12 compared intranasal colloidal silver nasal spray to a saline control in a double-blind crossover randomised controlled trial. No significant difference was found in the change of SNOT-22 scores between both groups after a 6-week trial of therapy (colloidal silver, +1.0 vs saline, −2.8, p = 0.373).
Ooi et al. Reference Ooi, Richter, Bennett, Macias-Valle, Vreugde and Psaltis13 performed a single-blinded pilot study comparing colloidal silver sinonasal irrigation with saline irrigation and 10–14 days of culture-directed oral antibiotics. While treatment with colloidal silver showed a trend toward SNOT-22 score improvement after 10 days of twice daily washes, this change was not statistically significant (colloidal silver, −5.8 (95 per cent CI: −0.2 to +11.9) vs control, –0.6 (95 per cent CI: −6.7 to +5.40)).
Honey
Three studies reported the effect of intranasal honey-containing preparations on SNOT-22 scores.
In a clinician-only blinded randomised controlled trial of patients with recalcitrant chronic rhinosinusitis, Ooi et al. Reference Ooi, Jothin, Bennett, Ooi, Vreugde and Psaltis14 tested the efficacy of a twice-daily sinonasal rinse containing 16.5 per cent Manuka honey. The study showed no significant change between pre- and post-treatment scores in the honey group (−4.4 (95 per cent CI: −13.1 to 4.4)) or the saline control group (−6.3 (95 per cent CI: −13.5 to 0.8)). Furthermore, neither arm was found to be statistically superior (control vs Manuka honey = −1.7 (95 per cent CI: −20 to 16.6); p = 0.85).
Lee et al. Reference Lee, Humphreys, Purcell and Davis15 demonstrated similar results for Manuka honey. They found that while 30 days of twice daily, Manuka honey-containing saline rinses did give a clinically significant improvement, this change was not different to that of saline control washes (control, −12 (95 per cent CI: −20 to −1) vs Manuka honey, −12.5 (95 per cent CI: −22 to −6); p = 0.57).
Hashemian et al. Reference Hashemian, Baghbanian, Majd, Rouini, Jahanshahi and Hashemian16 tested a 35 per cent thyme honey-containing nasal spray in a double-blind randomised controlled trial. Similarly to Lee et al.,Reference Lee, Humphreys, Purcell and Davis15 they found a significant improvement in SNOT-22 scores in the honey group (−27.8; p < 0.01) but this was not statistically different when compared with the saline control group (p = 0.86).
Baby shampoo
Farag et al. Reference Farag, Deal, McKinney, Thorp, Senior and Ebert17 reported a surgeon-only, blind randomised controlled trial of 40 patients with chronic rhinosinusitis, comparing 1 per cent baby shampoo sinonasal washes with hypertonic saline control rinses. All patients underwent functional endoscopic sinus surgery (FESS) as part of the trial and were treated with three-times daily washes for between one and seven weeks post-operatively. Although both groups showed improvements in the two validated quality of life scores used (p < 0.0001), no statistical difference was found between patients treated with baby shampoo versus hypertonic saline irrigations with either of the patient reported outcome measures (SNOT-22, p = 0.09; Rhinosinusitis Outcome Measure-31, p = 0.5).
In a non-controlled, non-randomised trial, Chiu et al.Reference Chiu, Palmer, Woodworth, Doghramji, Cohen and Prince18 administered 1 per cent baby shampoo sinonasal irrigations to 15 chronic rhinosinusitis patients who were symptomatic despite optimal medical therapy and previous sinus surgery. The patients’ SNOT-22 scores were measured before and after treatment. An average improvement of −11.1 was noted across all participants; however, a decrease in score was found in only 7 of 15 patients. Furthermore, 10 of 15 participants received concomitant antibiotics, and 2 of 15 were on oral prednisone during the trial period.
Xylitol
Xylitol is a naturally occurring sugar alcohol, found in many fruits and vegetables and widely used as a sugar additive or sweetener in the food industry.Reference Jain, Lee, Hardcastle, Biswas, Radcliff and Douglas19
Lin et al. Reference Lin, Tang, Wei, Dai and Sun20 performed a double blind randomised controlled trial in which daily sinonasal washes with 5 per cent weight per volume xylitol were compared with a saline control. After 30 days, daily washes with xylitol showed a significant improvement in SNOT-22 scores (p < 0.001) while no change was noted in the saline control group. The mean SNOT-22 score change for each cohort was not reported.
Weissman et al. Reference Weissman, Fernandez and Hwang21 studied the same preparation of xylitol sinonasal washes used twice daily for 10 days in a crossover randomised controlled trial. A significant reduction in SNOT-20 scores was noted during the xylitol phase of the trial (mean SNOT-20 change: −2.43), as opposed to a worsening of symptoms during the saline control phase (mean SNOT-20 change: +3.93). The difference in treatment effect was statistically significant (p = 0.04).
Bacteriophage cocktail
Bacteriophages are naturally occurring viruses which infect and lyse narrow families of bacteria with high specificity.Reference Petrovic Fabijan, Lin, Ho, Maddocks, Ben Zakour and Iredell22
In a phase 1, first-in-humans, open-label clinical trial, Ooi et al. Reference Ooi, Drilling, Morales, Fong, Moraitis and Macias-Valle23 investigated the use of a bacteriophage cocktail on patients with recalcitrant chronic rhinosinusitis due to Staphylococcus aureus. Three cohorts (3 patients per cohort) received serial doses of twice-daily sinonasal irrigations of a mixture of 3 natural lytic phages belonging to the myoviridae family. The treatment regime was administered for either 7 or 14 days, depending on the cohort. Two cohorts showed a mean improvement in SNOT-22 scores pre- and post-treatment (8.4, −10) while the third slowed a slight deterioration (+1.3). No control arm was utilised.
Sodium hyaluronate
Hyaluronic acid and its sodium salt, sodium hyaluronate are natural polysaccharides abundant in skin and connective tissues.Reference Marcuzzo, Tofanelli, Boscolo Nata, Gatto and Tirelli24 Two double-blind randomised controlled trials evaluated the use of sodium hyaluronate washes after FESS.
Mozzanica et al. Reference Mozzanica, Preti, Gera, Bulgheroni, Cardella and Albera25 performed a double-blind randomised controlled trial of 56 patients post-FESS who were randomised to either normal saline sinonasal washes or sodium hyaluronate containing saline washes. Washes were performed twice daily for six weeks post-operatively. Although an improvement in mean SNOT-22 scores was noted at three and six weeks post-operatively in both cohorts, there was no significant difference between the sodium hyaluronate group and the control group at three weeks (p = 0.933) or six weeks (p = 0.175).
Cantone et al. Reference Cantone, Castagna, Sicignano, Ferranti, Rega and Di Rubbo26 performed a double-blind randomised controlled trial of 122 chronic rhinosinusitis patients post-FESS. Participants in the investigational arm used an intranasal administration of sodium hyaluronate prepared via a nebuliser ampoule for nasal douche. After 60 days of twice daily treatment, mean SNOT-22 scores changes were better in the sodium hyaluronate group compared with a saline control (sodium hyaluronate, −20.2 vs control, −7.7, p < 0.05).
Transcutaneous ultrasound
The effect of transcutaneous ultrasound was investigated in one study. In a small, non-controlled trial, Young et al. Reference Young, Morton and Bartley27 administered pulsed ultrasound to the skin over the maxillary and frontal sinuses in chronic rhinosinusitis patients for 9 minute sessions performed 2 to 3 times per week. On average, SNOT-20 scores were found to improve by 24.0 per cent after 3 sessions (p < 0.0001) and 34.1 per cent after 6 sessions (p < 0.0001).
Quality assessment
Results of the quality assessment measure for each of the included studies are included in Table 2. The mean summary score as calculated by the Standard Quality Assessment Criteria for Evaluating Papers from a Variety of FieldsReference Kmet, Lee and Cook11 was 0.78 (range, 0.46–0.96).
Meta-analysis
Due to the small number of included trials and the clinical and methodological heterogeneity of these studies, a meta-analysis was not performed.
Discussion
Chronic rhinosinusitis is most appropriately regarded as a multifactorial chronic inflammatory disorder. The prevailing, but unproven, pathophysiological hypothesis is that dysfunctional interactions at the mucosal surface drive multiple interacting inflammatory mechanisms resulting in variable patterns of tissue inflammation and clinical phenotype.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma9
The role of biofilms in chronic rhinosinusitis disease evolution is unclear, and identifying causality in the bacterial contribution to sinonasal homeostasis and chronic rhinosinusitis remains a challenge. Modern molecular microbiome data lend support for the ‘dysbiosis hypothesis’Reference Knight, Vrbanac, Taylor, Aksenov, Callewaert and Debelius28 in chronic rhinosinusitis in which the collective sinonasal microbiome becomes deranged, with an abundance of opportunistic pathogens and depletion of commensal organisms.Reference Rom, Bassiouni, Eykman, Liu, Paramasivan and Alvarado7,Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma9
A number of mechanisms have been proposed to explain the pathogenic role of bacterial biofilms in chronic rhinosinusitis. It has been suggested that biofilms provide a highly organised and robust superstructure in which bacteria are shielded from host defences and conventional antibiotics. In this way, they are able to reduce their metabolic rates and curb their propensity for antibiotic susceptibility and potentially prolong the sinonasal mucosal inflammatory response.Reference Lam, Schleimer and Kern29 Furthermore, biofilms downregulate antibacterial peptides in the nasal mucosa resulting in destruction of nasal mucosa and impaired mucociliary clearance.Reference Fastenberg, Hsueh, Mustafa, Akbar and Abuzeid30 However, conversely, other findings suggest that biofilms do not precede an abnormal inflammatory response, but rather arise as a secondary effect of the antecedent inflammatory milieu in chronic rhinosinusitis in which chronic mucociliary dysfunction and a static mucous blanket are present.Reference Lee and Lane31
The question remains as to whether an anti-biofilm centric strategy reduces the burden of disease. In this study, a sizeable and varied array of anti-biofilm specific topical therapies were identified. Although some treatments showed improved outcomes, no therapy was identified as confidently efficacious beyond placebo. Of the seven treatments identified, intranasal xylitol was the only one to show a statistically significant reduction in symptom scores compared with placebo in more than one trial.Reference Lin, Tang, Wei, Dai and Sun20,Reference Weissman, Fernandez and Hwang21 This result is reflected in current treatment guidelines which suggest consideration of xylitol washes in some subsets of recalcitrant disease.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma9 Despite this, the SNOT-22 changes in studies of xylitol, and indeed most of the other therapies, were below the minimal clinically important difference of 12 points,Reference Phillips, Hoehle, Caradonna, Gray and Sedaghat32 suggesting a weak therapeutic effect where it was shown. Conflicting treatment effects were shown in some therapies (bacteriophage cocktail) while others showed no significant benefit compared with placebo (colloidal silver, baby shampoo and honey). Some of these negative results may be attributed to selection bias with the majority of eligible patients having undergone prior sinonasal surgery (97 per cent), likely selecting for a more severe disease population who were less likely to show large changes in response to adjuvant therapies.
Studies have shown that biofilms represent either markers or drivers of severe chronic rhinosinusitis, and it stands to reason, at least from first principles, that eliminating them is likely to be beneficial. The results from this study appear to disagree with this hypothesis. In a sense this is not surprising as the approach to treat chronic rhinosinusitis by targeting presumed aetiological factors is at odds with the observation that it is typically an adult-onset disorder with a wide spectrum of disease presentations. Illnesses of these sorts have a long pre-morbid period, allowing ample lead time for complex host–environment interactions to play out with high variability. The 2020 European Position Paper on Rhinosinusitis and Nasal PolypsReference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma9 has moved away from a diagnostic paradigm identifying the presence or absence of nasal polyposis and towards one based on whether the disease is ‘primary’ or ‘secondary’. In primary chronic rhinosinusitis, the disease is further divided by endotype dominance, either type 2 (T-helper cell associated disease) or non-type 2 disease. Indeed, the most promising emerging therapies for chronic rhinosinusitis are framed by this endotype approach and implicitly appreciate the complex, interweaved and potentially unseen driving factors of chronic rhinosinusitis. These therapies, most notably monoclonal antibodies such as dupilumab, dampen the downstream inappropriate sinonasal tissue response through suppression of specific mediators in the type 2 inflammatory pathway and have shown very promising results.Reference Bachert, Mannent, Naclerio, Mullol, Ferguson and Gevaert33,Reference Bachert, Han, Desrosiers, Hellings, Amin and Lee34
This review is limited by a number of factors. Follow-up times varied significantly between trials with some patients receiving only two weeks of treatment, which is significantly shorter than the current post-operative standard of care. Furthermore, there were few numbers of trials identified per treatment, and population size was generally small. As such, the durability of improved symptom scores is not clear. Finally, the concomitant use of oral antibiotics and intranasal and oral corticosteroids may have confounded true treatment effects in those studies in which they were present.
Future research may best be undertaken within the diagnostic paradigm devised by the European Position Paper on Rhinosinusitis and Nasal Polyps 2020. In addition to more robust trials, future studies may stratify patients by endotype to identify if any are disproportionately affected by high biofilm burden and respond more favourably to anti-biofilm therapies. Furthermore, anti-biofilm therapies may act synergistically when used in conjunction with other medical therapies (antibiotic, steroid, biological or otherwise), and exploring these effects may be a fruitful avenue for research.
Conclusion
Robust evidence supporting the use of various anti-biofilm therapies in chronic rhinosinusitis is lacking. Further high quality, human, in vivo trials studying the effect of anti-biofilm therapies in chronic rhinosinusitis are needed to address the deficiencies of the current evidence base.
Competing interests
None declared
Appendix 1. Medical Subject Headings used in search strategy







