Introduction
Cognitive alterations are a central and heterogeneous trait in psychotic disorders (Kahn & Keefe, Reference Kahn and Keefe2013; Keri & Janka, Reference Keri and Janka2004) related to both their development and outcome (Bora & Murray, Reference Bora and Murray2014; Dickson, Laurens, Cullen, & Hodgins, Reference Dickson, Laurens, Cullen and Hodgins2012; Khandaker, Barnett, White, & Jones, Reference Khandaker, Barnett, White and Jones2011; Reichenberg et al., Reference Reichenberg, Weiser, Rapp, Rabinowitz, Caspi, Schmeidler and Davidson2005). Severity of cognitive alterations varies widely between patients, and several domains may be affected, including verbal learning, working memory and processing speed (Islam et al., Reference Islam, Habtewold, van Es, Quee, van den Heuvel, Alizadeh and Bruggeman2018; Keri & Janka, Reference Keri and Janka2004; Nuechterlein et al., Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004; Palmer, Dawes, & Heaton, Reference Palmer, Dawes and Heaton2009).
Both familial (i.e. shared genetic and environmental) vulnerability and patient-specific factors drive cognitive deficits in psychotic disorders. First-degree relatives of patients with psychosis show variable degrees of cognitive alterations, which may be traced back to shared familial risk (Islam et al., Reference Islam, Habtewold, van Es, Quee, van den Heuvel, Alizadeh and Bruggeman2018; Meijer, Simons, Quee, & Verweij, Reference Meijer, Simons, Quee and Verweij2012; Mucci et al., Reference Mucci, Galderisi, Green, Nuechterlein, Rucci, Gibertoni and Maj2018; Szöke et al., Reference Szöke, SchÜRhoff, Mathieu, Meary, Ionescu and Leboyer2005).
To disentangle effects of familial cognitive vulnerability on outcome from patient-specific vulnerability and illness-related cognitive alterations in psychosis, cognitive functioning of siblings unaffected by a psychotic disorder may be used as a proxy measure. Put differently, a cross-sibling design may be used to study associations between siblings cross-trait (e.g. the association between cognition in unaffected siblings and symptoms in their probands affected by psychosis) (Lataster, Collip, Lardinois, van Os, & Myin-Germeys, Reference Lataster, Collip, Lardinois, van Os and Myin-Germeys2010). The cognitive phenotypes under study (i.e. familial cognitive vulnerability as expressed by sibling cognition) are derived from a mixture of genetic and environmental risks (Gottesman & Gould, Reference Gottesman and Gould2003).
A recent study in unaffected siblings of psychotic disorder patients found three sibling cognitive subtypes in a cluster analysis to model familial cognitive risk (Quee, Alizadeh, Aleman, & van den Heuvel, Reference Quee, Alizadeh, Aleman and van den Heuvel2014). In the ‘normal’ subtype siblings, cognitive performance was comparable to healthy controls, and significantly better than their probands on all measures. ‘Mixed’ subtype siblings had impaired performance on episodic memory and performance comparable to healthy controls on the other measures. Siblings with an ‘impaired’ cognitive subtype performed significantly worse than healthy controls and comparable to their affected proband on all measures. Notably, sibling cognitive subtypes were associated with distinguishable profiles in affected probands: patients with a sibling of the ‘impaired’ cognitive subtype performed significantly worse on cognitive tests than probands of siblings of the other two subtypes. Additionally, they had an earlier onset of psychosis and were less likely to be in remission in a cross-sectional analysis. These results suggest that the more cognitive impaired the healthy sibling is, the worse the illness outcome in their affected relative is.
Beyond cross-sectional associations, familial cognitive risk may be an important marker of symptomatic course of patients with schizophrenia spectrum disorders. Familial cognitive risk as assessed in healthy siblings may indicate risk of poor long-term outcome in patients. To evaluate the impact of familial cognitive vulnerability on symptomatic outcome of psychosis, longitudinal evidence is needed.
In the current study, we use a cross-sibling, cross-trait approach and investigate the prognostic validity of familial cognitive subtypes for long-term symptomatic outcomes of psychosis. We investigated multi-cross-sectional associations between the cognitive phenotype of unaffected siblings and repeated measures of symptomatic outcomes in their proband affected by psychosis. Additionally, we investigated whether symptomatic course over time differs between familial cognitive subtypes.
We hypothesized that patients with non-affective psychosis at high familial cognitive risk (i.e. probands with a sibling with an ‘impaired’ cognitive subtype) would be more likely to have an unfavorable symptomatic outcome across repeated measures over a 6-year period. We expected that the effect of sibling cognitive subtype would be most profound on symptomatic dimensions conceptually close to cognition, i.e. disorganization (Feigenson, Gara, Roche, & Silverstein, Reference Feigenson, Gara, Roche and Silverstein2014; Ventura, Thames, Wood, Guzik, & Hellemann, Reference Ventura, Thames, Wood, Guzik and Hellemann2010). Second, we expected that patients at high familial cognitive risk show less long-term symptomatic improvement over time (i.e. at 3- and 6-year follow-up) compared to other patients.
Methods
Study design
The Genetic Risk and Outcome of Psychosis (GROUP) study is a longitudinal multicenter cohort study of 1119 patients with a non-affective psychotic disorder, 1059 non-affected siblings and 586 unrelated healthy control subjects in the Netherlands and Belgium, described elsewhere in detail (Korver, Quee, Boos, Simons, & de Haan, Reference Korver, Quee, Boos, Simons and de Haan2012). In short, in- and out-patients consecutively presenting at selected representative mental health services in representative geographical areas (four university medical centers and 36 associated mental healthcare institutions) and their unaffected siblings were recruited for the study between 8 January 2004 and 6 February 2008. Inclusion criteria were: (1) a diagnosis of non-affective psychotic disorder according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria; (2) age 16–50 years (extremes included); (3) Dutch language proficiency and (4) ability to provide informed consent. Siblings were recruited through their proband based on aforementioned criteria 2–4 and the absence of a lifetime psychotic disorder. Data were collected at baseline and follow-up at 3 and 6 years by trained investigators. In the current study, a sample of patients representative for the full GROUP cohort with a sibling that has been assigned a cognitive subtype by Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014) was selected (n = 629, see online Supplementary flow chart). The study protocol was approved by the Medical Ethics Committee of the Academic Medical Center in Utrecht and subsequently by those of the other participating centers. All participants provided written informed consent. Release 7.0 of the GROUP database was used in all analyses.
Symptomatic outcome
Symptom severity was assessed at baseline, and at 3- and 6-year follow-up with the Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987). Symptomatic remission was defined according to the Remission in Schizophrenia Working Group (RSWG) criteria, with scores of 3 or less on specific PANSS items (Andreasen et al., Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger2005). The PANSS five-factor structure according to van der Gaag et al. (Reference van der Gaag, Hoffman, Remijsen, Hijman, de Haan, van Meijel and Wiersma2006) was used to obtain dimensional symptomatic outcomes, including positive symptoms (items; score range: 9; 1–55), negative symptoms (10; 2–62), disorganization (10; 10–70), excitement (8; 8–56) and emotional distress (8; 8–56). This five-factor structure of the PANSS has been shown to provide a better fitting representation of the symptom structure in schizophrenia than its original three-factor structure in confirmatory factor analysis (Stefanovics, Elkis, Zhening, Zhang, & Rosenheck, Reference Stefanovics, Elkis, Zhening, Zhang and Rosenheck2014).
Sibling cognitive subtype
Cognitive performance of unaffected siblings was expressed by three distinct sibling cognitive profiles (‘normal’, ‘mixed’ and ‘impaired’) identified by Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014) in a cluster analysis on baseline neurocognitive performance. Analyses were based on cognitive domains known to be impaired in schizophrenia and comparable to those included in the MATRICS consensus battery (Meijer et al., Reference Meijer, Simons, Quee and Verweij2012; Nuechterlein et al., Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004). The following tests were included: total scores and intra-individual variability scores from the continuous performance test (CPT-HQ), assessing attention and vigilance (Nuechterlein & Dawson, Reference Nuechterlein and Dawson1984), ‘block design’, ‘digit symbol’, ‘arithmetic’ and ‘information’ subtest scores from the Wechsler Adult Intelligence Scale-III (WAIS-III), assessing perceptual reasoning, processing speed and verbal comprehension subdomains of intellectual and executive functioning (Blyler, Gold, Iannone, & Buchanan, Reference Blyler, Gold, Iannone and Buchanan2000), and ‘immediate recall’ and ‘delayed recall’ scores from the Word Learning Task (WLT) (Brand & Jolles, Reference Brand and Jolles1985), measuring episodic memory. A detailed description of the cluster analysis method to identify the sibling cognitive profiles is provided in Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014). In short, test scores were first standardized to obtain a z-score per test for each individual. Then, hierarchical cluster analysis with the objective approach of Duda & Hart was used to evaluate the number of clusters (three in this case) and to provide starting values for subsequent k-means clustering using the Ward method (Duda & Hart, Reference Duda and Hart1973; Everitt, Reference Everitt2011). This clustering method was then employed to partition the cases into three clusters based on the scores on the aforementioned cognitive tests. The resulting sibling cognitive profiles per subtype (normal, mixed and impaired), described in the introduction are illustrated in online Supplementary Fig. S1 [reprint from Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014) with permission].
Covariates
Potential confounders were selected a priori based on their associations with cognition and symptomatic outcome barring covariates presumed to lie within the causal pathway between familial cognitive risk and clinical outcome, such as patient cognition or educational attainment. This prevents underestimation of the association of interest between familial cognitive risk and symptomatic outcome by overadjustment bias (Ananth & Schisterman, Reference Ananth and Schisterman2017; Schisterman, Cole, & Platt, Reference Schisterman, Cole and Platt2009). Apart from age and sex, we also included ethnicity, cannabis use and antipsychotics use, based on their association with both cognition and symptomatic outcome (Lambert, Karow, Leucht, Schimmelmann, & Naber, Reference Lambert, Karow, Leucht, Schimmelmann and Naber2010; Omachi & Sumiyoshi, Reference Omachi and Sumiyoshi2018; Stouten, Veling, van der Helm, Laan, & van der Gaag, Reference Stouten, Veling, van der Helm, Laan and van der Gaag2013; Volkow et al., Reference Volkow, Swanson, Evins, DeLisi, Meier, Gonzalez and Baler2016). Ethnicity was dichotomized (white/non-white). Cannabis use (yes/no) was assessed at each measurement with urinalysis, with a 50 ng/l urine cannabis concentration cut-off. Antipsychotics use (yes/no/unknown) was assessed at each measurement.
Statistical analyses
We compared baseline data of patients grouped by sibling cognitive subtype on demographic characteristics and symptomatic outcomes with analysis of variance and χ2 tests, and baseline data of patients with complete data with those lost to follow-up with t tests and χ2 tests. We visualized symptomatic outcome trajectories of patients grouped by sibling cognitive subtype in line-plots for dichotomous symptomatic outcome (remission) and the five PANSS symptom dimensions (Fig. 1).

Fig. 1. Symptomatic trajectories on PANSS dimensions of patients grouped by sibling cognitive subtype. Panels (A) symptomatic remission; (B) positive symptoms; (C) negative symptoms; (D) disorganization; (E) excitement and (F) emotional distress. X axis: time (baseline; 3-year follow-up; 6-year follow-up). Y axis: mean PANSS dimensional score (A–E); proportion in symptomatic remission (F); whiskers denote 95% CIs adjusted for within-subject variability by period according to Morey (Reference Morey2008). Cognitive subtype – lines/points; normal subtype – solid/circle; mixed subtype – dotted/triangle; impaired subtype – two dash/box.
We assessed multi-cross-sectional associations between the sibling cognitive subtype as defined by Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014) at baseline and repeated measures of symptomatic outcomes of probands at baseline, 3- and 6-year follow-up. We corrected for selected covariates, using (generalized) linear mixed effects implemented in the lme4 package in R version 3.5.2 (Bates, Machler, Bolker, & Walker, Reference Bates, Machler, Bolker and Walker2015; R Core Team, 2016), with a logit link-function for the dichotomous outcome of symptomatic remission. We added random intercepts for subjects and by-subject random slopes for the effect of time. Time, age, sex, ethnicity, cannabis use and antipsychotics use were added en bloc as fixed effects in the model, followed by sibling cognitive subtype. PANSS dimensional models were fitted with restricted maximum likelihood under the assumption of data missing at random, which we tried to make tenable by adding the aforementioned set of covariates, and by running a sensitivity analysis on complete cases. For our second hypothesis regarding the moderating effect of time on the relationship between familial cognitive profile and symptomatic outcome, a time × sibling cognitive subtype interaction term was added to the models. In case model terms suggested interaction effects between time and a sibling cognitive subtype, as expressed by an interaction term with p < 0.10, we explored post-hoc differences between subtypes per measurement. We report pairwise comparisons between sibling cognitive subtypes using estimated marginal means of symptomatic outcomes. To assess model fit, we used likelihood-ratio tests (online Supplementary Table S2). For symptomatic remission, we provide p values based on Wald Z-statistics. To minimize type-I error, p value computation of model variables on symptom dimensions follow the Kenward–Roger approach (Luke, Reference Luke2017), and Tukey's method for post-hoc pairwise comparisons.
Results
Sample characteristics
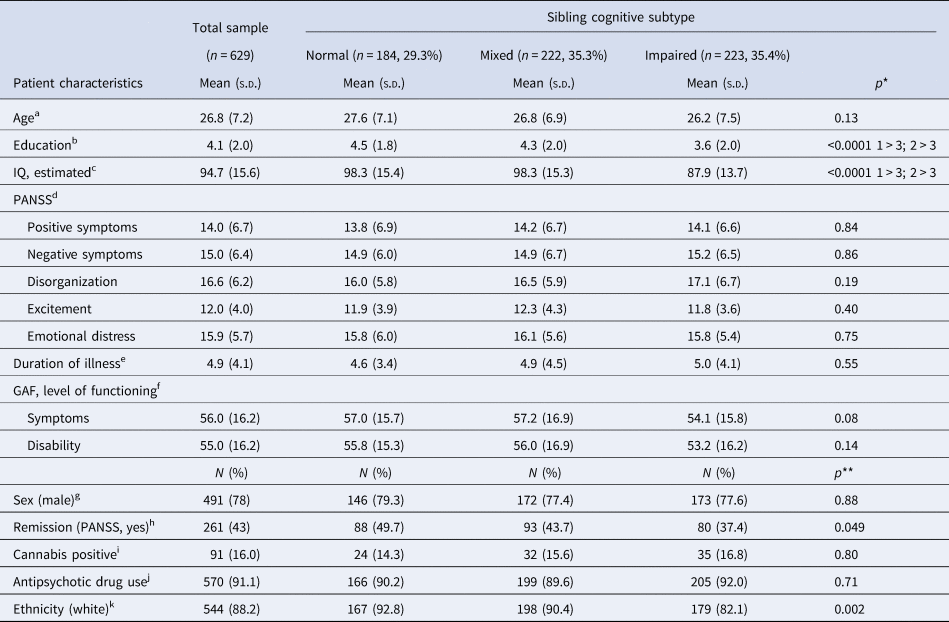
Baseline characteristics of the study sample (n = 629) are listed in Table 1. Patients from the three sibling subtypes significantly differed by mean IQ, education level, ethnicity and symptomatic remission. The distribution of patients lost to follow-up at 3 and/or 6 years (n = 253 in total, n = 144 at 3 years, n = 225 at 6 years) did not significantly differ between the sibling cognitive subtypes. Overall, dropouts were less likely to be of a white ethnicity and did have higher baseline symptom levels, and a lower level of general functioning, education and IQ (online Supplementary Table S1), indicating that the most severely affected patients were lost to follow-up. Missing data on selected covariates and outcomes at all time points resulted in 596–602 cases available for analyses depending on the outcome of interest (online Supplementary flow chart). Proband symptoms improved in all sibling cognitive subtypes from baseline to 3-year follow-up, and remained relatively stable between 3 and 6 years (Fig. 1).
Table 1. Baseline characteristics of all patients and patient characteristics per sibling cognitive profile
s.d., standard deviation; PANSS, Positive and Negative Syndrome Scale; Cigs/day, number of cigarettes per day; CAPE, community assessment of psychic experiences; GAF, global assessment of functioning; WHO-QoL, World Health Organization-Quality of Life.
a Data were missing for one patient.
b Range, 0 = no education; 1–7 = primary school – B.Sc.; 8 = M.Sc. and above. Data were missing for five patients.
c Estimated IQ based on WAIS-III subscales at baseline; missing for 13 patients.
d Five-factor structure of the PANSS, denoting five symptomatic dimensions: positive symptoms (1–55), the negative symptoms (2–62); disorganization (10–70); excitement (8–56) emotional distress (8–56). PANSS baseline data on separate dimensions were missing for 40 patients or less.
e Data were missing for 84 patients.
f Data were missing for 119 patients.
g No data were missing.
h Cross-sectional remission (without time criterion). Data were missing for 25 patients at baseline.
i Data were missing for 48 patients at baseline.
j Antipsychotic drug use unknown for 57 patients at baseline.
k Data were missing for 12 patients at baseline.
*Two-sided p values were computed by a one-way ANOVA using type-I sums of squares, with pairwise comparisons using Tukey's test executed in case p < 0.05.
**Two-sided p values were computed by a Pearson's χ2 test.
Multi-cross-sectional associations between sibling cognitive subtype and symptomatic outcome
The odds to be in symptomatic remission for patients with a sibling of the impaired subtype were 2.8 times smaller [i.e. the inverse of the reported odds ratio (OR) of 0.36 in Table 2], relative to a patient with a sibling of the normal cognitive subtype. We found no significant difference in symptomatic remission between patients with siblings of a normal and a mixed cognitive subtype. Regarding the multi-cross-sectional associations between the sibling cognitive subtype and dimensional symptomatic outcomes in patients (Table 2), we found a significant association between the impaired cognitive subtype and the disorganization dimension of the PANSS. No other PANSS dimensions were significantly associated with sibling cognitive profile, but we observed a non-significant tendency for patients with a sibling with impaired cognitive subtype to score higher on these symptom dimensions compared to the other subtypes. The standard errors of the sibling cognitive subtype effect estimates showed substantial within-group variability.
Table 2. Results of linear mixed models regarding the multi-cross-sectional association between sibling cognitive subtype and patient symptom severitya

PANSS, Positive and Negative Syndrome Scale.
n = number of cases included in analysis.
a The following fixed effects were entered to the models: sibling subtype, time, age, gender, cannabis use, antipsychotics use and ethnicity. As random effects, intercepts for subjects were added and by-subject random slopes for the effect of time. Baseline was the reference value for time. Normal sibling cognitive profile was the reference value for the sibling cognitive profile.
b Using Wald-Z statistic for remission and calculated with the Kenward–Roger approach for continuous outcomes.
c Estimates on response scale, ORs between brackets.
d Model fitted with Nelder–Mead optimizer.
A sensitivity analysis on complete cases (n = 372) showed comparable estimates for the effect of sibling cognitive subtype on all outcomes, indicating the validity of the assumption of data missing at random. In this analysis, both the effect of the impaired sibling cognitive subtype on symptomatic remission [estimate: −0.85, OR 0.43, 95% confidence interval (CI) 0.25–0.74], and PANSS disorganization (estimate = 1.44, s.e. = 0.52, p = 0.006) remained statistically significant. The trend of symptomatic improvement between baseline and 3-year follow-up remained visible in the complete case analysis, albeit attenuated.
Longitudinal associations between sibling cognitive subtype and symptomatic outcome
We explored the effect of time on the relationship between sibling cognitive subtype and symptomatic outcome in their probands suggested by our data (Fig. 1). Models with time × sibling cognitive subtype interaction effects indicated that differences on both symptomatic remission and dimensional outcomes between the impaired and other sibling cognitive subtypes emerged over time (Table 3). The standard errors of the sibling cognitive subtype effect estimates showed substantial within-group variability. In post-hoc comparisons between the impaired and other two sibling cognitive subtypes (Table 4, see online Supplementary Table S3 for estimated marginal means based on the models), patients with a sibling of the impaired cognitive subtype showed significantly less remission at baseline than those with a normal sibling cognitive subtype, and this difference became more pronounced at 3- and 6-year follow-up, compared to baseline. In the last two measurements, patients with a sibling of the impaired subtype were also significantly less likely to be in remission than patients with a sibling of the mixed cognitive subtype. On dimensional outcomes, the largest between-group differences were found for disorganization, with significantly less decrease in symptom severity in patients with a sibling of the impaired subtype, compared to patients with a sibling of the normal cognitive subtype at 3 years. At 6-year follow-up, this difference became more pronounced, and the difference between probands of the mixed and impaired sibling cognitive subtype also reached statistical significance. Lastly, pairwise comparisons showed that at 6-year follow-up, patients with a sibling of the impaired cognitive subtype showed less decrease in positive symptoms, negative symptoms and excitement compared to the normal cognitive subtype group.
Table 3. Results of linear mixed models regarding longitudinal associations between sibling cognitive subtype and patient symptom severitya

PANSS, Positive and Negative Syndrome Scale.
n = number of cases included in analysis.
a The following fixed effects were entered to the models: sibling subtype, time, age, gender, cannabis use, antipsychotics use and ethnicity. As random effects, intercepts for subjects were added and by-subject random slopes for the effect of time.
b Calculated with the Kenward–Roger approach for continuous outcomes.
c Model fitted with Nelder–Meads optimizer.
Table 4. Post-hoc tests of between group differences based on estimated marginal means of (generalized) linear mixed models regarding longitudinal associations between sibling cognitive subtype and patient symptom severitya

PANSS, Positive and Negative Syndrome Scale; Estimate, difference between estimated marginal means.
a Results are averaged over the levels of gender, cannabis use, antipsychotics use and ethnicity with proportional weights.
b p values adjusted by the Tukey's method for comparing a family of three estimates.
c Values back-transformed to ORs from the logit scale; tests performed on the log OR scale.
Discussion
As hypothesized, probands affected by psychosis at high familial cognitive risk (modeled by the impaired sibling cognitive subtype) were almost three times less likely to be in symptomatic remission across all time points in multi-cross-sectional analyses. Ondimensional outcomes, high familial cognitive risk was associated with more disorganization. Regarding long-term symptomatic improvement over time, as expected, patients at high familial cognitive risk showed less symptomatic remission compared to the mixed and normal cognitive subtype at 3- and 6-year follow-up. Accordingly, they showed less improvement on dimensional outcomes, for which differences concerning disorganization were most substantial at 6-year follow-up. High familial cognitive risk negatively affected symptomatic improvement on the disorganization dimension most, which was to be expected as these are conceptually closest. However, differences also appeared on positive, negative and excitement dimensions, suggesting a small effect of familial cognitive risk on these dimensions over time. The results indicate two distinguishable patient profiles, comprised of the subgroup at high familial cognitive risk and the other two sibling cognitive subtypes respectively, as no differences emerged between the normal and mixed cognitive subgroups.
Notably, we observed a significant symptomatic improvement between baseline and 3-year follow-up. This may reflect the context in which the GROUP sample was collected, that is, when probands needed clinical or outpatient treatment. Some improvement is to be expected at follow-up after a fixed period. Dropout of the more severely affected patients may have strengthened the pattern observed.
Long-term effects of familial cognitive risk on outcome
The findings of this study support the prognostic validity of high familial cognitive risk for symptomatic outcomes. Moreover, findings indicate that high familial cognitive risk is especially associated with long-term outcome, as differences especially emerge at 6-year follow-up. This suggested trend over time has also been found in the association between high familial risk for psychosis and worse outcome, which also appears to be dependent on longer follow-up periods (Esterberg, Trotman, Holtzman, Compton, & Walker, Reference Esterberg, Trotman, Holtzman, Compton and Walker2010; Kakela et al., Reference Kakela, Panula, Oinas, Hirvonen, Jaaskelainen and Miettunen2014).
Our findings support the notion that differential familial cognitive loading may lead to distinguishable illness profiles, which has beenbased on cross-sectional studies, both inside and outside the GROUP framework (Bigdeli et al., Reference Bigdeli, Nuechterlein, Sugar, Subotnik, Kubarych, Neale and Asarnow2019; Quee et al., Reference Quee, Alizadeh, Aleman and van den Heuvel2014). To the best of our knowledge, no cross-sibling, cross-trait study investigated the longitudinal relationship between sibling cognition and symptomatic outcome in probands affected by psychosis. In line with our findings, within-trait, cross-sibling associations of cognition over time show covariation of cognition in patients affected by psychosis and unaffected siblings (Islam et al., Reference Islam, Habtewold, van Es, Quee, van den Heuvel, Alizadeh and Bruggeman2018), pointing at the familial nature of cognitive traits. Furthermore, cross-trait, within-sibling investigations showed that poor cognition and poor outcome are related within subjects, which has been well described in patients, and at a subclinical level, in unaffected siblings and persons at clinical high risk (Chang et al., Reference Chang, Ming Hui, Yan Wong, Wa Chan, Ming Lee and Hai Chen2013; Green, Kern, & Heaton, Reference Green, Kern and Heaton2004; Liu et al., Reference Liu, Wang, Jin, Lyu, Liu, Guo and Davis2019).
Of the separate symptomatic dimensions in our study, the relationship between familial cognitive risk and disorganization stood out. This is in line with earlier research in patient samples testing the association between cognition and symptomatic dimensions (Ventura et al., Reference Ventura, Thames, Wood, Guzik and Hellemann2010). Symptomatic dimensions of psychosis including disorganization appear as continua in the general population, where patients form the extremes (Compton, Chien, & Bollini, Reference Compton, Chien and Bollini2007; Feigenson et al., Reference Feigenson, Gara, Roche and Silverstein2014; Lataster, Verweij, & Viechtbauer, Reference Lataster, Verweij and Viechtbauer2014; van Os & Reininghaus, Reference van Os and Reininghaus2016). Taking from this perspective, disorganization is both a dimension of psychotic illness severity but also a characteristic co-varying with cognition in the general population.
Familial cognitive risk as an inseparable mix of genetic and environmental factors
Using cognitive functioning of siblings unaffected by a psychotic disorder as a proxy for familial vulnerability helps to separate shared genetic and environmental factors from patient specific factors. These include both non-shared genetic and environmental factors, such as early childhood infections (Khandaker et al., Reference Khandaker, Dalman, Kappelmann, Stochl, Dal, Kosidou and Karlsson2018), and illness-related cognitive alterations, including those associated with medication use (Omachi & Sumiyoshi, Reference Omachi and Sumiyoshi2018). These factors may lead to adverse outcomes in their own right. However, familial cognitive risk as expressed in our study by sibling cognitive subtypes still represents an inseparable mixture of familial genetic and environmental risk factors. This merits a discussion of potential pathways from familial cognitive risk to symptomatic outcomes. The effect of familial cognition on long-term outcome of psychosis may be mediated early, through neurodevelopmental patterns (Bora, Reference Bora2015), driven by shared genetic and environmental factors (Blokland et al., Reference Blokland, Mesholam-Gately, Toulopoulou, Del Re, Lam, DeLisi and Petryshen2017; Tucker-Drob, Briley, & Harden, Reference Tucker-Drob, Briley and Harden2013). Familial genetic variants explain part of the variance in adolescent cognitive development (Dickinson et al., Reference Dickinson, Zaidman, Giangrande, Eisenberg, Gregory and Berman2019). Shared environmental circumstances, including shared trauma, or family financial stability may also add to development of familial cognition (Gottschling et al., Reference Gottschling, Hahn, Beam, Spinath, Carroll and Turkheimer2019; Hakulinen, Webb, Pedersen, Agerbo, & Mok, Reference Hakulinen, Webb, Pedersen, Agerbo and Mok2020; Mansueto, Schruers, Cosci, & van Os, Reference Mansueto, Schruers, Cosci and van Os2019; Spano, Reference Spano2018). Cognition then may mediate the relationship between these exposures and symptomatic outcomes in psychosis, exerting the strongest effect on disorganization (Mansueto et al., Reference Mansueto, Schruers, Cosci and van Os2019).
Additionally, familial cognition may play a role later, through resilience (the capacity to recover from, and adapt to difficulties) of the familial support structure (Fu, Czajkowski, Rund, & Torgalsboen, Reference Fu, Czajkowski, Rund and Torgalsboen2017; Robinson, Woerner, McMeniman, Mendelowitz, & Bilder, Reference Robinson, Woerner, McMeniman, Mendelowitz and Bilder2004; Zahid & Ohaeri, Reference Zahid and Ohaeri2010). We speculate that high familial cognitive risk affects outcome in patients in two ways. First, through increased disorganization symptoms in patients and second, through lower overall familial functioning. This constellation of lower personal and contextual resources may lead to a higher risk of poor long-term outcome.
Based on the above, we suggest that interventions targeting exposures that influence cognition at the family level, such as family socio-economic status, may be beneficial (Hackman & Farah, Reference Hackman and Farah2009; Jongsma et al., Reference Jongsma, Gayer-Anderson, Lasalvia, Quattrone, Mule, Szoke and Kirkbride2018). Moreover, the reported longitudinal effect suggests that patients with psychosis at high familial risk may represent a subgroup with prolonged need for care.
It should be noted that our results indicate substantial within-group variability, as often is the case in research on heterogeneous and multifactorial illnesses. Future research may elucidate pathways from familial cognitive risk to outcome and substantiate aforementioned suggestions on intervention. This includes investigating differences in familial genetic profiles and in familial environmental exposures between patients with cognitively impaired v. unimpaired unaffected siblings.
Limitations
The GROUP sample is relatively high functioning and the most severely affected patients tended to be lost to follow-up. This may have led to underestimation of the effect of familial cognitive risk on outcome. By correcting for ethnicity, which is unevenly distributed among the sibling subgroups and among study completers v. dropouts, we tried to correct for this effect.
As touched upon earlier, cognitive alterations may have non-familial causes. Probands with a psychotic disorder may have endured childhood social adversities not shared with their sibling (Aas et al., Reference Aas, Dazzan, Mondelli, Melle, Murray and Pariante2014). As cognitive profiles of probands at low familial cognitive risk are significantly altered compared to their unaffected sibling, whereas cognitive profiles of probands at high familial cognitive risk are not, non-familial causes of cognitive alterations may especially be at play in patients at low familial cognitive risk, as argued by Quee et al. (Reference Quee, Alizadeh, Aleman and van den Heuvel2014). This may have attenuated outcome differences between patients at low or high familial cognitive risk.
Methodological limitations include the following. As mentioned in our Introduction, the sibling cognitive subtypes we used as a proxy for familial cognitive risk were constructed in a cluster analysis, which reduces the multidimensional concept of cognition to discrete subtypes. This inherently leads to information-loss. Using categorical variables for cognition moreover suggests a switch effect, whereas the level of cognition is thought to be described best as a continuous distribution (Caspi & Moffitt, Reference Caspi and Moffitt2018). We argue that at this expense, using discrete subtypes may add interpretability, especially when using cognition in a cross-trait, cross-sibling approach. Lastly, we employed a cross-sectional measure of sibling cognition, based on evidence from the GROUP cohort that cognitive trajectories in unaffected siblings are stable (Islam et al., Reference Islam, Habtewold, van Es, Quee, van den Heuvel, Alizadeh and Bruggeman2018).
Proband educational attainment was not entered in our analyses as a covariate, under the assumption that it lies on the causal pathway between familial cognitive vulnerability and proband symptomatic outcome. It may be used in future research to estimate the part of familial cognitive risk that is mediated through premorbid cognition in probands, by using it as a proxy for premorbid cognition (Ananth & Schisterman, Reference Ananth and Schisterman2017; Schisterman et al., Reference Schisterman, Cole and Platt2009). Caution is to be taken when using such a proxy in future research, as the relationship between educational attainment and cognition is complex and bidirectional: longer education tends to yield better cognitive abilities, and higher cognitive abilities yield higher education levels (Deary & Johnson, Reference Deary and Johnson2010). Furthermore, in psychosis research, using educational attainment as a proxy for premorbid cognition may induce bias because cognitive decline before transgression to psychosis may affect educational attainment (Bora & Murray, Reference Bora and Murray2014).
Familial cognitive risk as modeled in our study did not include social cognitive domains. Social cognition also shows familial patterns, and has been related to outcomes of psychosis (Eack et al., Reference Eack, Mermon, Montrose, Miewald, Gur, Gur and Keshavan2010; Fett & Maat, Reference Fett and Maat2013). Future research may focus on the long-term effect of high familial risk for social cognitive deficits. A relationship similar to our findings may well emerge, since social cognition covaries with neurocognition (McCleery et al., Reference McCleery, Green, Hellemann, Baade, Gold, Keefe and Nuechterlein2015). Furthermore, cross-sectional evidence suggests that the clearest effect on symptomatic outcome may also be found on the disorganization dimension (Fett & Maat, Reference Fett and Maat2013).
Conclusion
High familial cognitive vulnerability is a risk factor for worse symptomatic outcomes, especially in the longer term, and may be a marker of a distinct clinical vulnerability profile. The most important strength of the cross-trait, cross-sibling approach is that it separates familial cognitive vulnerability from illness-related cognitive alterations that may lead to adverse outcomes in their own right. Future research needs to elucidate pathways from high familial cognitive vulnerability to illness and, ultimately, to worse long-term outcomes. Cross-sibling pathways from higher levels of familial cognitive vulnerability to worse long-term outcomes may be informative in identifying cognition-related environmental and genetic risks that impact psychotic illness heterogeneity over time.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720000719.
Acknowledgement
We are grateful for the generosity of time and effort by the patients, their families and healthy subjects. Furthermore we would like to thank all research personnel involved in the GROUP project, in particular: Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen and Erna van ’t Hag.
Financial support
The infrastructure for the GROUP study is funded through the Geestkracht program of the Dutch Health Research Council (Zon-Mw, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly and Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia Psycho-medical Center, The Hague. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGzE, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Virenze riagg, Zuyderland GGZ, MET ggz, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal and Delta).







