Introduction
Osteogenesis imperfecta is a rare hereditary systemic disorder that affects connective tissue, and its prevalence is between 7 and 10 per 100,000 live births.Reference Van Dijk and Sillence1 Osteogenesis imperfecta mainly affects bone but also any tissue that contains collagen, and is predominantly characterised by low bone mineral density that results in bone fragility, recurring fractures, and bone deformation.Reference Van Dijk, Cobben and Kariminejad2, Reference Marini, Forlino and Bächinger3
Most cases of osteogenesis imperfecta are due to autosomal dominant inheritance of COL1A1 and COL1A2 gene mutations. These genes normally code for type I collagen chains.Reference Thiele, Cohrs and Flor4 However, recent studies have shown that osteogenesis imperfecta can also be caused by several other mutations in genes that are involved in collagen biosynthesis and osteoblast function, with dominant, recessive, or X-linked inheritance.Reference Marini, Forlino and Bächinger5
In the heart, its valves, tendinous cords, fibrous rings, and the interventricular septum all contain type I collagen, with approximately 75% of the mitral valve’s collagen being type I. Collagen fibres in the ventricular myocardium contribute both to its rigidity and to maintaining myocyte architecture.Reference Ashournia, Johansen, Folkestad, Diederichsen and Brixen6 The aorta and most arteries are also rich in collagen types III and I, and many cardiac problems are associated with collagen matrix accumulation, depletion, or restructuring, such as those caused by type III and V collagen alterations, fibrillin I alterations, and those responsible for the aortic diseases associated with Marfan syndrome and Ehlers–Danlos syndrome.Reference Ashournia, Johansen, Folkestad, Diederichsen and Brixen6–Reference Hernández, Saavedra, Teresa and Vela8
Osteogenesis imperfecta has been described primarily as a bone disorder, but recent studies have suggested that its morbidity and mortality are often related to secondary cardiac and respiratory problems, although skeletal deformities are still the main problem. Myocardial dysfunction and valve disease have been described in many osteogenesis imperfecta cases, mainly in adults.Reference Migliaccio, Barbaro and Fornari9–Reference Tournis and Dede12
Little is known about the early development of cardiac problems in osteogenesis imperfecta and their eventual risks. The objective of this research was to study echocardiography-based cardiovascular findings in a cohort of children with the disease.
Subjects and methods
Subjects
This cross-sectional comparative study was conducted in patients with osteogenesis imperfecta (0–18 years old) who were being followed at the Reference Center for Osteogenesis Imperfecta at the Hospital de Clínicas de Porto Alegre, in Porto Alegre, Brazil, from December 2015 to August 2017.
For each patient, diagnosis was based on their clinical manifestations and radiological findings, according to criteria established by the expanded Sillence Classification published in Nosology and Classification of Genetic Skeletal Disorders: 2015 Revision.Reference Bonafe, Cormier-daire and Hall13
More than 55% of included cases also had molecular diagnoses using the next-generation sequencing gene panel that included 100% coverage of the COL1A1 and COL1A2 genes, or through Sanger sequencing for the specific mutation c.-14C>T in the IFITM5 gene for osteogenesis imperfecta type 5.
For the control group, echocardiograms from healthy children (referred to the Hospital de Clínicas de Porto Alegre who were suspected of having heart murmurs) were evaluated and then paired with the osteogenesis imperfecta group according to body surface area. These controls did not have a history of cardiac disease, chronic pulmonary disease, or other chronic disorders.
Demographic data that included sex, age, height, and weight were collected. Body surface areas were calculated using the Haycock formula.
Echocardiography
All echocardiography were performed by the same paediatric cardiologist using iE33 equipment (Soma Technology, Bloomfield, USA). Two-dimensional M-mode echocardiography was performed in subjects placed in the left decubitus position. Three loops were recorded using the parasternal long-axis view; measurements were made perpendicular to the long axis between the tips of the mitral leaflets and the tips of the papillary muscles, as suggested by the American Society of Echocardiography guidelines.Reference Lang, Bierig and Bandano14 The dimensions of the left ventricular interventricular septum and left ventricular posterior wall were measured at end diastole at onset of the QRS complex in the electrocardiogram. Left ventricular mass was calculated using linear M-mode measurements derived from two-dimensional echocardiography. Examinations were recorded and analysed by a single paediatric cardiologist, who was blinded to the study objectives, minimising any intra-observer variability. Measurements were divided by body surface area according to the recommendations of the American Society of Echocardiography guidelines.
Statistical analyses
Quantitative variables were described either as means and standard deviations, or as medians and interquartile intervals, while categorical variables were described as absolute or relative frequencies. Paired t-tests were used to compare subgroups within the same cohort and for comparisons to controls paired by body surface area. T-tests for independent samples were used to compare cohort groups among themselves. Chi-square tests were used to compare echocardiographic findings between osteogenesis imperfecta cases and controls. All analyses were performed using SPSS software (v. 18.0; SPSS Inc., Chicago, Illinois, USA).
Results
In total, 54 children with osteogenesis imperfecta and 54 healthy children were included, matched according to body surface area. Group demographic characteristics are summarised in Table 1.
Table 1. Characteristics of patients with osteogenesis imperfecta and control subjects

OI, Osteogenesis imperfecta; BSA, body surface area
* p-Value for OI total sample and controls. Paired t-test was used to compare groups
For the osteogenesis imperfecta group, 35 individuals had a type 1 diagnosis (considered a mild form of the disease), 18 had osteogenesis imperfecta type 3 or 4, and 1 had type 5. Patients with types 3, 4, and 5 (19 individuals with moderate–severe forms of the disease) were pooled for statistical analysis purposes. Of the 54 children with osteogenesis imperfecta, 30 (55.5%) had a molecular diagnosis: 29 had collagen type I mutations (COL1A1, n = 20 [69%]; COL1A2, n = 9 [31%]), and 1 had the IFTM5 gene mutation. When analysed by type, 11 patients with osteogenesis imperfecta type 1 had quantitative mutations of the COL1A1 gene; the 6 osteogenesis imperfecta type 3 patients had qualitative mutations (COL1A1, n = 5; COL1A2, n = 1); and the 12 patients with type 4 disease had qualitative mutations (COL1A1, n = 4; COL1A2, n = 8).
Echocardiogram data values were increased in the osteogenesis imperfecta group compared to those for the body surface-area-matched controls (Table 2), with significant differences for aortic diameter (2.31 versus 2.14 cm) (p < 0.001), left atrial diameter (2.76 versus 2.46 cm; p < 0.001), left ventricular end-diastolic diameter (3.93 versus 3.79 cm; p = 0.045), left ventricular end-systolic diameter (2.37 versus 2.25 cm; p = 0.025), and right ventricular diameter (1.56 versus 1.33 cm; p = 0.001).
Table 2. Echocardiographic measurements of subjects with osteogenesis imperfecta and controls

OI, Osteogenesis imperfecta
These differences were even more evident (Table 3) when the osteogenesis imperfecta group was subdivided further according to disease severity level: mild (type 1) compared to moderate–severe (types 3 + 4 + 5). The mild group showed significant differences compared to the control group with respect to aortic diameter (2.37 versus 2.28 cm; p < 0.001); left atrial diameter (2.82 versus 2.63 cm, p = 0.002); left ventricular end-diastolic diameter (4.10 versus 4.04 cm, p = 0.013); left ventricular end-systolic diameter (2.47 versus 2.43 cm, p = 0.026); and right ventricular diameter (1.64 versus 1.28 cm; p < 0.001). Taken together, these results suggest larger left ventricles in patients with osteogenesis imperfecta type 1 compared to the control group. The osteogenesis imperfecta group also had significantly smaller left ventricular posterior wall thickness (0.69 cm) compared to the control group (0.71 cm) (p > 0.001).
Table 3. Echocardiographic measurements of osteogenesis imperfecta type 1 and 3/4/5 compared to their matched controls
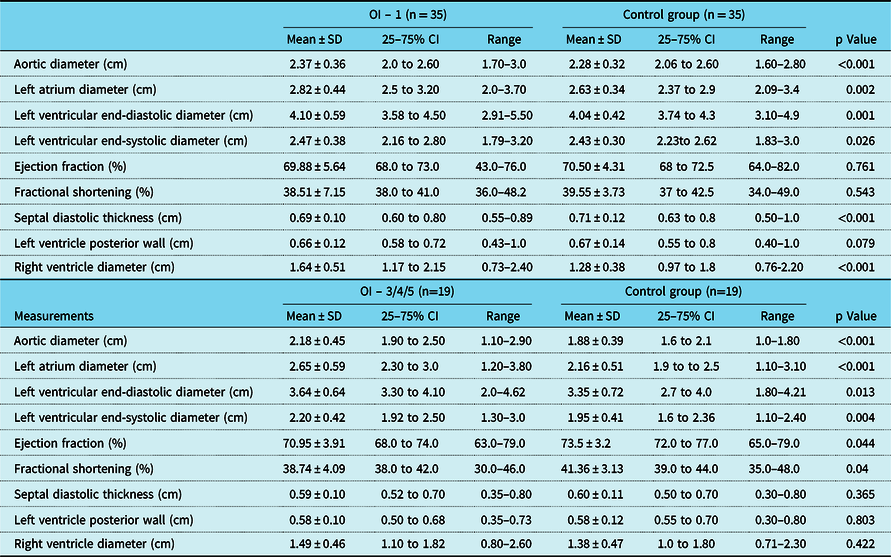
OI, osteogenesis imperfecta
Comparisons of data from moderate–severe cases of osteogenesis imperfecta (types 3 + 4 + 5) and from the control group showed several significant differences: aortic diameter (2.18 versus 1.88 cm, p < 0.001, respectively), left atrial diameter (2.65 versus 2.16 cm, p < 0.001, respectively), left ventricular end-diastolic diameter (3.64 versus 3.35 cm, p = 0.013, respectively), and left ventricular end-systolic diameter (2.20 versus 1.95 cm, p = 0.004, respectively). Ejection fractions (70.9 versus 73.5%, p = 0.044, respectively) and fractional shortening (38.7 versus 41.4%, p = 0.040, respectively) were smaller in moderate–severe osteogenesis imperfecta cases compared to controls. Other measured parameters were not different between these moderate–severe cases and the control group.
No cases of osteogenesis imperfecta had structural or valvular cardiac manifestations. However, 26 (48.1%) of these cases had physiological tricuspid regurgitation under conditions of normal pulmonary artery systolic pressure. In contrast, this finding was observed in eight control subjects (14.8%) (p < 0.001). Moreover, eight (14.8%) osteogenesis imperfecta patients had physiological mitral and tricuspid regurgitation, compared to only two subjects in the control group (3.7%, not statistically significant). All valvular regurgitations seen in the osteogenesis imperfecta group were slight (grade 1 out of 4) and not haemodynamically significant. In addition, among the 54 subjects with osteogenesis imperfecta, 3 (5.5%) had a patent oval foramen.
Discussion
Many studies have examined cardiovascular problems in adults with osteogenesis imperfecta, but few studies have investigated the development of cardiac problems, and their course, in children with the disease.
For the aortic diameter results, subjects in the mild osteogenesis imperfecta subgroup were within normal reference ranges. However, compared to the control group, left atrial diameter, left ventricular end-diastolic and end-systolic diameters, and right ventricular diameter were all significantly larger in the mild osteogenesis imperfecta group. Patients in the moderate–severe subgroup also had left atrial diameter, aortic diameter, left ventricular end-diastolic and end-systolic diameters that were within normal reference ranges, but were larger compared to the control group, while ejection fraction and fractional shortening parameter values were also considered normal in this subgroup, but were significantly smaller than control group values.
A comparable study that analysed 58 children with osteogenesis imperfecta found no cardiac abnormalities in those with type 1 disease but did report cardiac alterations in children with type 3 disease, including larger aortic root diameter and left ventricular posterior wall thickness with a standard deviation of +2, corrected for body surface area.Reference Vetter, Maierhofer and Miiller15 In our study, aortic diameter was significantly larger in patients with osteogenesis imperfecta compared to that of controls.
Recent studies of children with osteogenesis imperfecta have shown variable cardiac findings, including increased aortic diameter, diastolic dysfunction, and electrophysiological alterations.Reference Thiele, Cohrs and Flor4, Reference Hernández, Saavedra, Teresa and Vela8, Reference Vetter, Maierhofer and Miiller15
Rush et al.Reference Rush, Li and Goodwin7 conducted a cohort study with 100 children diagnosed with osteogenesis imperfecta that also paired 100 controls using body surface area. They reported larger aortic diameter, larger left ventricle internal diameter, and smaller left ventricle mass in children with osteogenesis imperfecta type 1 compared to their controls. However, this study reported aortic dilatation only in patients with osteogenesis imperfecta types 3 and 4, with normal left ventricle dimensions, compared to their control group. A likely explanation for such changes in osteogenesis imperfecta patients is greater myocardial tissue stiffness and decreased elasticity, leading to echocardiographic changes and altered myocardial relaxation.Reference Frommelt16, Reference Lamanna, Fayers, Clarke and Parsonage17
Thiele et al.Reference Thiele, Cohrs and Flor4 described an Aga2 mouse model with a dominant type of frameshift mutation in COL1A1 that presented with both a skeletal phenotype and cardiac alterations that included right ventricle hypertrophy, larger left ventricular end-systolic dimensions, and a smaller ejection fraction. As the second arm of that study, the echocardiography results of 46 children and young adults with types 3 and 4 osteogenesis imperfecta showed that 78% of subjects presented with one or more abnormal cardiac findings, including valve dysfunction and increased size of the left atrium. Although the human findings were heterogeneous, the mouse data suggest that mutant collagen could be responsible for the cardiac disorders. The skeletal phenotype of these mice was more severe than that seen in most humans with type 1 osteogenesis imperfecta. Consequently, mouse haploinsufficiency (and lower collagen transcription) may be a cross-species explanation for thinner and larger left ventricles in human type 1 osteogenesis imperfecta.
Although few echocardiography studies have been published for the paediatric population with osteogenesis imperfecta, many adult disease studies have confirmed the prevalence of valve diseases. Mitral and aortic valvulopathies are more common in patients with osteogenesis imperfecta than in the general population, and echocardiographic signs of valvulopathy are more prevalent in adults with osteogenesis imperfecta than in children with the disease.Reference Folkestad18 Valvular regurgitation has previously been documented in adult studies of types 1, 3, and 4 disease, with 95% of subjects demonstrating either isolated or multivalvular tricuspid, mitral, or aortic regurgitation, and the incidence of mitral valve prolapse in patients with the disease has been reported to be approximately 3–8%.Reference Vetter, Maierhofer and Miiller15, Reference Lamanna, Fayers, Clarke and Parsonage19
Aortic insufficiency is usually due to intrinsic valvular dysfunction, or it may be secondary to dilation of the aortic root. Mitral regurgitation also seems to be due to intrinsic valve involvement. Lamanna et al. described 49 patients with osteogenesis imperfecta referred for aortic and/or mitral valvular replacement surgery.Reference Lamanna, Fayers, Clarke and Parsonage19
In the present paediatric study, no cases of valvular prolapse or aortic regurgitation were found; however, physiological tricuspid regurgitation was the most common finding and occurred in almost half (48.1%) of the patients. While not statistically significant, 14.8% of patients in the disease group had physiological mitral and tricuspid regurgitation, but only 3.7% of the control group.
Radunovic et al.Reference Radunovic and Steine11 reported increased left ventricular end-diastolic diameter, and both mitral and aortic regurgitation in adult osteogenesis imperfecta patients compared to controls. The present results have demonstrated that left ventricular dimensions and wall thickness were similar for the disease and control groups. However, the disease group had larger dimensions compared to controls. Similar results have been reported for both adult and paediatric osteogenesis imperfecta patients.Reference Migliaccio, Barbaro and Fornari9, Reference Vetter, Maierhofer and Miiller15
A systematic review of 68 studies, and a total of 499 subjects (mostly adults), found that the most common cardiac abnormalities among patients with osteogenesis were valve diseases and increases aortic diameters. These results support the hypothesis that patients with osteogenesis imperfecta have a higher risk of cardiac pathology compared to healthy controls.Reference Ashournia, Johansen, Folkestad, Diederichsen and Brixen6
Valve disease prevalence in children with osteogenesis imperfecta is uncertain and has not been investigated as well as in other collagen diseases such as Marfan and Ehlers–Danlos syndromes.Reference Radunovic, Wekre, Diep and Steine10, Reference Mcdonnell, Gorman and Mandel20, Reference Sponseller21 With regard to aortic dilatation, abnormal type I collagen synthesis can change its biomechanical properties and lead to progressive aortic enlargement, that is, most evident in type 3 osteogenesis imperfecta. Similar to other hereditary collagen diseases, such dilatation can contribute to the development of valve regurgitation over time.Reference Radunovic and Steine11
Although the osteogenesis imperfecta subjects in this study did not present with cardiac manifestations, the echocardiogram differences reported here suggest that long-term manifestations cannot be ruled out. Therefore, we recommend lifelong monitoring. In this study, the incidence of patent oval foramen in patients with osteogenesis imperfecta was similar to the normal population and it should be considered only a minor anomaly. The simultaneous incidence of osteogenesis imperfecta and other congenital cardiopathies, such as Ebstein anomaly, aortic stenosis, and tetralogy of Fallot, have been described in the literature.Reference Vetter, Maierhofer and Miiller15, Reference Almassi, Hughes and Bartlett22, Reference Eufemia, Versacci and Zambrano23
This study provides evidence supporting the hypothesis that children with osteogenesis imperfecta can have slight alterations in cardiac anatomy that can progress throughout life. Longitudinal follow-up studies of this population are therefore necessary to further evaluate these findings, and any contributions they may have for morbidity and mortality in osteogenesis imperfecta.
This study has limitations, including the sample size. Multi-centered studies with larger cohorts will be necessary to determine the true prevalence of cardiac abnormalities among children with osteogenesis imperfecta, especially as the current findings were mostly within normal limits, even though they were significantly increased compared to our control population. Because the natural history of cardiac manifestations in osteogenesis imperfecta is not clearly understood, we suggest that patients undergo routine regular echocardiography to assess possible cardiac changes. Furthermore, due to the difficulty of establishing normal parameter ranges in patients with low height, bone deformities, and abnormal physical proportions, we adjusted values for comparison between groups based on pairings by body surface area (using height and weight).
Conclusion
The children with osteogenesis imperfecta in this study had normal cardiac function. However, compared to the healthy control group, we found many echocardiography parameters are significantly different. In addition, when compared to controls, a significant percentage of children with osteogenesis imperfecta had physiological tricuspid regurgitation.
Considering that collagen type I disorders cause the majority of these cases, and that cardiovascular system involvement is an extra-skeletal manifestation that can initially occur without symptoms, we strongly suggest children with osteogenesis imperfecta are evaluated for cardiac impairment. The echocardiogram is an imaging modality that is both accessible and non-invasive and therefore is ideal for regular disease course follow-up throughout these patients’ lives.
Acknowledgements
We thank all the patients and their relatives for their contributions to this research.
Financial Support
This study was financially supported by Fundo de Incentivo à Pesquisa e Eventos (FIPE)/Hospital das Clínicas de Porto Alegre (#15-0602), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) (#2014-255/13-0). BS and TMF were supported by National Council for Scientific and Technological Development – CNPq (#306245/2016-7).
Conflict of Interest
None.
Ethical Standards
The Ethics and Research Committee from Hospital de Clínicas de Porto Alegre (number 15-0602) approved this study, and all the patients’ guardians signed consent forms. The study was performed in accordance with the 1975 Declaration of Helsinki.





