INTRODUCTION
Cryptosporidium spp. are common causes of enteric infection in both immunocompetent and immunocompromised hosts, and at least 25 different species and numerous genotypes of Cryptosporidium have been described (Chalmers, Reference Chalmers2012). Most of the genotypes have been named after the hosts in which they were originally detected, although their taxonomy is under constant revision. The majority of human cryptosporidiosis cases are caused by the five species Cryptosporidium hominis, Cryptosporidium parvum, Cryptosporidium meleagridis, Cryptosporidium felis and Cryptosporidium canis, of which the first two are most common. However, 10 additional species have also been reported in humans (i.e. Cryptosporidium ubiquitum, Cryptosporidium cuniculus, Cryptosporidium viatorum, Cryptosporidium suis, Cryptosporidium andersoni, Cryptosporidium muris, Cryptosporidium fayeri, Cryptosporidium scrofarum, Cryptosporidium tyzzeri and Cryptosporidium bovis), as well as four genotypes designated Cryptosporidium skunk genotype, Cryptosporidium horse genotype, C. hominis monkey genotype and Cryptosporidium chipmunk genotype I (Xiao, Reference Xiao2010; Chalmers, Reference Chalmers2012). Furthermore, it has been observed that the immune status of the host is not necessarily linked to infection with more uncommon species/genotypes (Chalmers et al. Reference Chalmers, Elwin, Thomas and Joynson2002; Elwin et al. Reference Elwin, Hadfield, Robinson and Chalmers2012a).
In an earlier investigation (Svenungsson et al. Reference Svenungsson, Insulander, De Jong and Lebbad2009), we discovered that there is considerable under-diagnosis of cryptosporidiosis in Sweden, and thus we performed another study in Stockholm County to gain a better understanding of the epidemiology of human cryptosporidiosis in Sweden (Insulander et al. Reference Insulander, Silverlas, Lebbad, Karlsson, Mattsson and Svenungsson2013). A total of 194 isolates were successfully genotyped, which identified C. parvum (n = 111), C. hominis (n = 65), mixed C. hominis/C. parvum (n = 1), C. meleagridis (n = 11) and C. felis (n = 2). In addition, we found patients who were infected with C. viatorum (n = 2) and Cryptosporidium chipmunk genotype I (n = 2). The objective of the present investigation was to extend the molecular characterization and knowledge of the epidemiology of these two unusual Cryptosporidium species/genotypes from the Swedish dataset.
MATERIALS AND METHODS
The present study included four of the patients who had been assessed in our previous investigation (Insulander et al. Reference Insulander, Silverlas, Lebbad, Karlsson, Mattsson and Svenungsson2013); two of these subjects were infected with Cryptosporidium chipmunk genotype I, and the other two were infected with C. viatorum. The patients answered a questionnaire concerning travel abroad within 2 weeks of the onset of the disease, the symptoms displayed, and the date of onset of diarrhoea. Approval was obtained from the ethics committee of Karolinska Institutet, Stockholm, Sweden.
Cryptosporidium oocysts were initially identified by modified Ziehl–Neelsen staining. Confirmation of oocysts was obtained after staining with an anti-Cryptosporidium FITC-conjugated monoclonal antibody (Aqua-Glo, Waterborne Inc., New Orleans, LA, USA). The stool specimens were also analysed for additional intestinal parasites and cultured on selective media to detect bacterial enteropathogens.
DNA extraction and investigation of the Cryptosporidium oocyst wall protein (COWP) and the small sub-unit rRNA (SSU rRNA) genes using PCR and restriction fragment length polymorphism (RFLP) analysis were performed as previously described (Spano et al. Reference Spano, Putignani, Mclauchlin, Casemore and Crisanti1997; Xiao et al. Reference Xiao, Morgan, Limor, Escalante, Arrowood, Shulaw, Thompson, Fayer and Lal1999, Reference Xiao, Bern, Limor, Sulaiman, Roberts, Checkley, Cabrera, Gilman and Lal2001; Insulander et al. Reference Insulander, Silverlas, Lebbad, Karlsson, Mattsson and Svenungsson2013). The isolates were further examined after sequencing of the SSU rRNA and COWP amplicons. We also conducted PCR analysis of the actin gene, the 70-kDa heat shock protein (HSP70) gene and the 60-kDa glycoprotein (GP60) gene, and subsequently carried out standard Sanger bi-directional sequencing of the different isolates (Sulaiman et al. Reference Sulaiman, Morgan, Thompson, Lal and Xiao2000, Reference Sulaiman, Lal and Xiao2002; Alves et al. Reference Alves, Xiao, Sulaiman, Lal, Matos and Antunes2003). Sequences were edited and analysed using the BioEdit Sequence Alignment Editor version 7.0.9.0. The sequences were compared with isolates in the GenBank database using the Basic Local Alignment Search Tool (BLAST; NCBI http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Phylogenetic analysis was performed on SSU rRNA, actin, HSP70 and COWP gene sequences from the species/genotypes considered in the present study, as well as sequences from other known Cryptosporidium species/genotypes. In addition to the analyses of the individual genes, we constructed a concatenated tree consisting of SSU rRNA, actin and HSP70 gene sequences. Multiple sequence alignments were carried out using the MAFFT program with default settings (Katoh and Toh, Reference Katoh and Toh2008). The alignments were used to construct distance trees by the neighbour-joining method with MEGA5 software (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). The maximum composite likelihood model was employed with gaps/missing data treated as pairwise deletions. To estimate robustness, bootstrap proportions were computed after 1000 replications.
Representative nucleotide sequences have been deposited in GenBank under accession numbers JX978269–JX978276, JX984441 and JX984442.
RESULTS
Patients
Specimens from all four patients were positive for Cryptosporidium by modified Ziehl–Neelsen staining, and this was confirmed by staining with the anti-Cryptosporidium monoclonal antibody. One patient was co-infected with Giardia duodenalis. No infections with bacterial enteropathogens were identified by culture. None of the investigated patients had any known immune deficiency disease.
Both patients infected with C. viatorum had travelled outside Sweden prior to infection. The first of these subjects (isolate designated Swec025) was a 35-year-old pregnant female who fell ill during a visit to Kenya in July 2006. She suffered from diarrhoea, abdominal pain, nausea, fever and headache, and also experienced weight loss of 4 kg; duration of the symptoms was 9 days. The other patient (isolate Swec066) was a 26-year-old female who became ill in connection with a trip to Guatemala in March 2007, and she was co-infected with G. duodenalis. She had diarrhoea, abdominal pain and nausea; her symptoms lasted 10 days, and she was treated with metronidazole for the Giardia infection.
The two patients with Cryptosporidium chipmunk genotype I infection had not travelled outside Sweden prior to infection. One of these subjects (isolate Swec096) was a 2-year-old girl, who was probably infected in the Stockholm archipelago in September 2007. She had frequent diarrhoea, severe abdominal pain, nausea and appetite loss, and the duration of symptoms was 6 days. The other patient (isolate Swec176) was a 56-year-old male who was infected in Dalarna County in Central Sweden in August 2008. He complained of diarrhoea, abdominal pain, nausea and fever, and his symptoms lasted approximately 1 week. Neither of these patients reported contact with animals, but they had eaten unwashed home-grown berries and vegetables, respectively.
Genetic characterization
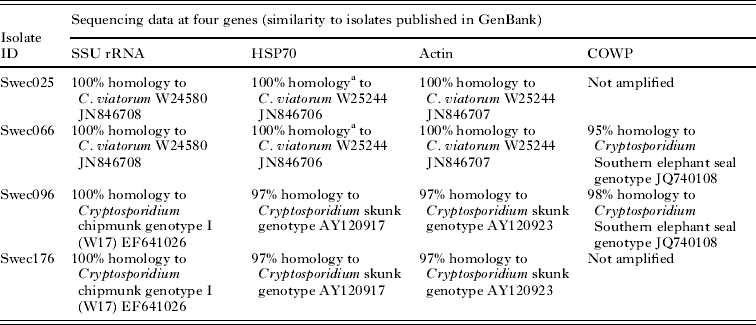
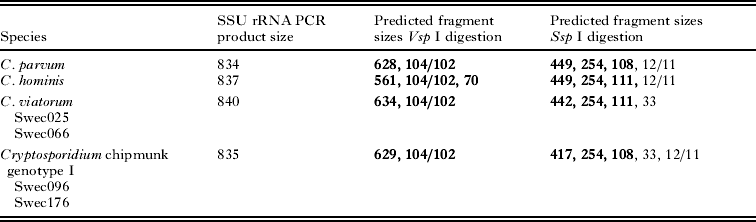
The combined results of molecular characterization are shown in Table 1. Sequences of the SSU rRNA PCR products from isolates Swec025 and Swec066 showed complete homology with C. viatorum isolate W24580 (GenBank Accession No. JN846708) but exhibited a single nucleotide difference in comparison with the C. viatorum type isolate W25244 (GenBank Accession No. JN846705). No ambiguous positions, as previously reported by Elwin et al. (Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b), were observed in the SSU rRNA C. viatorum sequences. Isolates Swec096 and Swec176 were completely homologous to Cryptosporidium chipmunk genotype I (GenBank Accession No. EF641026). The RFLP profiles of the SSU rRNA PCR products are presented in Table 2 and Supplementary Fig. S1 (online version only).
Table 1. Molecular characterization of C. viatorum and Cryptosporidium chipmunk genotype I isolates

a Sequence coverage 361/1921bp.
Table 2. VspI and SspI digestion of SSU rRNA products. Restriction fragment length in base pairs; visible bands in bold

Two isolates, Swec066 (C. viatorum) and Swe096 (Cryptosporidium chipmunk genotype I), were successfully amplified at the COWP locus. The closest match for both sequences was Cryptosporidium Southern elephant seal genotype (GenBank Accession No. JQ740108), with 95 and 98% homology for C. viatorum and Cryptosporidium chipmunk genotype I, respectively. The RFLP profile of the C. viatorum isolate (predicted fragments after RsaI digestion of the COWP product: 372, 129, 34 and 18 bp) did not match any known Cryptosporidium spp., whereas the profile of Cryptosporidium chipmunk genotype I isolate (predicted fragments: 266, 147, 106 and 34 bp) corresponded to the published COWP RFLP pattern of C. wrairi and the Cryptosporidium ferret genotype (Xiao et al. Reference Xiao, Limor, Morgan, Sulaiman, Thompson and Lal2000).
All four isolates were successfully amplified and sequenced at the actin locus. The two C. viatorum isolates were indistinguishable from each other, and showed complete sequence homology with the C. viatorum type isolate W25244 (GenBank Accession No. JN846707). The two chipmunk isolates were also indistinguishable from each other, and their closest match in GenBank was the Cryptosporidium skunk genotype (Table 1).
At the HSP70 locus 1920 to 1930 bp were successfully sequenced from all four isolates. For both of the C. viatorum isolates, the HSP70 sequences showed complete homology with those in the C. viatorum type isolate W25244 (GenBank Accession No. JN846706). However, due to the short sequence length of the W25244 isolate, only 18% (361 of 1921 bp) of our sequences were covered. The two C. viatorum isolates analysed in our study had 11 repeats of a 12-bp segment with single nucleotide polymorphisms (SNPs) at the 3rd and the 6th base: GG(A/C/T)GG(A/C/T)ATGCCA at the 3′ end. The sequences differed from each other by two SNPs, both located in the repetitive part of the gene. The two Cryptosporidium chipmunk genotype I sequences were identical in the repetitive region, which consisted of 12 repeats of 12 bp with single polymorphisms at the 3rd base (GG(A/T)GGTATGCCA), but differed from each other by a single nucleotide (G to T) at position 1424 from the start of the sequence, resulting in an amino acid change (lysine to asparagine). For these two isolates, the closest match in GenBank was the Cryptosporidium skunk genotype (Table 1). None of the four isolates were amplified at the GP60 locus despite repeated attempts.
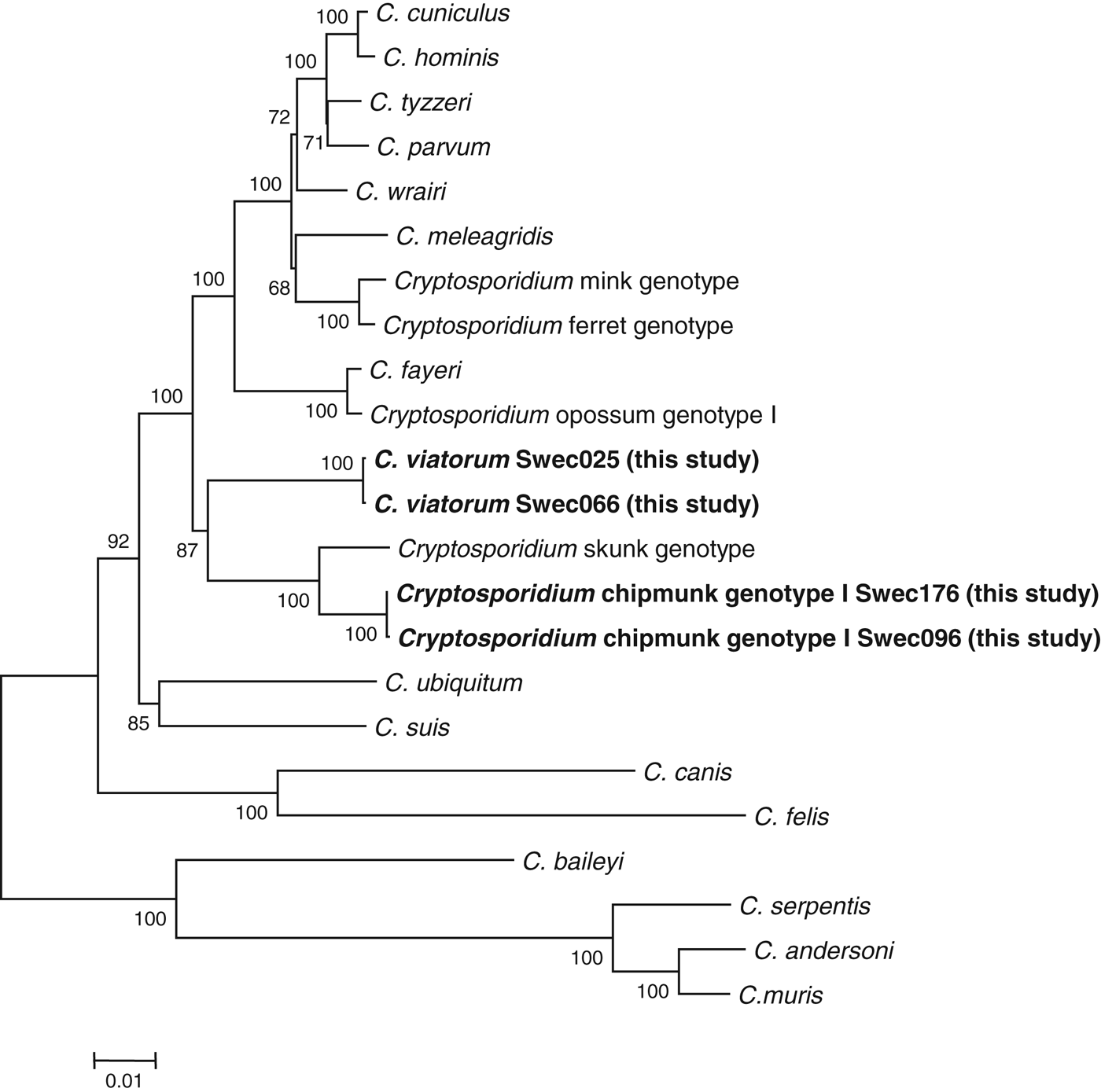
We constructed four individual phylogenetic trees (SSU rRNA, COWP, actin and HSP70) and also a tree based on the concatenated nucleotide sequences of the SSU rRNA, actin and HSP70 genes (Fig. 1 and Supplementary Figs S2–S5, online version only). In the concatenated tree and in the individual trees based on the actin and COWP loci, the two Cryptosporidium species/genotypes described in our study grouped together. In the tree based on SSU rRNA, C. viatorum showed a closer relationship with C. fayeri than with any other species/genotypes, whereas Cryptosporidium chipmunk genotype I grouped with Cryptosporidium skunk genotype.

Fig. 1. Phylogenetic relationship between Cryptosporidium sequences found in the present study and sequences retrieved from the GenBank database. The tree was created using a concatenated sequence based on the partial sequences of the actin, HSP70 and SSU rRNA genes. There were ∼3500 base positions in the final dataset. Evolutionary distances were inferred by the neighbour-joining method. Bootstrap values ⩾50% from 1000 replicates are indicated at each node. The scale bar indicates an evolutionary distance of 0·01 nucleotides per position in the sequence.
DISCUSSION
This report describes two unusual Cryptosporidium species/genotypes involved in human cryptosporidiosis: C. viatorum and Cryptosporidium chipmunk genotype I. The recently identified species C. viatorum has previously only been reported in 10 travellers, all of whom visited the Indian subcontinent (Elwin et al. Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b). The two patients positive for this species in our study had acquired their infections in Kenya and Guatemala, respectively, demonstrating that C. viatorum is not restricted to Asia but appears to be cosmopolitan. Elwin et al. (Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b) reported that the C. viatorum-infected patients they investigated had diarrhoea, abdominal pain, nausea and fever, symptoms that are typical for human cryptosporidiosis and that also correspond well with our findings. However, one of our patients was co-infected with G. duodenalis, which might have influenced the symptomatology.
Genetic characterization of the C. viatorum isolates from the Indian subcontinent revealed minor variations at the SSU rRNA locus in the form of ambiguous nucleotides at a specific position (Elwin et al. Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b). The two C. viatorum isolates in our study showed no ambiguities, and they were identical to each other and to the published W24580 isolate. Interestingly, the RFLP profiles of C. viatorum at this locus were almost identical to those of C. parvum (Table 2 and Supplementary Fig. S1, online version only), which highlights the necessity of sequencing to achieve correct species identification. At the HSP70 locus, the C. viatorum isolates differed from each other in the repetitive region at the 3′ end of the gene, an area that is highly polymorphic and has been used for subtyping of other Cryptosporidium species, such as C. meleagridis and C. hominis (Glaberman et al. Reference Glaberman, Sulaiman, Bern, Limor, Peng, Morgan, Gilman, Lal and Xiao2001; Sulaiman et al. Reference Sulaiman, Lal and Xiao2001; Peng et al. Reference Peng, Meshnick, Cunliffe, Thindwa, Hart, Broadhead and Xiao2003; Abe, Reference Abe2010; Silverlas et al. Reference Silverlas, Mattsson, Insulander and Lebbad2012). Inasmuch as the only published C. viatorum HSP70 sequence in GenBank (Accession No. JN846706) covers only 18% of the sequences in the isolates assessed in our study and does not include the 3′ end of the gene, we were not able to compare the polymorphic parts of this locus. Nevertheless, our results do indicate that the two patients carried different HSP70 genotypes of C. viatorum, which suggests geographical variation in this species considering that one of the patients was infected in Africa and the other in South America. Unfortunately, despite repeated efforts, we were unable to amplify GP60, which is the most polymorphic and also the most commonly used gene for subtyping of Cryptosporidium isolates. Failure to amplify C. viatorum at this locus has also been reported by Elwin et al. (Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b) and might be due to mismatches at the primer binding sites, because the primers we used are based on C. hominis and C. parvum sequences. This problem may be resolved by whole genome sequencing of an extended number of Cryptosporidium species, which possibly will determine target sequences suitable for the design of universal or species-specific GP60-primers.
The findings of our phylogenetic analysis of the SSU rRNA locus corroborate the results obtained by Elwin et al. (Reference Elwin, Hadfield, Robinson, Crouch and Chalmers2012b) showing that, of all the known Cryptosporidium species, C. viatorum is closest related to C. fayeri. Notably, the phylogenetic assessments of the actin and COWP loci, as well as the analysis of the concatenated tree, show a different pattern in which C. viatorum and Cryptosporidium chipmunk genotype I are more closely related to each other than to any of the established Cryptosporidium species. It is evident that more isolates, preferably from different geographical areas, must be obtained and analysed to provide a better understanding of the epidemiology of this species. So far, no animal reservoir for C. viatorum has been described, but we cannot exclude that future studies will identify animal hosts in different parts of the world.
The first known cases of humans infected with Cryptosporidium chipmunk genotype I in Sweden were also included in our study. This genotype was initially detected in the USA in storm water in the state of New York and was reported as genotype W17 (Jiang et al. Reference Jiang, Alderisio and Xiao2005), and it was later also found in two sporadic cases of human cryptosporidiosis in the state of Wisconsin (Feltus et al. Reference Feltus, Giddings, Schneck, Monson, Warshauer and McEvoy2006). In 2007, Feng et al. (Reference Feng, Alderisio, Yang, Blancero, Kuhne, Nadareski, Reid and Xiao2007) identified the same genotype in fecal specimens from eastern chipmunk, eastern squirrel and deer mouse in the watershed of the New York City drinking water supply, and it was subsequently named chipmunk genotype I. The first report of this genotype in Europe concerned red squirrels and showed that 2 out of 70 animals were infected with Cryptosporidium chipmunk genotype I, whereas 15 were infected with Cryptosporidium ferret genotype (Kvac et al. Reference Kvac, Hofmannova, Bertolino, Wauters, Tosi and Modry2008). Cryptosporidium chipmunk genotype has also been found in an HIV-positive patient in France (ANOFEL, 2010), although the investigators did not indicate whether this patient was infected in France or abroad, or if the infection involved chipmunk genotype I, II or III. The paucity of publications concerning human infection with Cryptosporidium chipmunk genotype I means that data on symptoms are limited. The two patients in our study who were positive for this genotype showed classical symptoms of Cryptosporidium infection, including diarrhoea and abdominal pain for about 1 week. They were both infected in late summer and stated that they had not been in contact with animals but had eaten unwashed berries or vegetables from their gardens. Considering that Cryptosporidium chipmunk genotype I has been found in red squirrels in Italy (Kvac et al. Reference Kvac, Hofmannova, Bertolino, Wauters, Tosi and Modry2008), it seems plausible that the two patients in our study were infected through ingestion of food or water contaminated by animals of this species. However, to our knowledge, no studies have been performed to evaluate the occurrence of Cryptosporidium oocysts in red squirrels in Sweden.
Our results indicate that more unusual Cryptosporidium species/genotypes can be the cause of both imported and domestic cases of cryptosporidiosis. This study has also provided supplementary sequence data on C. viatorum (COWP and HSP70 genes) and Cryptosporidium chipmunk genotype I (actin, HSP70 and COWP genes), for which no information (or only information regarding short sequences) has previously been deposited in the GenBank database. Genotyping tools can help explain the complex epidemiology and host specificity of uncommon causes of human cryptosporidiosis. As data are accumulated, it will also become possible to improve risk analysis in cases that potentially involve Cryptosporidium spp.
ACKNOWLEDGEMENTS
We thank Dr Lihua Xiao for valuable advice on sequence interpretation.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S003118201300084X.