INTRODUCTION
Palliative Care in Nursing Homes
As the world's population is ageing rapidly, the number of people staying in nursing homes has increased (World Health Organization, 2011). Since many nursing home residents are facing chronic (progressive) diseases (e.g., dementia, hypertension, and diabetes), they are more vulnerable to a decline in health status and eventual death (van Dijk et al., Reference van Dijk, Mehr and Ooms2005). As a result, the importance of nursing homes as locations for palliative care has also increased (Ersek & Carpenter, Reference Ersek and Carpenter2013).
Palliative care aims to “improve the quality of life of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of an early identification, impeccable assessment, and treatment of pain and other problems, physical, psychosocial, and spiritual” (World Health Organization, 2002). This definition emphasizes the need to adopt a holistic model of assessment and care (Rosser & Walsh, Reference Rosser and Walsh2014) that highlights the concept of “total pain”. Total pain looks not merely at the physical components of pain but also at the emotional, cultural, psychological, social, spiritual, and existential aspects (Bendelow & Williams, Reference Bendelow and Williams1995). Given the diversity in individual suffering, it is important for care professionals to understand and address the different palliative care needs (Goldstein & Morrison, Reference Goldstein and Morrison2012). However, identification of these needs in nursing homes is challenging due to a lack of care professional knowledge on palliative care practices, low staffing levels, a lack of available time for residents, and a lack of adequate screening, among other factors (Wowchuk et al., Reference Wowchuk, McClement and Bond2007). Palliative care needs and symptoms are therefore often over- or underestimated and poorly addressed, especially in residents with dementia (Hermans et al., Reference Hermans, Cohen and Spruytte2016a ). Research has shown that a comprehensive assessment tool can support evaluation and identification of residents' needs in a palliative care setting (Mcllfatrick & Hasson, Reference Mcllfatrick and Hasson2014).
The interRAI Palliative Care Instrument and the BelRAI Web Application
The multinational research consortium interRAI developed the interRAI Palliative Care instrument (the interRAI PC) in 2003 in order to provide standardized comprehensive information on the different needs, strengths, and preferences of adults receiving palliative care (Hirdes et al., Reference Hirdes, Ljunggren and Morris2008; Smith et al., Reference Smith, Steel and Fries2010). The instrument was designed as part of the interRAI Suite of Instruments (e.g., interRAI Home Care, interRAI Acute Care, interRAI Long-Term Care Facilities) (visit interRAI.org). The 74-item instrument consists of 17 sections, covering 8 domains: symptoms or conditions, cognitive competency and communication mood, functional status, preferences, social relations, spirituality, services, and treatments (Steel et al., Reference Steel, Ljunggren and Topinková2003). The interrater reliability was found to be 0.77 in all domains (average κ = 0.83) (Hirdes et al., Reference Hirdes, Ljunggren and Morris2008). The value of κ was ≥ 0.80 for more than half of the questions (Steel et al., Reference Steel, Ljunggren and Topinková2003). The interRAI PC is the most comprehensive assessment that has been validated for nursing home residents with palliative care needs (Hermans et al., Reference Hermans, De Almeida Mello and Spruytte2014a ).
In Belgium, the interRAI instruments are completed on the secured online web application: BelRAI (visit belrai.org). This web application enables multidisciplinary completion of the instruments and supports exchange of client data between healthcare settings, thereby improving the continuity of care (Vanneste & Declercq, Reference Vanneste, Declercq, Müller and Kubitschke2014; Hermans et al., Reference Hermans, De Almeida Mello and Spruytte2014a ). The interRAI PC instrument was implemented on the BelRAI web application in 2012 (Hermans et al., Reference Hermans, De Almeida Mello and Spruytte2014a ). The outcomes of the interRAI PC are Client Assessment Protocols (CAPs) and scales. CAPS provide an alert for specific problems and information about the risk of their appearance or the potential for improvement. Every CAP is linked to guidelines that inform care professionals about how to approach problems in order to resolve them, reduce the risk of deterioration, or increase the opportunity to improve or maintain function (Carpenter & Hirdes, Reference Carpenter and Hirdes2013). The scales of the interRAI instruments are coherent calculations of client characteristics and conform to internationally validated scales (Declercq et al., Reference Declercq, Gosset and Paepen2009). The standardized overview of the CAPS of the interRAI PC can be employed to support care planning and facilitate the dialogue with clients and their family members (Steel et al., Reference Steel, Ljunggren and Topinková2003; Bernabei et al., Reference Bernabei, Landi and Onder2008; Hermans et al., Reference Hermans, Spruytte and Cohen2016b ). Research shows that data gathered from the interRAI PC may improve our understanding of palliative care clients. Integrating the interRAI PC outcomes into the care planning process may allow for a higher quality of care since person-specific needs would be addressed better (Freeman et al, Reference Freeman, Hirdes and Stolee2014). However, to our knowledge, no studies have yet been conducted to evaluate whether the needs and symptoms of people potentially requiring palliative care are better met when using the interRAI PC.
Research Aims
The main objective of our study is to evaluate whether the palliative care needs of nursing home residents are better met and whether symptoms associated with the palliative care situation are reduced after using the interRAI PC over the course of a year. The BelRAI project was commissioned in 2006 by the Federal Ministry of Public Service, Health, Food Chain Safety, and Environment in order to test the feasibility of the BelRAI web application and the use of the interRAI instruments in Belgium (Declercq et al., Reference Declercq, Gosset and Paepen2009). During this project, about 20 Belgian nursing homes received training on the BelRAI web application and the interRAI Long-Term Care Facilities instrument (interRAI LTCF). At that point in time, several nursing homes still used the interRAI LTCF in day-to-day practice to assess and tackle the needs of their residents. The secondary aim of our study is therefore to evaluate whether or not prior experience with the interRAI influences the effect of using the interRAI PC. Because several Belgian nursing homes already use the interRAI LTCF, we hypothesize a smaller effect of the interRAI PC in these nursing homes than in the nursing homes that were not using the interRAI LTCF (Dimitrov & Rumrill, Reference Dimitrov and Rumrill2003).
METHODS
Design
Our study has a quasi-experimental pretest–posttest design and is part of a complex intervention to evaluate the use of the interRAI PC in nursing homes. The protocol of this study was published elsewhere (Hermans et al., Reference Hermans, Spruytte and Cohen2014b ). The SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence) guidelines were used for reporting.
Setting
Calls for participation in the intervention group were sent out by all four umbrella organizations of nursing homes in Flanders (the Dutch-speaking region of Belgium) and at a national conference for nursing home staff. The care professionals from 15 nursing homes agreed to participate in the study to implement the interRAI PC in their nursing home (intervention group). Based on the list of nursing homes in Flanders from the National Institute for Health and Disability Insurance, 15 other nursing homes were matched to these intervention nursing homes in terms of facility size and geographic region and were contacted about participation in the control group.
Eligibility
Of the 15 control and 15 intervention nursing homes, residents aged 65 and older who were anticipated to be in the last year of their lives were included in our study. The latter identification was based on the “surprise question” (Would you be surprised if this person was to die within 6 to 12 months?) (Hubbard, Reference Hubbard2011). Research has shown the “surprise question” to be a feasible, effective, and simple screening tool to identify people with greatly increased risk of mortality in the coming year (Moss et al., Reference Moss, Lunney and Culp2010). For our study, the answers to the surprise question were discussed for every resident of the nursing home during multidisciplinary team meetings.
Data Collection
Pretest
At baseline, care professionals from the multidisciplinary nursing home staff of 15 intervention and 15 control nursing homes filled out the Palliative care Outcome Scale (POS) for all residents identified as eligible. The POS is a 10-item multidimensional scale that covers the physical, psychological, emotional, spiritual, practical, and informational domains of life (Aspinal et al., Reference Aspinal, Hughes and Higginson2002; Cicely Saunders Institute, 2012). The POS can be utilized to evaluate the palliative care needs and symptoms of people with and without dementia (Brandt et al., Reference Brandt, Deliens and van der Steen2005). Based upon a validation study in specialist palliative care settings throughout the United Kingdom, the POS was found to be internally consistent (patient version α = 0.70, staff version α = 0.65). Furthermore, the POS has shown moderate to good construct validity (Spearman's ρ = 0.43–0.80) and good test–retest reliability for 7 of its 10 items (Hearn & Higginson, Reference Hearn and Higginson1999). The first 8 items of the POS are scored on a 5-point Likert scale ranging from 0 (no problem) to 4 (an overwhelming problem). Items 9 (wasted time) and 10 (personal affairs) are scored on a 3-point scale: 0 (good), 2 (moderate), and 4 (bad). Individual POS item scores of 0 or 1 require less clinical attention than items scores of 2, 3, or 4 (Cicely Saunders Institute, 2012). The POS has already been employed in several studies with a pretest–posttest design in order to evaluate differences in palliative care needs after implementing an intervention (Bajwah et al., Reference Bajwah, Ross and Wells2015).
There are two versions of the POS: the POS–patient version is to be filled out by the patient, and the POS–staff version is to be filled out by a staff member. Agreement between both versions was found to be acceptable for 8 of 10 items (Hearn & Higginson, Reference Hearn and Higginson1999). It is not feasible to obtain the POS–patient version from all nursing home residents. Especially for people with dementia, filling out a structured questionnaire is not always possible. Since our study includes residents with and without dementia, the POS–staff version was used for all residents for reasons of comparability. Nurses and nursing assistants were informed on the use of the POS and received a POS manual during an introductory meeting. Depending on the degree of cognitive impairment of the nursing home resident, the POS–staff version was completed individually by nurses and nursing assistants who knew the resident well or in consultation with the nursing home resident or a relative.
Intervention
Care professionals from the intervention nursing homes received training on the use of the interRAI PC and the BelRAI web application. Over the course of the year, these care professionals filled out the interRAI PC every three months for all residents identified as eligible. Based on the CAPS results from the interRAI PC assessment and the accompanying manuals, care professionals were able to evaluate, adapt, and design individual care plans. All steps of the intervention were described in the study protocol (see Hermans et al., Reference Hermans, Spruytte and Cohen2014b ). The control nursing homes did not complete the interRAI PC and provided care as usual.
Posttest
At posttest, care professionals from both the intervention and control nursing homes again completed the POS for all nursing home residents anticipated to be in the last year of life in order to evaluate whether their palliative care needs and symptoms were reduced after using the interRAI PC.
Data Analyses
Our analyses were conducted in SPSS and STATA 11.2. We respectively used the Mann–Whitney U test and the Wilcoxon signed-rank test in order to compare the following data.
Primary Outcomes
-
• Pretest data from the intervention and control nursing homes.
-
• Pre- and posttest data from the control nursing homes.
-
• Pre- and posttest data from the intervention nursing homes.
-
• Posttest data from the control and intervention nursing homes.
Secondary Outcomes
-
• Pre- and posttest data from the intervention nursing homes that were already working with the interRAI LTCF instrument.
-
• Pre- and posttest data from the intervention nursing homes that were not working with the interRAI LTCF instrument.
Generalized linear mixed models (GLMMs) were employed to adjust for clustering by nursing homes. GLMMs combine the properties of two statistical frameworks: linear mixed models, which incorporate random effects, and generalized linear models, which handle nonnormal data by using link functions and exponential family (e.g., normal, Poisson, or binomial) distributions. GLMMs are the best tool for analyzing nonnormal data since they provide a more flexible approach (Bolker et al., Reference Bolker, Brooks and Clark2008). They provide a broad range of models for the analysis of grouped data. Two tests were performed for our study: a linear model to test for fixed-, between-, and random-effects adjusted for the cluster, and a generalized linear mixed-effect regression. Both tests yielded the same results.
Ethics Statement
Approval to conduct our research was granted by the Belgian Commission for the Protection of Privacy and the University Hospital Leuven Medical Ethics Committee (file no. B322201421986). All nursing home residents with palliative care needs or their representatives were asked to sign an informed consent agreement. Refusing to participate did not affect the care services offered to a resident. After residents or their representatives decided to participate, they could withdraw their consent at any time. A formal procedure was undertaken to enable caregivers to fill out the interRAI PC on a secured online web application (belrai.org).
RESULTS
Setting
Care professionals from 15 nursing homes agreed to participate in the study and implement the interRAI PC in their nursing home (intervention nursing homes). Of the 15 intervention nursing homes, all participated in the pretest and 12 in the posttest. Four intervention nursing homes had been working with the interRAI LTCF since 2006. Eight nursing homes did not have prior experience with the interRAI LTCF.
Of the 15 matched control nursing homes who participated in the pretest, 9 did so in the posttest. Dropouts were due to the following reasons: a lack of time to complete the interRAI PC, a lack of staff, discharge of contact persons, and renovation of the nursing home, among others.
Nursing Home Residents
In total, 429 nursing home residents were identified as eligible and signed an informed consent agreement. The pretest consisted of 273 nursing home residents: 133 in the intervention group and 140 in the control group.
The posttest included 156 nursing home residents, 83 of whom were included in the intervention condition and 73 in the control group. Specific data on the characteristics of the residents can be found in the Appendix (see the Supplementary Materials).
Since the surprise question was employed to identify residents with palliative care needs, most residents who were included in the pretest had died before the posttest was conducted. The posttest sample thus included different subjects than those in the pretest sample.
Primary Outcomes (see Table 1 and 2)
At baseline, significant differences were found between the POS scores of the 15 control and intervention nursing homes on the items “pain” (ρ = –0.492; 95% confidence interval [CI 95%] = –0.90, –0.08; p = 0.019); “other symptoms” (ρ = –0.577; CI 95% = –0.95, –0.21; p = 0.002); “family anxiety” (ρ = –1.055; CI 95% = –1.58, –0.52; p < 0.001); and total POS score (ρ = –2.66; CI 95% = –4.76, –0.56; p = 0.013) (Table 1). No differences were found between the posttest POS scores of the control and intervention nursing home residents (Table 1).
Table 1. Control nursing homes and intervention nursing homes at baseline and at posttest

a By GLMM (controlled for gender, age, dementia diagnosis).
Table 2. Comparison of pre- and posttest POS scores in the control nursing homes and comparison of pre- and posttest POS scores in the intervention nursing homes

a By GLMM (controlled for gender, age, dementia diagnosis).
We found no significant differences between the pre- and posttest POS scores of the control nursing homes (n = 9) (Table 2). Posttest POS scores in the intervention nursing homes (n = 12) were significantly higher on item 9 (“wasted time”) (ρ = 0.14; CI 95% = 0.05, –0.23; p = 0.002), indicating that more time was wasted on appointments related to care for residents (e.g., waiting for transport, repeated examinations). No other significant differences between the pre- and posttest POS scores of the intervention nursing homes were found (Table 2).
Secondary Outcomes (see Table 3)
No significant differences were identified between the pre- and posttest POS scores of the intervention nursing homes that were already working with the interRAI LTCF (n = 4).
Table 3. Comparison of pre- and posttest POS scores in the intervention nursing homes
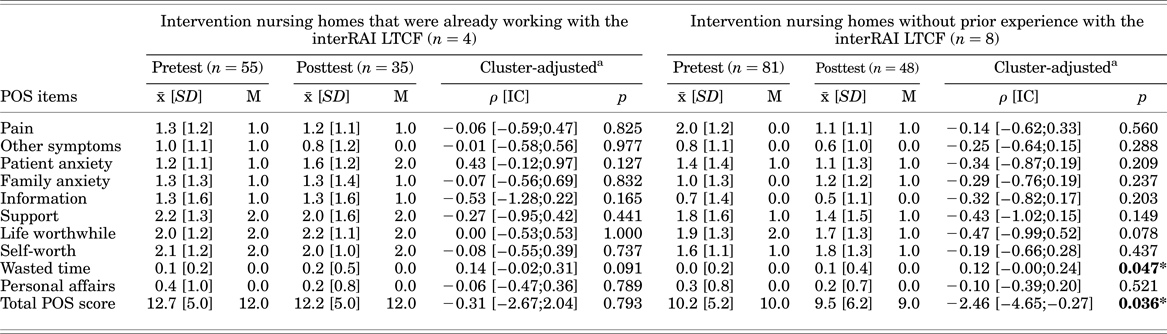
a By GLMM (controlled for gender, age, dementia diagnosis).
Total POS scores in the intervention nursing homes where care professionals did not have prior experience with the completion of the interRAI instruments (n = 8) were significantly lower in the posttest (ρ = –2.01; CI 95% = –3.89, –0.14; p = 0.036), even after adjusting for clustering (Table 3).
The results of the Mann–Whitney U test were consistent with those obtained from the GLMM analyses.
DISCUSSION
Our study found that the use of the interRAI PC in nursing homes over the course of one year is not associated with reduced needs and symptoms among residents anticipated to be in the last year of their lives. Care professionals at the nursing homes using the instrument even indicated that more time was wasted on appointments concerning the care of residents (e.g., waiting for transport, repeated examinations) than prior to implementation of the instrument. There are a number of reasons for the negative results regarding care professionals experiencing a waste of time after implementing the interRAI PC. Research shows that filling out the interRAI instruments is an extensive, laborious, and time-consuming process. Care professionals do not always have sufficient time to complete these instruments, especially not in a nursing home, where there is such a heavy workload (Hermans et al., Reference Hermans, Spruytte and Cohen2016b ; Devriendt et al., Reference Devriendt, Wellens and Flamaing2013; Vanneste & Declercq, Reference Vanneste, Declercq, Müller and Kubitschke2014). A study in Belgian nursing homes showed that it takes about a year to integrate the use of the interRAI PC in the day-to-day practices of a nursing home (Hermans et al., Reference Hermans, Spruytte and Cohen2016b ). After this period, the perceived and actual waste of time may be reduced. The implementation of complex interventions requires time and practice, as several elements need to be taken into account. What works in one setting may not be as effective or may be harmful in another (National Institute for Health and Clinical Excellence, 2007). Moreover, in this study evaluating the interRAI PC, a number of additional burdens may have been imposed that would not apply in real-life implementations and that may have increased the amount of perceived wasted time. For instance, as user involvement is essential in implementation research, key users have to be involved in all stages of the process and in evaluation of the intervention (Craig et al., Reference Craig, Dieppe and Macintyre2006). Also, all nursing home residents with palliative care needs or their representatives need to be asked for an informed consent agreement, which would not be the case in real-life implementations.
Why we detected no reduction in residents' needs and symptoms in the posttest also warrants further discussion. It took about a year to implement the interRAI PC and the BelRAI web application in the nursing homes (Hermans et al., Reference Hermans, Spruytte and Cohen2016b ), and some nursing homes did not have sufficient time to discuss and work with the interRAI PC results (CAPs and scales) beyond merely registering. Hence, they did not use the results to develop, evaluate, and adjust care plans. Ideally, clinicians need to react when CAPs are triggered by the collaborative process of deciding whether or not the triggered issues should be addressed in the plan of care (Freeman et al. Reference Freeman, Hirdes and Stolee2014). This would suggest that our study was only able to capture a preliminary effect of the interRAI PC and that future research should evaluate in a longer-term design whether effective and appropriate use of the interRAI PC CAPS is associated with reduced needs and symptoms among residents receiving palliative care.
Interestingly, we did find reduced unmet needs and symptoms after the intervention in those nursing homes that had no prior experience with the use of the interRAI LTCF, but we did not find this effect in nursing homes with prior experience. Our hypothesis about a ceiling effect seems to be confirmed. One explanation could be that using an interRAI instrument reduces residents' needs, independent of what specific interRAI instrument is being used, because it provides an overall picture of the person's needs and leads to a better observation of the nursing home residents and of how care professionals act to fulfill their clients' needs (Devriendt et al., Reference Devriendt, Wellens and Flamaing2013; Hermans et al., Reference Hermans, Spruytte and Cohen2016b ; Vanneste & Declercq, Reference Vanneste, Declercq, Müller and Kubitschke2014). This may suggest that it would be useful to add specific palliative care items as a supplement to the other interRAI instruments rather than working with a separate instrument for use in palliative care situations.
STRENGTHS AND LIMITATIONS OF THE STUDY
This is the first study to evaluate whether the use of the interRAI Palliative Care instrument is associated with reduced palliative care needs in the nursing home setting. Our study had a pretest–posttest design with quasi-random assignment to the control and intervention groups. The 15 control nursing homes matched the intervention nursing homes in terms of number of residents and geographic region. Another strength of the study was the use of generalized linear mixed models to adjust for clustering by nursing homes.
However, the limitations of our study also need to be acknowledged. First of all, sampling bias might have occurred as it was not possible to conduct a randomized controlled trial (due to ethical and practical considerations). Because of the strong commitment requirements, all nursing homes that volunteered to participate were included. Furthermore, it was impossible to refuse care professionals to fill out the interRAI PC since the instrument is accessible to all nursing homes through the BelRAI online web application. All volunteering nursing homes were thus included in the study. Sampling bias might also have occurred as, in spite of the matching of control and intervention nursing homes, intervention nursing homes scored better from baseline and onward. This might be due to the fact that these nursing homes are generally more innovative and actively search for methods to improve the quality of care. Finally, some limitations were imposed on the analyses as the population in the pretest was different from that in the posttest.
CONCLUSIONS
After completing the interRAI PC over the course of one year, no reduction in residents' needs and symptoms were detected in the intervention nursing homes. We did find an effect in the subgroup of intervention nursing homes where care professionals did not have prior experience with the interRAI LTCF instrument, as there were fewer needs and symptoms reported after using the interRAI PC over the course of the year. Future research should aim to replicate our study in a longer-term design in order to evaluate whether integrating the use of the interRAI PC in the day-to-day operations of nursing homes supports regular observation of the resident and hence leads to earlier detection of needs and symptoms.
ACKNOWLEDGMENTS
This study is part of the Flanders Study to Improve End-of-Life Care and Evaluation Tools (FLIECE), a collaboration between the Vrije Universiteit Brussels, the University of Ghent, the University of Leuven, and VU Medical Center Amsterdam. This project is funded by the Flemish National Agency for Innovation in Science and Technology (SBO IWT no. 100036). We would also like to thank Joni Gilissen for her contributions to the data collection. Furthermore, we are grateful to prof. Geert Molenberghs for his advice on the statistical analyses.
DISCLOSURES
The authors hereby declare that they have no conflicts of interest to disclose.
SUPPLEMENTARY MATERIALS
To view supplementary materials for this article, please visit https://doi.org/10.1017/S1478951517000153.





