Introduction
Microorganisms are widely distributed in animals and in foods of animal origin. The major causes of concern and product recalls associated with meat and poultry products are Escherichia coli O157:H7, Salmonella enteritidis, Campylobacter jejuni and Listeria monocytogenes (Sofos, Reference Sofos2008). These pathogens are found in animal feces (Hutchinson et al., Reference Hutchinson, Walters, Avery, Munro and Moore2005) and contamination of carcasses and food products by animal feces is the major method for transmitting foodborne pathogens to consumers (Oliver et al., Reference Oliver, Patel, Callaway and Torrence2008). Although foodborne outbreaks in recent years appear to have shifted from being primarily associated with foods of animal origin to increasingly being associated with fresh produce (CDC, 2008), shellfish (Pontrelli et al., Reference Pontrelli, Boccia, Di Renzi, Massari, Giugliano, Celentano, Taffon, Genovese, Di Pasquale, Scalise, Rapicetta, Croci and Salmaso2008) and ingredients (e.g. peanut butter) (CDC, 2009), product recalls and foodborne outbreaks indicate that meat safety continues to be a concern and challenge for food and health authorities and industries. Intervention is possible at many points along the production chain, but on-farm control points are likely to be most cost-effective (Humphrey et al., Reference Humphrey, O'Brien and Madsen2007). Recently, the U.S. Department of Agriculture (USDA) awarded a $2.5 million grant to a university to develop strategies to reduce the shedding or release of Shiga toxin-producing E. coli by cattle (STEC) (USDA, 2011). This important investment indicates the priority being assigned to reducing the microbial load at the farm level in order to prevent contamination further down the food chain.
Even though most bacterial foodborne outbreaks were traced to improper food handling practices, Nørrung and Buncic (Reference Nørrung and Buncic2008) noted that the original sources of foodborne pathogens that cause most meatborne bacterial diseases are asymptomatic farm animals that carry and shed pathogens in the feces. In many cases, farmed ruminants carrying zoonotic pathogens in the gastrointestinal tract show no signs of infection (Adam and Brülisauer, Reference Adam and Brülisauer2010). Transfer of bacteria from the hide and gut contents to the carcass can occur during hide removal and evisceration in the abattoir (McEvoy et al., Reference McEvoy, Doherty, Finnerty, Sheridan, McGuire, Blair, McDowell and Harrington2000; Huffman, Reference Huffman2002; Reid et al., Reference Reid, Small, Avery and Buncic2002). The majority of pathogenic bacteria that can spread at slaughter by cross-contamination were traced back to production on the farm rather than originating from the slaughter plant (Autio et al., Reference Autio, Säteri, Fredriksson-Ahomaa, Rahkio, Lundén and Korkeala2000; Wegener et al., Reference Wegener, Hald, Lo, Wong, Madsen, Korsgaard, Bager, Gerner-Smidt and Mølbak2003). The contamination cycle in food-producing animals occurs through the ingestion of feeds and water that are contaminated with feces. The use of untreated manure as fertilizer and spread of slurry on grazing fields also contribute to the spread of microbial pathogens. Stresses on animals due to poor management (Nørrung and Buncic, Reference Nørrung and Buncic2008), and quantity and quality of animal feed increase the susceptibility to infections and the shedding of foodborne pathogens (Adam and Brülisauer, Reference Adam and Brülisauer2010). Oliver et al. (Reference Oliver, Patel, Callaway and Torrence2008) suggested that all these environmental and management factors must be considered when identifying farm practices and critical control points on the farm where contamination occurs.
Elder et al. (Reference Elder, Keen, Siragusa, Barkocy-Gallagher, Mohammad and Laegreid2000) reported that fecal shedding by cattle is correlated with carcass contamination. This association between fecal prevalence and carcass contamination indicates a role for control of microbial pathogens in cattle on the farm to reduce the risk of human infection from ingestion of undercooked beef or cross-contamination of other foods. Traditionally, much of the research effort was aimed at improving the safety of meat products after slaughter and during processing (Elder et al., Reference Elder, Keen, Siragusa, Barkocy-Gallagher, Mohammad and Laegreid2000) and several post-slaughter steps that reduce the level and frequency of E. coli O157:H7 on beef have been implemented. However, consumers are still sickened by foodborne disease outbreaks. Hence, increased emphasis is being laid on pre-slaughter intervention strategies (Callaway et al., Reference Callaway, Anderson, Edrington, Elder, Genovese, Bischoff, Poole, Jung, Harvey and Nisbet2003). Hynes and Wachsmuth (Reference Hynes and Wachsmuth2000) assert that ‘strategies that reduce foodborne pathogenic bacterial populations in the animal prior to slaughter could produce the most significant reduction in human exposures to the organism and therefore reduction in related illnesses and deaths. It should be noted that activities at the farm level that affect excretion of E. coli O157:H7 by cattle will affect not only fecal contamination of beef but will also affect contamination of the environment, including water that receives runoff from farms. This environmental contamination frequently leads to human infections through bathing, and irrigation and washing of fruits and vegetables.
LeJeune and Wetzel (Reference LeJeune and Wetzel2007) also reported that intervention strategies that target the pathogen in live animals on the farm before slaughter may have the largest impact on improving beef safety. However, Sofos (Reference Sofos2008) noted that pathogen control in animals during the pre-harvest stage is difficult due to limitations in the existing scientific information. It is recognized that on-farm interventions are not likely to eliminate E. coli O157:H7 from cattle presented for slaughter (Arthur et al., Reference Arthur, Keen, Bosilevac, Brichta-Harhay, Kalchayanand, Shackelford, Wheeler, Nou and Koohmaraie2009). An understanding of the possible sources of on-farm infection is important for effective control. For example, Davies (Reference Davies and Mead2005) stated that once pathogens are introduced onto the farm, it is important to understand their spread, the involvement of other farm animals and wildlife, contamination of equipment and personnel, and airborne spread and survival in environmental niches. Only then can the organisms be effectively controlled. Hence, this paper seeks to review the scientific literature reporting potential sources of on-farm infections among food-producing animals and intervention strategies adopted to reduce the risk of contamination.
Foodborne outbreaks traced to beef products
Table 1 shows the relative frequency of foodborne outbreaks in beef due to E. coli O157:H7. Foodborne disease caused by E. coli O157:H7 infection decreased by 44% in the US in 2010 compared to 1996–1998. This is translated as ⩽1 case of E. coli per 100,000 and reaching this goal was a huge success (Table 2). The goal for 2020 is a 50% reduction compared to the 3-year baseline period of 2006–2008. Many factors likely contributed to the decrease in incidence of E. coli O157:H7 infections, such as improved detection and investigation of outbreaks which leads to prompt recalls and enhanced knowledge on sources of contamination (CDC, 2011). Interventions applied pre- and post-slaughter also contributed to the decline in E. coli O157 infections (Gyles, Reference Gyles2007; CDC, 2011).
Table 1. Relative frequency of beef as the implicated source of E. coli outbreaks reported internationally between 1988 and 2007 (Greig and Ravel, Reference Greig and Ravel2009)
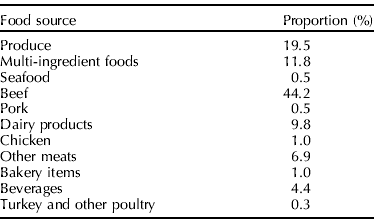
Table 2. Number of STEC O157:H7 infections per 100,000 persons per year in the US (CDC, 2011)

Cattle are the major reservoir for E. coli O157:H7 (Nørrung and Buncic, Reference Nørrung and Buncic2008). It is estimated that 20 STEC O157 illnesses occur for every one that is reported (Mead et al., Reference Mead, Slutsker, Dietz, McCaig, Breese, Shapiro, Griffin and Tauxe1999). E. coli O157:H7 has been more researched than other serotypes of STEC because it is the serotype most frequently involved in outbreaks and in severe disease. Its ability to ferment sorbitol is a convenient marker for screening for this pathogen and has facilitated detection of this pathogen in patients and in animal feces (Bettelheim, Reference Bettelheim2007).
Sources and transmission routes of E. coli O157:H7 in beef cattle
There are several recent reviews that critically discuss the risk factors and the transmission and prevalence of E. coli O157:H7 in cattle (Oliver et al., Reference Oliver, Patel, Callaway and Torrence2008; Ellis-Iversen et al., Reference Ellis-Iversen, Cook, Smith, Pritchard and Nielen2009; Berry and Wells, Reference Berry and Wells2010). The prevalence of E. coli in water, feed, hide and soil are of major importance. We have illustrated and summarized some of the sources and transmission routes of E. coli O157:H7 in beef cattle (Fig. 1).

Fig. 1. Potential on-farm contamination of beef cattle with E. coli O157:H7.
Water and feed
LeJeune et al. (Reference LeJeune, Besser and Hancock2001) demonstrated that cattle water troughs can be reservoirs of E. coli O157:H7 on farms and serve as a source of infection for cattle. This is in agreement with Hancock et al. (Reference Hancock, Besser, Rice, Ebel, Herriott and Carpenter1998) who had earlier reported that E. coli O157:H7 were able to survive in water trough sediments for at least 4 months and appeared to multiply especially in warm weather. However, improved water trough hygiene did not reduce the risk of E. coli O157:H7 in young cattle (Ellis-Iversen et al., Reference Ellis-Iversen, Smith, Van Winden, Paiba, Watson, Snow and Cook2008, Reference Ellis-Iversen, Cook, Smith, Pritchard and Nielen2009). Similar to water contamination, the hygiene of animal feed plays a key role in microbial contamination in livestock (Crump et al., Reference Crump, Griffin and Angulo2002) since feed can be a vehicle for transmitting E. coli O157:H7 to cattle (Hancock et al., Reference Hancock, Besser, LeJeune, Davis and Rice2001; Davis et al., Reference Davis, Hancock, Rice, Call, DiGiacomo, Samadpour and Besser2003; Horchner et al., Reference Horchner, Brett, Gormley, Jenson and Pointon2006). Fenlon and Wilson (Reference Fenlon and Wilson2000) also demonstrated that E. coli can multiply in feed. E. coli O157:H7 inoculated in laboratory silage (made from rye grass) increased from an initial level of 103 to 107 colony-forming units (CFU) g−1 within 13 days. E. coli O157:H7 was isolated from the oral cavities of 74.8% of cattle examined and it has been suggested that feed may be contaminated by E. coli O157:H7 from cattle saliva (Keene and Elder, Reference Keene and Elder2002). Cattle return rumen contents to their mouths to be chewed again and further digested and this may be the most probable source of E. coli O157:H7 in the animals’ mouths (Tkalcic et al., Reference Tkalcic, Zhao, Harmon, Doyle, Brown and Zhao2003). Other possible sources include fecal contamination by wildlife (Fischer et al., Reference Fischer, Zhao, Doyle, Goldberg, Brown, Sewell, Kavanaugh and Bauman2001; Renter et al., Reference Renter, Sargeant, Hygnstorm, Hoffman and Gillespie2001), including birds (Shere et al., Reference Shere, Bartlett and Kaspar1998; Nielsen et al., Reference Nielsen, Skov, Madsen, Lodal, Jespersen and Baggesen2004), rodents (Nielsen et al., Reference Nielsen, Skov, Madsen, Lodal, Jespersen and Baggesen2004) and insects (Ahmad et al., Reference Ahmad, Nagaraja and Zurek2007). However, LeJeune et al. (Reference LeJeune, Hancock, Wasteson, Skjerve and Urdahl2006) did not find significant correlation between the magnitude of feed contamination and E. coli O157:H7 prevalence in cattle.
Diez-Gonzalez et al. (Reference Diez-Gonzalez, Callaway, Kizouliz and Russell1998) argued that grain-diet promoted acid production in the colon, which leads to increased acid-resistant generic E. coli strains in the faeces. The authors demonstrated that hay-fed cattle had a lower concentration of volatile fatty acids in their colons and that acid shock killed more than 99.99% of the E. coli. When diets were supplemented with grain, acids accumulated, colonic pH declined and this selectively favored E. coli resistant to extreme acid shock. There has been much debate since the intervention of switching to a forage-based diet before slaughter was first described. It was noted that the authors did not investigate E. coli O157:H7 and that the results with generic E. coli could not be extrapolated to E. coli O157:H7. Although grain-fed cattle harbored more acid-resistant generic E. coli than did forage-fed cattle (Diez-Gonzalez et al., Reference Diez-Gonzalez, Callaway, Kizouliz and Russell1998; Hovde et al., Reference Hovde, Austin, Cloud, Williams and Hunt1999), the acid resistant E. coli O157:H7 was not suppressed by a forage diet (Hovde et al., Reference Hovde, Austin, Cloud, Williams and Hunt1999; Grauke et al., Reference Grauke, Kudva, Yoon, Hunt, Williams and Hovde2002). Van Baale et al. (Reference Van Baale, Sargeant, Gnad, DeBey, Lechtenberg and Nagaraja2004) also demonstrated that feeding forage actually increased the shedding of E. coli O157:H7 in cattle. Cattle fed forage diets were culture positive for E. coli O157:H7 in the feces for longer duration than cattle fed a grain diet. Judging from the various dietary interventions, Callaway et al. (Reference Callaway, Carr, Edrington, Anderson and Nisbet2009) emphasized that dietary manipulations is a potentially powerful method to reduce E. coli populations in cattle but further research is essential to clarify the effect of different diets on the bovine gastrointestinal system (Wood et al., Reference Wood, McKendrick and Gettinby2006).
Besides implementing HACCP in animal feed-processing plants, management of feed in the farm must involve reducing exposure to wildlife excreta (Daniels et al., Reference Daniels, Hutchings and Greig2003). Oliver et al. (Reference Oliver, Patel, Callaway and Torrence2008) concluded that the contamination cycle occurs when cattle ingest contaminated feed and water, followed by shedding of foodborne pathogens in feces, which then contaminate feeds and animal drinking water, causing new infections and reinfection of animals. In order to break this infection−reinfection cycle (Fig. 2), on-farm foodborne control programms based on the critical points of transmission can be designed to reduce the introduction of foodborne pathogens into processing plants and the human food chain (Sargeant et al., Reference Sargeant, Gillespie, Oberst, Phebus, Hyatt, Bohra and Galland2000; Oliver et al., Reference Oliver, Patel, Callaway and Torrence2008). Methods for both pre-harvest and post-harvest control of E. coli O157:H7 have been widely studied, but the development of a simple and universal effective mitigation strategy remains elusive. The most successful strategy will involve the implementation of both pre- and post-harvest measures (Wood et al., Reference Wood, McKendrick and Gettinby2007).

Fig. 2. On-farm control measures that have been suggested.
Role of hide for pathogen transmission
Hide cleanliness and prevalence of foodborne pathogens may be associated with the pen feedlot condition. Smith et al. (Reference Smith, Blackford, Younts, Moxley, Gray, Hungerford, Milton and Klopfenstein2001) observed that higher percentages of cattle in muddy feedlot pens shed E. coli O157:H7 compared to cattle in normal pen conditions. These researchers reasoned that the muddy feedlot pens may facilitate fecal – oral transmission. Similarly, Cobbold and Desmarchelier (Reference Cobbold and Desmarchelier2002) suggested that pen floors and hides were the main source of STEC transmission to dairy calves. Bach et al. (Reference Bach, Selinger, Stanford and McAllister2005) also suggested that faeces on pen floors are a more significant source of infection than are feed or drinking water. Reid et al. (Reference Reid, Small, Avery and Buncic2002) found that the brisket area contains the highest concentration of bacteria on the hide compared to the rump or flank area. This is in agreement with McEvoy et al. (Reference McEvoy, Doherty, Finnerty, Sheridan, McGuire, Blair, McDowell and Harrington2000) who demonstrated that the total viable bacterial counts were significantly higher at the brisket. This may be attributable to the fact that the brisket area is in contact with the floor when cattle are resting. This is also the site where the initial cut is made during the hide-removal process and there is a high probability of transferring pathogens from the hide to the carcass (McEvoy et al., Reference McEvoy, Doherty, Finnerty, Sheridan, McGuire, Blair, McDowell and Harrington2000). High-level fecal shedding of E. coli O157:H7 has also been linked to increased hide contamination (Arthur et al., Reference Arthur, Keen, Bosilevac, Brichta-Harhay, Kalchayanand, Shackelford, Wheeler, Nou and Koohmaraie2009; Stephens et al., Reference Stephens, McAllister and Stanford2009).
There is evidence that super shedding cattle have a large impact on the overall contamination of animals due to the increased animal density and confined spaces associated with farm and lairage environments (Arthur et al., Reference Arthur, Brichta-Harhay, Bosilevac, Kalchayanand, Shackelford, Wheeler and Koohmaraie2010). Cattle that excrete exceptionally high numbers of E. coli O157 have been referred to as high-level shedders or ‘super-shedders’ (Chase-Topping et al., Reference Chase-Topping, McKendrick, Pearce, MacDonald, Matthews, Halliday, Allison, Fenlon, Low, Gunn and Woolhouse2007; Berry and Wells, Reference Berry and Wells2010). High shedding has been defined as counts of E. coli O157:H7 that are ≥103 (Low et al., Reference Low, McKendrick, McKechnie, Fenlon, Naylor, Currie, Smith, Allison and Gally2005) or 104 CFU g−1 of feces (Omisakin et al., Reference Omisakin, MacRae, Ogden and Strachan2003; Ogden et al., Reference Ogden, MacRae and Strachan2004). Matthews et al. (Reference Matthews, Low, Gally, Pearce, Mellor, Heesterbeek, Chase-Topping, Naylor, Shaw, Reid, Gunn and Woolhouse2006) reported that 20% of the E. coli O157:H7 infected cattle were responsible for 80% of the transmission of the organism in Scottish cattle farms. Another study reported similar findings, where 9% of the animals shedding E. coli O157:H7 produced over 96% of the total E. coli O157:H7 fecal load for the group (Omisakin et al., Reference Omisakin, MacRae, Ogden and Strachan2003). Cobbold et al. (Reference Cobbold, Hancock, Rice, Berg, Stilborn, Hovde and Besser2007) showed that the cattle that did not shed E. coli O157:H7 were five times more likely to be housed in a pen that did not contain a super-shedder. Matthews et al. (Reference Matthews, Low, Gally, Pearce, Mellor, Heesterbeek, Chase-Topping, Naylor, Shaw, Reid, Gunn and Woolhouse2006) suggested that the spread of E. coli O157:H7 between cattle could be controlled if one could prevent super shedding in the 5% of individuals that are the main source of contamination. Ultimately, significant reductions may be made by targeting the super-shedders (Chase-Topping et al., Reference Chase-Topping, Gally, Low, Matthews and Woolhouse2008). Measures that reduce the carriage and shedding of E. coli O157:H7 also have the potential to reduce secondary transmission through feed, drinking water or direct contact and grooming (Gyles, Reference Gyles2007). Chase-Topping et al. (Reference Chase-Topping, Gally, Low, Matthews and Woolhouse2008) presented a comprehensive discussion of super-shedding and the risk for human infection.
Wild and domestic animals and insects
Wildlife fecal contamination can serve as a potential source of infection to livestock (Daniels et al., Reference Daniels, Hutchings and Greig2003). Animals that have been shown to carry E. coli O157:H7 include, but are not limited to, wild deer (Sargeant et al., Reference Sargeant, Hafer, Gillespie, Oberst and Flood1999; Fischer et al., Reference Fischer, Zhao, Doyle, Goldberg, Brown, Sewell, Kavanaugh and Bauman2001; Renter et al., Reference Renter, Sargeant, Hygnstorm, Hoffman and Gillespie2001), rats (Cizek et al., Reference Cizek, Alexa, Literak, Hamrik, Novak and Smola1999; Nielsen et al., Reference Nielsen, Skov, Madsen, Lodal, Jespersen and Baggesen2004) and raccoons (Shere et al., Reference Shere, Bartlett and Kaspar1998). Birds are another singular and important source of transmission and birds found positive for E. coli O157:H7 include pigeons (Shere et al., Reference Shere, Bartlett and Kaspar1998), gulls (Wallace et al., Reference Wallace, Cheasty and Jones1997) and starlings (Nielsen et al., Reference Nielsen, Skov, Madsen, Lodal, Jespersen and Baggesen2004). A study by Scaife et al. (Reference Scaife, Cowan, Finney, Kinghorn-Perry and Crook2006) in Norfolk, UK found 20.62% (20/97) of fecal samples collected from wild rabbits were positive for STEC O157. Ahmad et al. (Reference Ahmad, Nagaraja and Zurek2007) showed that houseflies are capable of transmitting E. coli O157:H7 to cattle. Fecal samples from all calves exposed to inoculated flies were positive. The pathogen counts were as high as 1.5×105 CFU per fly. This high concentration of E. coli O157:H7 suggested that houseflies are not simply mechanical vectors, but that the pathogen likely multiplied in the gastrointestinal tract of the houseflies (Alam and Zurek, Reference Alam and Zurek2004). Although cattle are considered the main reservoir of E. coli O157:H7, strains of E. coli O157:H7 may be introduced into cattle populations through feed (Daniels et al., Reference Daniels, Hutchings and Greig2003) and water contaminated with the feces of wild and domestic animals (Wetzel and LeJeune, Reference Wetzel and LeJeune2006). In most instances it is impossible to keep wild animals out of the farm but it is important for farms and farm workers to be aware that wild animals can also act as vectors for infection via the fecal−oral route.
On-farm intervention strategies
The distribution of E. coli O157:H7, its persistence in the environment, and its ability to infect and reinfect cattle and wildlife make eradication an unrealistic goal. Traditional means of controlling infectious agents, such as eradication, involving testing and removal of carrier animals are not feasible for this pathogen. An achievable objective is to reduce the magnitude or prevalence of E. coli O157:H7 in feces and to break the contamination cycle (LeJeune and Wetzel, Reference LeJeune and Wetzel2007). Koohmaraie et al. (Reference Koohmaraie, Arthur, Bosilevac, Guerini, Shackelford and Wheeler2005, Reference Koohmaraie, Arthur, Bosilevac, Brichta-Harhay, Kalchayanand, Shackelford and Wheeler2007) suggested that the post-harvest is the most logical and effective step to maximally reduce E. coli O157:H7 (and other pathogens) but it is evident that reductions in the pre-harvest stages will reduce environmental contamination and enhance the effectiveness of post-harvest measures.
Farm management practices – especially the maintenance of feed and water may be the most practical means of reducing infectious agents in cattle (Hancock et al., Reference Hancock, Besser, LeJeune, Davis and Rice2001). Oliver et al. (Reference Oliver, Patel, Callaway and Torrence2008) suggested that all environmental and management factors must be considered when identifying farm practices and critical control points on the farm where contamination occurs. According to Collins and Wall (Reference Collins and Wall2004), it is the primary producer who should take all reasonable measures to reduce the entry and prevalence of E. coli O157 on his/her farm. They need to adopt approaches on the farm with the objective of eliminating or minimizing carriage and shedding of zoonotic agents by cattle. However, although some measures have shown promise it is difficult to pinpoint a single practice to a producer or a feedlot operator that can be predicted to consistently reduce the prevalence and/or concentration of E. coli O157:H7 in cattle in a cost-effective manner (Koohmaraie et al., Reference Koohmaraie, Arthur, Bosilevac, Guerini, Shackelford and Wheeler2005). Regardless of the challenges, Loneragan and Brashears (Reference Loneragan and Brashears2005) reported that the potential of on-farm control exists. Table 3 shows a summary of some of the control measures tested in vitro or in animal trials and on farms. Some have been effective under artificial conditions but require further investigation to evaluate the method and its feasibility in implementation at the farm level. We have also illustrated and summarized some of the management measures that have been tested in animal trials and at the farm level to reduce and control E. coli O157:H7 in beef cattle (Fig. 2).
Table 3. On-farm E. coli O157:H7 control methods investigated in experimental animal trials or on farms

Feed
A number of studies have identified animal feed as a potential source of infection of cattle with STEC O157 (Hancock et al., Reference Hancock, Besser, LeJeune, Davis and Rice2001; Van Donkersgoed et al., Reference Van Donkersgoed, Berg, Potter, Hancock, Besser, Rice, LeJeune and Klashinsky2001; Dodd et al., Reference Dodd, Sanderson, Sargeant, Nagaraja, Oberst, Smith and Griffin2003). In 2004, Codex Alimentarius introduced the Code of Practice on Good Animal Feeding to establish a feed safety system for food-producing animals. The objective of the Code is to help ensure the safety of food through adherence to good animal feeding practices at the farm and good manufacturing practices during the processing and handling of feed and feed ingredients. It also states that ‘where appropriate, Hazard Analysis Critical Control Point (HACCP) principles should be followed to control hazards that may occur’.
Dietary intervention has been suggested to offer a simple and practical means of reducing the prevalence of E. coli O157:H7 in the hindgut (Fox et al., Reference Fox, Depenbusch, Drouillard and Nagaraja2007). Berg et al. (Reference Berg, McAllister, Bach, Stilborn, Hancock and LeJeune2004) showed that cattle fed corn-based diets shed more generic E. coli than do cattle fed barley-based finishing diets. The more extensively cereal grains were processed, the more starch was digested in the rumen and this reduced the amount entering the lower digestive tract (Huntington, Reference Huntington1997). Since corn is less digestible in the rumen compared to barley (Huntington, Reference Huntington1997), this provided more undigested starch in the large intestine and resulted in increased fermentation and reduced fecal pH. Corn-fed cattle were found to have a lower mean fecal pH value (pH 5.85) compared to barley-fed cattle (pH 6.51) (Buchko et al., Reference Buchko, Holley, Olson, Gannon and Veira2000; Berg et al., Reference Berg, McAllister, Bach, Stilborn, Hancock and LeJeune2004). Feeding dry-rolled grain diet to cattle reduced the prevalence of E. coli O157:H7 by 35% as compared to steam-flaked grain diet. It is possible that dry-rolling allows more substrate to reach the hindgut where it increased fermentation and volatile fatty acid production and made the hindgut inhospitable to the survival of E. coli O157:H7 (Fox et al., Reference Fox, Depenbusch, Drouillard and Nagaraja2007; Depenbusch et al., Reference Depenbusch, Nagaraja, Sargeant, Drouillard, Loe and Corrigan2008). However, a number of studies demonstrated that this approach is not likely to be effective for E. coli O157:H7.
In calves, increased risk has been associated with feeding colostrum from a bucket compared to suckling. The reduced risk among calves that did suckle colostrum from the mother could be explained by the increased protection from maternal antibodies (Rugbjerg et al., Reference Rugbjerg, Nielsen and Andersen2003). The researchers hypothesized that calves that suckle colostrum from the cow and stayed longer with the mother were in some way protected from infection with E. coli O157. However, this needs to be confirmed by further studies.
The fermentation of cereal grains to produce ethanol results in a co-product called distillers’ grains (DG). The co-product is fed either as wet distillers’ grain (WDG) (approximately 30% dry matter) or dried distillers’ grains (DDG) (approximately 90% dry matter) (Spiehs et al., Reference Spiehs, Whitney and Shursinm2002). DG were shown to increase daily weight gain in finishing cattle due to the condensed nutrients and hence were used in ruminant diets (Ham et al., Reference Ham, Stock, Klopfenstein, Larson, Shain and Huffman1994). Cattle fed diets including 25% of DDG or 40% of WDG with solubles (WDGS) had a higher prevalence of E. coli O157:H7 in their feces. It is possible that (i) feeding dried distillers grains results in decreased starch concentration in the hindgut, which may alter the ecology and favor the growth of E. coli O157:H7 or (ii) components of the brewers’ grain may stimulate the bacterial growth (Jacob et al., Reference Jacob, Fox, Drouillard, Renter and Nagaraja2008; Wells et al., Reference Wells, Shackelford, Berry, Kalchayanand, Guerini, Varel, Arthur, Bosilevac, Freetly, Wheeler, Ferrell and Koohmaraie2009). Cattle fed 20 or 40% of WDGS were also found to have prolonged survival of inoculated E. coli O157:H7 compared to those fed 0% WDGS. The slurries obtained from cattle fed 20 or 40% WDGS had lower concentrations of L-lactate and pH values between 6.0 and 8.0 (Varel et al., Reference Varel, Wells, Berry, Spiehs, Miller, Ferrell, Shackelford and Koohmaraie2008). L-lactate has a significant antimicrobial effect on E. coli O157:H7 as well as non-O157 E. coli (McWilliam Leitch and Stewart, Reference McWilliam Leitch and Stewart2002).
Essential oils have been shown to inhibit foodborne pathogens in pure culture (Burt, Reference Burt2004). The addition of plant phenolic acids such as cinnamic acid, coumaric acid and ferulic acid to feces increased the death rate of E. coli O157:H7 (Wells et al., Reference Wells, Berry and Varel2005). Addition of orange peel and pulp to inoculated ruminal fluid reduced E. coli O157:H7 from 105 to 102 CFU ml−1. This may be the result of the antimicrobial action of essential oils such as limonene found in the peel (Callaway et al., Reference Callaway, Carroll, Arthington, Pratt, Edrington, Anderson, Galyean, Ricke, Crandall and Nisbet2008a). It is still unknown as to which constituents or mixtures of essential oils are responsible for their antimicrobial activity (Espina et al., Reference Espina, Somolinos, Lorán, Conchello, Garcìa and Pagán2011). The major chemical component of most citrus oils is limonene with sweet orange containing 68–98% and lemon 45–76% (Svoboda and Greenaway, Reference Svoboda and Greenaway2003). Further research is still needed to determine the mechanisms of action and whether the antimicrobial activity can be expressed in the livestock's lower gastrointestinal tract (Callaway et al., Reference Callaway, Carroll, Arthington, Pratt, Edrington, Anderson, Galyean, Ricke, Crandall and Nisbet2008a). In addition, candidate plant compounds with antimicrobial activity or grasses used as cattle forages, which contain phenolic acids can be used as potential dietary additives or manure treatments (Wells et al., Reference Wells, Berry and Varel2005). Doyle and Erickson (Reference Doyle and Erickson2011) suggested that the active components of antimicrobial compounds may not be reaching the E. coli colonization sites in animals; hence, encapsulation of these ingredients may warrant further investigation. Numerous studies have been carried out on dietary interventions to determine the optimum method of reducing the prevalence of E. coli O157:H7 in beef cattle. Since E. coli O157:H7 is a normal flora of cattle it is a daunting task to reduce its presence in the intestine.
Probiotics and direct-fed microbials
Probiotics or direct-fed microbials are preparations of live bacteria fed to a host to elicit beneficial health effects in the host (Schrezenmeir and de Vrese, Reference Schrezenmeir and de Vrese2001). Several lactic acid bacteria (LAB), most commonly Lactobacillus, Enterococcus and Streptococcus, have been tested as probiotic agents or competitive exclusion products (CEP) for livestock (Brashears et al., Reference Brashears, Jaroni and Trimble2003b). Competitive exclusion cultures consist of a mixture of undefined microbes and are usually isolated from the gastrointestinal tract of the animal species that will be treated, while probiotics are well-defined strains that have been cultured separately prior to application (Doyle and Erickson, Reference Doyle and Erickson2011).
The genus Lactobacillus is one of the most commonly used genera of probiotic organisms added to a range of feeds (Gaggìa et al., Reference Gaggìa, Mattarelli and Biavati2010). A specific strain, Lactobacillus acidophilus NP51, reduced the prevalence of E. coli O157:H7 by 49% in animals receiving NP51 compared to controls (Brashears et al., Reference Brashears, Galyean, Loneragan, Mann and Killinger-Mann2003a). Peterson et al. (Reference Peterson, Klopfenstein, Erickson, Folmer, Hinkley, Moxley and Smith2007a) reported that by administering L. acidophilus strain NP51 in feed daily for 2 years, fecal shedding of E. coli O157:H7 decreased by 35% in beef cattle. In another study, steers fed a standard steam-flaked corn-based finishing diet containing L. acidophilus NP51 showed a reduction of E. coli O157:H7 fecal shedding by 57% (Younts-Dahl et al., Reference Younts-Dahl, Galyean, Loneragan, Elam and Brashears2004) while a combination of L. acidophilus NP51 and Propionibacterium freudenreichii reduced fecal shedding of E. coli O157 by 32% compared to the control group (Tabe et al., Reference Tabe, Oloya, Doetkott, Bauer, Gibbs and Khaitsa2008). Selected cultures containing E. coli strains whose colicins killed E. coli O157:H7 (Schamberger and Diez-Gonzalez, Reference Schamberger and Diez-Gonzalez2002) or mixtures of probiotic E. coli (Tkalcic et al., Reference Tkalcic, Zhao, Harmon, Doyle, Brown and Zhao2003; Zhao et al., Reference Zhao, Tkalcic, Doyle, Harmon, Brown and Zhao2003) have also been tested for probiotic potential against E. coli O157:H7. However, bacteria can become resistant to the antimicrobial mechanisms of probiotic organisms. Laboratory studies indicated that E. coli O157:H7 can become resistant to individual colicins; hence, multiple-colicinogenic strains may be required for effective treatments (Schamberger and Diez-Gonzalez, Reference Schamberger and Diez-Gonzalez2005).
The inhibition of E. coli O157:H7 by probiotics may result from the decrease in pH due to the production of organic acids by LAB. It is also speculated that other factors such as production of bacteriocins, hydrogen peroxide, low-molecular-weight metabolites such as diacetyl and CO2, or enzymes by LAB contribute to the inhibition (Brashears et al., Reference Brashears, Jaroni and Trimble2003b). Fujiwara et al. (Reference Fujiwara, Hashiba, Hirota and Forstner1997) revealed that bifidobacteria produce a proteinaceous molecule(s) which prevents the binding of E. coli Pb176, an enterotoxigenic E. coli (ETEC) strain to intestinal mucosa. Studies by Medellin-Peña and Griffiths (Reference Medellin-Peña and Griffiths2009) also showed that the probiotic L. acidophilus strain La-5 is capable of modifying E. coli O157:H7 virulence in vitro and in vivo. L. acidophilus La-5 secretes a molecule(s), which was able to reduce E. coli O157:H7 attachment to gastro-intestinal epithelial cells (Medellin-Peña and Griffiths, Reference Medellin-Peña and Griffiths2009) and directly inhibit the transcription of O157:H7 genes involved in colonization (Medellin-Peña et al., Reference Medellin-Peña, Wang, Johnson, Anand and Griffiths2007). From the above studies, the potential of probiotics to reduce E. coli O157:H7 is promising and many commercial products are currently in use.
Prebiotics
Prebiotics are ‘nondigestible food ingredients such as fructooligosaccharides (FOS), inulin and galactooligosaccharides (GOS), that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon’ (Gibson and Roberfroid, Reference Gibson and Roberfroid1995; Gaggìa et al., Reference Gaggìa, Mattarelli and Biavati2010). Oligosaccharides are of interest because they are neither hydrolyzed nor absorbed in the upper part of the gastrointestinal tract but stimulate the growth and/or activity of desirable bacteria in the colon (Cummings et al., Reference Cummings, MacFarlane and Englyst2001). The use of prebiotics in cattle has been limited due to the ability of ruminants to degrade most prebiotics, but developments in rumen-protective technologies may allow prebiotics to be used in feedlot and dairy cattle (Callaway et al., Reference Callaway, Edrington, Anderson, Harvey, Genovese, Kennedy, Venn and Nisbet2008b; Doyle and Erickson, Reference Doyle and Erickson2011). There is a concern that prebiotics may promote satiety and this may decrease feed intake and weight gain in animals (Cani et al., Reference Cani, Neyrinck, Maton and Delzenne2005). Ultimately, cattle in feedlots need to be fed energy-dense diets to improve growth and produce a high-quality product and any pathogen reduction benefit from a diet should not come at an increased cost for the farm (Berry and Wells, Reference Berry and Wells2010).
Bacteriophage
Bacteriophages are obligate parasites that prey upon specific host bacteria (Greer, Reference Greer2005). Phages have narrow target spectra and this allows them to be used as potential alternatives to control selective pathogens in mixed microbial populations (Callaway et al., Reference Callaway, Anderson, Edrington, Elder, Genovese, Bischoff, Poole, Jung, Harvey and Nisbet2003; LeJeune and Wetzel, Reference LeJeune and Wetzel2007; Niu et al., Reference Niu, McAllister, Xu, Johnson, Stephens and Stanford2009a). Studies to date suggest that multiple bacteriophages administered in combination are more effective for eliminating E. coli O157:H7 (Bach et al., Reference Bach, McAllister, Veira, Gannon and Holley2002; O'Flynn et al., Reference O'Flynn, Ross, Fitzgerald and Coffey2004; Niu et al., Reference Niu, Johnson, Xu, McAllister, Sharma, Louie and Stanford2009b). A mixture of three O157-specific bacteriophages was shown to lyse liquid cultures of E. coli O157:H7 at 4 and 37°C. However, no individual phage was able to eliminate the culture (Kudva et al., Reference Kudva, Jelacic, Tarr, Youderian and Hovde1999). O'Flynn et al. (Reference O'Flynn, Ross, Fitzgerald and Coffey2004) also found that a mixture of bacteriophages reduced the numbers of E. coli O157:H7 in in vitro challenge tests. Similarly, in an in vivo study conducted by Callaway et al. (Reference Callaway, Edrington, Brabban, Anderson, Rossman, Engler, Carr, Genovese, Keen, Looper, Kutter and Nisbet2008c), the authors found that a cocktail of phages isolated from cattle feces reduced E. coli O157:H7 populations in the feces of sheep by 24 h after phage treatment.
Grauke et al. (Reference Grauke, Kudva, Yoon, Hunt, Williams and Hovde2002) identified the rectoanal junction of cattle as the predominant site for colonization by E. coli O157:H7. Hence, Sheng et al. (Reference Sheng, Knecht, Kudva and Hovde2006) applied a combination of two phage strains directly to the rectoanal junction site of the cattle. In addition, the researchers also continuously administered bacteriophage (106 plaque-forming units (PFU) ml−1) orally via drinking water. Both treatments reduced but did not eliminate E. coli O157:H7 in the inoculated steers. In another study, Rozema et al. (Reference Rozema, Stephens, Bach, Okine, Johnson, Stanford and McAllister2009) demonstrated that the oral administration of bacteriophage resulted in a lower level shedding of E. coli O157:H7 compared to rectal application. This may be due to the increased retention period within the digestive tract, which allows phages to replicate. Acid resistance of phage is critical in oral application to ensure that a sufficient amount of active phages reach the large intestine (Dini and de Urraza, Reference Dini and De Urraza2010). Stanford et al. (Reference Stanford, McAllister, Niu, Stephens, Mazzocco, Waddell and Johnson2010) developed polymer-encapsulated phages (Ephage) in combination with bolus or feed delivery systems. Ephage successfully released active phages when the capsule reached the large intestine and the pH was more than 7.0. However, Ephage did not reduce the shedding of E. coli O157:H7 in the treated steers.
Bacteriophage therapy appears to be most effective when E. coli O157:H7 populations are ≥104 CFU g−1. Hence, this method can be a strategic intervention option targeted at super shedders within a herd. Hide contamination and transmission can be reduced if fecal concentrations of E. coli O157:H7 are kept below 200 CFU g−1 (Arthur et al., Reference Arthur, Keen, Bosilevac, Brichta-Harhay, Kalchayanand, Shackelford, Wheeler, Nou and Koohmaraie2009). This can subsequently reduce the contamination load at the slaughter facilities (Rozema et al., Reference Rozema, Stephens, Bach, Okine, Johnson, Stanford and McAllister2009). However, there are still issues of gaining regulatory approval, development of phage resistance and the possibility of genetic materials being transferred to bacterial hosts (Joerger, Reference Joerger2003). In order to obtain the necessary regulatory approval for bacteriophage therapy, Bach et al. (Reference Bach, McAllister, Veira, Gannon and Holley2002) suggested sequencing the genome of bacteriophages, demonstrating that undesirable genes are not transferred from bacteriophage to non-target bacteria, and evaluating the effects of the bacteriophage on E. coli O157:H7 toxin production.
Vaccination
E. coli O157:H7 infection in cattle requires type-III secreted proteins (TTSP) which enable the bacteria to colonize the intestinal and recto-anal junction mucosa. Hence, a vaccine based on type-III secreted proteins of E. coli O157:H7 was developed by Potter et al. (Reference Potter, Klashinsky, Li, Frey, Townsend, Rogan, Erickson, Hinkley, Klopfenstein, Moxley, Smith and Finlay2004). The vaccine reduced the prevalence, duration and magnitude of E. coli O157:H7 fecal shedding in experimentally inoculated cattle. However, in early studies the vaccine did not significantly reduce the prevalence of fecal E. coli O157:H7 in feedlot cattle when tested in nine feedlots under commercial conditions (Van Donkersgoed et al., Reference Van Donkersgoed, Hancock, Rogan and Potter2005). After subsequent reformulations of the vaccine (e.g. using different dosage and adjuvant), the vaccine product effectively reduced the colonization of cattle by E. coli O157:H7 (Peterson et al., Reference Peterson, Klopfenstein, Moxley, Erickson, Hinkley, Rogan and Smith2007c; Smith et al., Reference Smith, Moxley, Peterson, Klopfenstein, Erickson, Bretschneider, Berberov and Clowser2009). A two-dose vaccination regime resulted in feedlot cattle being 98.3% less likely to have their terminal rectal mucosa colonized by E. coli O157:H7 (Peterson et al., Reference Peterson, Klopfenstein, Moxley, Erickson, Hinkley, Bretschneider, Berberov, Rogan and Smith2007b). Another study by Rogan et al. (Reference Rogan, Smith, Moxley, Potter and Strauss2009) showed that vaccination with the E. coli O157:H7 Type-III secretion proteins vaccine decreased the environmental pen-level prevalence of E. coli O157:H7. Similarly, Peterson et al. (Reference Peterson, Klopfenstein, Moxley, Erickson, Hinkley, Rogan and Smith2007c) found that vaccinating a majority of cattle within a pen reduced bacterial shedding among unvaccinated cattle sharing the pen.
Another development in vaccination against E. coli O157:H7 is the use of siderophore receptor and porin (SRP) proteins. The SRP protein vaccine reduced the burden of E. coli O157:H7 in cattle by targeting the SRP proteins of E. coli to disrupt their iron transport system (Fox et al., Reference Fox, Thomson, Drouillard, Thornton, Burkhardt, Emery and Nagaraja2009; Thomson et al., Reference Thomson, Loneragan, Thornton, Lechtenberg, Emery, Burkhardt and Nagaraja2009). The vaccine reduced fecal prevalence and fecal concentration of E. coli O157:H7. A three-dose vaccine resulted in a two-log reduction of E. coli O157:H7 in feces (Thomson et al., Reference Thomson, Loneragan, Thornton, Lechtenberg, Emery, Burkhardt and Nagaraja2009).
Husbandry
In the UK, cattle are graded before slaughter according to a five-point cleanliness scoring system. This is in accordance with the Meat Hygiene Service's Clean Livestock Policy with lower scores of 1–2 given to clean and dry animals and scores of 4–5 given to filthy and wet animals. Only livestock classed as categories 1 and 2 (clean and dry/slightly dirty and dry/damp are allowed to proceed to slaughter without further interventions (FSA, 2007)). This underscores the importance of hide cleanliness which helps to minimize the transfer of pathogens to the carcass during dressing. Providing sufficient clean and dry bedding will be the most effective means of preventing heavy soiling of the brisket area (Reid et al., Reference Reid, Small, Avery and Buncic2002). In another study, the housing of cattle on pens surfaced with pond ash (a by-product from coal combustion) or pens surfaced with soil did not affect fecal shedding of E. coli O157:H7 by cattle (Berry et al., Reference Berry, Wells, Arthur, Woodbury, Nienaber, Brown-Brandl and Eigenberg2010). An adequate design and layout of the resting and feeding area and the use of scrapers are also important hygienic measures. However, Barker et al. (Reference Barker, Amory, Wright, Blowey and Green2007) reported that even though automatic scrapers can improve hygiene in barns because of frequent scraping, they can also make cattle dirtier because of the wave of slurry that coats the claws and possibly the lower legs of cattle. The surface properties and cleanability of flooring and feeding surfaces of cattle pens may also affect food safety (Kymäläinen et al., Reference Kymäläinen, Kuisma, Määttä and Sjöberg2009; Määttä et al., Reference Määttä, Hellstedt, Kuisma, Kymäläinen, Mahlberg and Sjöberg2009). For example, coatings were found to improve cleanability of concrete (Kymäläinen et al., Reference Kymäläinen, Määttä, Puumala, Kaustell, Mattila, Joutsen, Kuisma, Hurme, Uusi-Rauva and Sjöberg2008).
Providing clean, dry bedding and maintaining animals in the same group showed a 48% reduction in E. coli O157:H7 burden over 4.5 months compared to 18% on the control farms (Ellis-Iversen et al., Reference Ellis-Iversen, Smith, Van Winden, Paiba, Watson, Snow and Cook2008). This study was in agreement with one by Ward et al. (Reference Ward, Hughes, Faull, Cripps, Sutherland and Sutherst2002) who showed that wet and dirty bedding with temperatures ranging from 15 to 45°C was conducive for E. coli growth (up to 106 CFU g−1). In addition to dry and clean bedding, the quantity of straw, diets that produce firmer feces, and stocking density are possible factors contributing to the cleanliness of the bedding. Indoor housing was also associated with a higher risk (Ellis-Iversen et al., Reference Ellis-Iversen, Cook, Smith, Pritchard and Nielen2009). The exclusion of wild animals from livestock is beneficial since it is possible that E. coli O157:H7 may be introduced into cattle populations through the environment, feed and water contaminated with wild animals’ feces (Daniels et al., Reference Daniels, Hutchings and Greig2003; Synge et al., Reference Synge, Chase-Topping, Hopkins, McKendrick, Thomson-Carter, Gray, Rusbridge, Munro, Foster and Gunn2003; Wetzel and LeJeune, Reference Wetzel and LeJeune2006).
Training and motivation as preventive control
The training and education of farmers should be considered as a primary preventive control. Training of farmers in farm food safety risk assessment could encourage the farmers to identify and control potential food safety hazards on farms (Soon et al., Reference Soon, Manning, Davies and Bainesin press). Ellis-Iversen et al. (Reference Ellis-Iversen, Cook, Watson, Nielen, Larkin, Wooldridge and Hogeveen2010) interviewed 43 cattle farmers from England and Wales and found that none of the farmers had implemented zoonotic control programs in their farm and less than 50% had an intention to do so. Although the farmers projected positive attitudes towards providing safe products, this study indicated that intention was often hindered by a lack of belief in self-efficacy. One way of promoting adoption of zoonotic control programs would be to simplify the advice on how to control several zoonotic agents. The ability to reduce or control multiple zoonotic agents using a few measures may appeal to farmers, hence may increase rate of adoption (Ellis-Iversen et al., Reference Ellis-Iversen, Cook, Watson, Nielen, Larkin, Wooldridge and Hogeveen2010).
Conclusion
In order to be acceptable, control measures need to provide significant reduction in carriage and shedding of E. coli O157:H7 and to be low cost. The majority of meatborne E. coli O157:H7 outbreaks can be traced back to production on the farm, highlighting the importance of trying to reduce carriage and shedding of this bacterium by cattle at the farm. The number of cases of E. coli O157:H7 infection in the US decreased to <1/100,000 persons by 2010 and the percentage attributable to contaminated beef is declining, but interventions at the farm have the potential to dramatically further reduce these numbers. Numerous interventions that could be applied at the farm level have been investigated over the past 20 years, but most have not been shown to be effective and practical.
Management measures such as provision of clean, dry bedding (Ellis-Iversen et al., Reference Ellis-Iversen, Smith, Snow, Watson, Millar, Pritchard, Sayers and Cook2007, Reference Ellis-Iversen, Smith, Van Winden, Paiba, Watson, Snow and Cook2008) and other steps that minimize fecal−oral spread of the bacterium in the herd appear to be able to reduce the prevalence of E. coli O157:H7 in cattle. The administration of L. acidophilus NP-51 appears promising (Brashears et al., Reference Brashears, Galyean, Loneragan, Mann and Killinger-Mann2003a; Younts-Dahl et al., Reference Younts-Dahl, Galyean, Loneragan, Elam and Brashears2004; Loneragan and Brashears, Reference Loneragan and Brashears2005; Peterson et al., Reference Peterson, Klopfenstein, Erickson, Folmer, Hinkley, Moxley and Smith2007a) and many probiotic preparations are in commercial production. Vaccines are also available and appear to reduce the shedding of E. coli O157:H7 by cattle but there may be issues associated with unrecovered cost to the farmers. There is good evidence that supershedders are an important target and efforts to simply identify these cattle may yield substantial benefits.
Acknowledgment
J. M. Soon gratefully acknowledges the financial support from the Ministry of Higher Education of Malaysia.







