Introduction
Obsessive–compulsive disorder (OCD) is characterized by recurrent and intrusive thoughts (obsessions; e.g. contamination fear) and repetitive behaviours or mental rituals (compulsions; e.g. excessive hand washing or counting) that the person feels compelled to perform to reduce anxiety accompanying the obsessions. Therefore, appraisal of emotionally relevant stimuli and emotion regulation might be altered in OCD.
Regulating initial responses to emotional stimuli is essential to mental health (Gross & Muñoz, Reference Gross and Muñoz1995). The neurobiological underpinning of emotional experience is based on the interplay between the instantaneous appraisal of salient stimuli, provided by limbic brain areas and prefrontal control mechanisms to adequately regulate emotional responses (Ochsner & Gross, Reference Ochsner, Gross, Gross and Buck2007). An imbalance of these processes has been suggested in affective disorders (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003). Likewise, anxiety has been linked to hyperactivity of the amygdala during symptom provocation in various anxiety disorders (for a review, see Holzschneider & Mulert, Reference Holzschneider and Mulert2011). Further, during emotion regulation, reduced activity in prefrontal areas involved in the cognitive control of emotions has been shown for social anxiety disorder (Goldin et al. Reference Goldin, Manber, Hakimi, Canli and Gross2009), generalized anxiety and panic disorder (Ball et al. Reference Ball, Ramsawh, Campbell-Sills, Paulus and Stein2013) and spider phobia (Hermann et al. Reference Hermann, Schafer, Walter, Stark, Vaitl and Schienle2009).
Although OCD is no longer classified as an anxiety disorder (American Psychiatric Association, 2013), exaggerated anxiety in OCD has also been linked to functional changes in brain areas implicated in emotion processing and regulation (Milad & Rauch, Reference Milad and Rauch2012). Thus, symptom provocation studies have demonstrated abnormal activity in both limbic and frontal brain areas during processing of OCD-relevant stimuli (e.g. Adler et al. Reference Adler, McDonough-Ryan, Sax, Holland, Arndt and Strakowski2000; Simon et al. Reference Simon, Kaufmann, Musch, Kischkel and Kathmann2010, Reference Simon, Adler, Kaufmann and Kathmann2014). Moreover, OCD symptomatology has been linked to self-reported emotion-regulation difficulties such as limited access to emotion-regulation strategies (de la Cruz et al. Reference de la Cruz, Landau, Iervolino, Santo, Pertusa, Singh and Mataix-Cols2013). However, in a recent functional magnetic resonance imaging study, OCD patients were able to successfully attenuate amygdala response to OCD-relevant stimuli in a visual distraction task (Simon et al. Reference Simon, Adler, Kaufmann and Kathmann2014). Distraction is a powerful strategy because emotional processing is early disrupted before elaborate stimulus processing takes place (Gross, Reference Gross1998). An alternative strategy is cognitive reappraisal, which involves reinterpreting the content of emotional stimuli to reduce their emotional impact. Because cognitive models propose that anxiety symptoms in OCD are mediated by dysfunctional appraisals of specific situations (Salkovskis, Reference Salkovskis1985; Calkins et al. Reference Calkins, Berman and Wilhelm2013), cognitive reappraisal might be impaired in OCD. That is, due to irrational beliefs (e.g. overestimation of threat), unwanted intrusive thoughts (e.g. ‘Did I turn off the stove?’) trigger negative automatic thoughts (e.g. ‘If not, my flat will explode and the neighbours will die.’), which cause distress and anxiety. Because individuals suffering from OCD seem unable to reappraise these feared scenarios as being highly unlikely, we suggest that patients with OCD show deficits when using cognitive reappraisal to reduce negative emotions elicited by symptom-provoking images.
Besides abnormal neural activity in limbic and prefrontal brain areas during threat processing, clinical anxiety is also characterized by exaggerated attention to threatening stimuli (Bar-Haim et al. Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and van IJzendoorn2007). Enhanced processing of phobic stimuli is reflected in the late positive potential (LPP; e.g. Kolassa et al. Reference Kolassa, Musial, Mohr, Trippe and Miltner2005; Leutgeb et al. Reference Leutgeb, Schafer and Schienle2009, Reference Leutgeb, Schafer and Schienle2011; Michalowski et al. Reference Michalowski, Melzig, Weike, Stockburger, Schupp and Hamm2009; Schienle et al. Reference Schienle, Kochel and Leutgeb2011) in the event-related potential (ERP). The LPP has its maximum over centro-parietal scalp sites and is enhanced for emotional compared with neutral pictures. The effect starts 300 ms following stimulus onset and lasts up to several seconds (Hajcak et al. Reference Hajcak, MacNamara and Olvet2010). Affective LPP modulations are related to increased haemodynamic responses in cortical areas involved in visual attention and subcortical brain structures implicated in emotional processing (Liu et al. Reference Liu, Huang, McGinnis-Deweese, Keil and Ding2012; Sabatinelli et al. Reference Sabatinelli, Keil, Frank and Lang2013). Thus, the LPP is thought to reflect increased attention and perceptual sensitivity to emotional stimuli (Schupp et al. Reference Schupp, Cuthbert, Bradley, Hillman, Hamm and Lang2004; Weinberg & Hajcak, Reference Weinberg and Hajcak2010).
Importantly, emotion-regulation strategies have been shown to attenuate the LPP in response to unpleasant pictures (Hajcak et al. Reference Hajcak, MacNamara and Olvet2010). Specifically, reduced LPP amplitudes were observed when participants were instructed to shift attention away from unpleasant pictures either via internal cognitive (e.g. Thiruchselvam et al. Reference Thiruchselvam, Blechert, Sheppes, Rydstrom and Gross2011; Thiruchselvam et al. Reference Thiruchselvam, Hajcak and Gross2012; Paul et al. Reference Paul, Simon, Kniesche, Kathmann and Endrass2013) or visual processes (e.g. MacNamara & Hajcak, Reference MacNamara and Hajcak2009; Wangelin et al. Reference Wangelin, Low, McTeague, Bradley and Lang2011; Wiens & Syrjanen, Reference Wiens and Syrjanen2013). Similarly, reappraising unpleasant pictures as less negative resulted in reduced LPP amplitudes (Hajcak & Nieuwenhuis, Reference Hajcak and Nieuwenhuis2006; Moser et al. Reference Moser, Krompinger, Dietz and Simons2009; Thiruchselvam et al. Reference Thiruchselvam, Blechert, Sheppes, Rydstrom and Gross2011; Paul et al. Reference Paul, Simon, Kniesche, Kathmann and Endrass2013; Schönfelder et al. Reference Schönfelder, Kanske, Heissler and Wessa2014).
This study investigated the LPP in response to disorder-relevant, generally aversive and neutral pictures while participants either maintained or reduced their emotional response using cognitive distraction or cognitive reappraisal. First, we tested whether patients with OCD show increased visual attention towards disorder-relevant stimuli as indexed by augmented LPP amplitudes. Second, we hypothesized that patients with OCD show impaired cognitive reappraisal of disorder-relevant stimuli that would be reflected in a reduced LPP attenuation and a smaller decrease in self-reported arousal compared with healthy controls (HCs). We included cognitive distraction as a second cognitive emotion-regulation strategy in order to investigate whether regulation deficits are specifically linked to cognitive reappraisal or generalized to other cognitive strategies as well (see Kanske et al. Reference Kanske, Heissler, Schönfelder and Wessa2012). Besides investigating the LPP in response to disorder-relevant compared with affectively neutral stimuli, we also included generally aversive stimuli to examine whether predicted deficits are specific to stimuli related to obsessive–compulsive (OC) symptoms.
Method
Participants
A total of 39 patients with a Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) diagnosis of OCD and 27 HCs participated in the study. Of the patients, 12 were not included in electroencephalographic (EEG) testing because they evaluated too few OCD-related pictures as symptom-inducingFootnote 1 Footnote †. Data of three OCD patients and three HCs were discarded due to excessive EEG artifacts, resulting in 24 OCD patients and 24 HCs for data analyses. Patients were recruited from the out-patient clinic of the Department of Psychology, Humboldt-Universität zu Berlin, Germany. Symptom severity was assessed using the Yale–Brown Obsessive–Compulsive Scale (Y-BOCS), the Y-BOCS Symptom Checklist (Goodman et al. Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989) and the Obsessive–Compulsive Inventory-Revised (OCI-R; Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic, Hajcak and Salkovskis2002). Depressive symptoms were assessed using the clinician-rated Montgomery–Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, Reference Montgomery and Åsberg1979). Prior to picture rating, depressive symptoms and trait and state anxiety were assessed using the Beck Depression Inventory-II (BDI-II, Beck et al. Reference Beck, Steer and Brown1996) and the State-Trait Anxiety Inventory (STAI; Spielberger, Reference Spielberger1983), respectively. Dispositional emotion regulation was measured with the Emotion Regulation Questionnaire (ERQ; Gross & John, Reference Gross and John2003) and the Cognitive Emotion Regulation Questionnaire (CERQ; Garnefski et al. Reference Garnefski, Kraaij and Spinhoven2001). Exclusion criteria for OCD patients comprised a Y-BOCS score ⩽12, a MADRS score >19, predominant hoarding symptoms and the presence of co-morbid disorders other than anxiety or Axis-II disorders (apart from borderline personality disorder). HCs were recruited from the participant database of the Institute of Psychology at the Humboldt-Universität zu Berlin and were matched case-by-case for gender, age, education and handedness and reported to be free of past or present psychiatric conditions. Participants with any history of neurological disorder were excluded. As shown in Table 1, the two groups did not statistically differ with regard to demographic variables. OCD patients reported higher scores of OCD symptoms (OCI-R), severity of depression (BDI-II) and both state and trait anxiety (STAI-S, STAI-T) than HCs.
Table 1. Demographic and clinical variables for patients with OCD and HC subjects

Data are given as mean (standard deviation) unless otherwise indicated.
OCD, Obsessive–compulsive disorder; HC, healthy control; df, degrees of freedom; WST, Wortschatztest; BDI-II, Beck Depression Inventory-II; OCI-R, Obsessive–Compulsive Inventory-Revised; MADRS, Montgomery–Åsberg Depression Rating Scale; Y-BOCS, Yale–Brown Obsessive Compulsive Scale; STAI-T, Trait version of the State-Trait Anxiety Inventory; STAI-S, State version of the State-Trait Anxiety Inventory.
Of the patients, nine received cognitive–behavioural therapy (CBT) at the time of the study, nine were taking selective serotonin reuptake inhibitors, and six had a co-morbid disorder including agoraphobia with panic disorder (n = 1), specific phobia (n = 1), social phobia (n = 1), adjustment disorder (n = 1) and OC personality disorder (n = 2). All participants reported normal or corrected-to-normal vision. They gave their written informed consent and received €35 for their participation.
Apparatus and stimuli
Using Presentation (Neurobehavioral Systems, USA), stimuli were presented on a black background of a 19-inch (48.26 cm) computer monitor, placed 80 cm in front of the participant. In total 40 neutral and 40 aversive pictures were selected from the International Affective Picture System (Lang et al. Reference Lang, Bradley and Cuthbert2008; see online Supplementary material S1 for picture numbers and normative ratings). OCD-related pictures were drawn from the Berlin OCD-Picture Set (Simon et al. Reference Simon, Kischkel, Spielberg and Kathmann2012), covering the following seven OCD themes: aggressive obsessions, religious obsessions, contamination/washing, checking, symmetry/ordering, hoarding, and counting. Stimuli subtended a visual angel of 8.5° horizontally and 6.7° vertically.
Experimental procedure
At 1 week (±2 days) prior to EEG testing, HC participants and OCD patients attended a picture-rating session and evaluated OCD-related, generally aversive and neutral pictures (on a nine-point rating scale regarding arousal, unpleasantness, and the potential to induce OC symptoms; see online Supplementary Table S1 and Supplementary material S2 for methods, statistical analysis and results). Based on these ratings, a final set of 24 pictures for each picture category was selected individually for each patient so that OCD-related pictures induced maximal OC symptoms and were maximally unpleasant and arousing while selected aversive and neutral pictures induced minimal OC symptoms with aversive pictures being maximally and neutral pictures being minimally unpleasant and arousing (see online Supplementary Table S2 for symptom dimensions of selected OCD-related pictures).
Emotion-regulation task
Participants were instructed to either maintain or reduce their emotional response elicited by subsequently presented pictures via cognitive reappraisal or cognitive distraction. When instructed to maintain emotions, participants should attend to and respond naturally to the picture without altering accompanying emotions. In the distraction condition, participants were asked to generate neutral thoughts or mental images unrelated to the picture (e.g. imagining the way to the supermarket). It was pointed out that cognitive distraction should be applied only after picture onset and not in advance. In the reappraisal condition, participants were asked to change the meaning of the picture to reduce the emotional impact (e.g. imagining the situation being unreal or assuming a different outcome than the one suggested).
At the beginning of each trial, a white fixation cross appeared at the centre of the screen for 1.5 s, followed by a 2-s cue word (maintain, distract, or reappraise in German). Immediately following, a picture of one of the three categories was presented for 5 s and participants were instructed to follow the task instruction specified by the preceding cue word. HCs saw the same stimuli as their respective matching patient. Following each picture, participants evaluated the arousal level of the presented picture on a nine-point rating scale (where 1 represented low and 9 represented high arousal). Each picture category was combined with all regulation instructions yielding nine experimental conditions. The task comprised four blocks of 54 trials each, and every block contained six trials in each of the nine experimental conditions. The 24 pictures of each picture category were repeated three times during the course of the experiment so that each picture was paired once with each regulation condition, resulting in 24 trials per condition. Trials were presented in a pseudo-random order with the constraint of no more than three consecutive trials with the same type of instruction or with the same picture category.
Participants completed 13 practice trials (three maintain, five distraction and five reappraisal trials) prior to the experiment until they clearly understood the task instructions. To ensure the appropriate use of strategies, participants explained their picture reinterpretation or their self-generated distraction during the practice trials.
EEG recording and analyses
Continuous EEG was recorded using an EasyCap electrode system (EASYCAP GmbH, Germany) from 61 electrodes, based on an equidistant electrode montage, and two external positions (below the left and right eyes). The ground electrode was attached to the right cheek. All channels were referenced to electrode Cz during data collection and impedances were below 5 kΩ. The EEG was amplified using two 32-channel BrainAmp amplifiers (Brain Products GmbH, Germany) in DC mode at a sampling rate of 1000 Hz.
EEG data were processed offline using BrainVision Analyzer 2.0 (Brain Products GmbH, Germany). Eye-movement artifacts were removed using the multiple source eye correction method (Berg & Scherg, Reference Berg and Scherg1994) implemented in BESA 5.2 (Brain Electrical Source Analysis, MEGIS Software GmbH, Germany). Data were re-referenced to the average of the left and right mastoids (T9 and T10), down-sampled to 500 Hz, and bandpass filtered from 0.05 to 30 Hz. EEG epochs were extracted time-locked to the onset of picture presentation and baseline corrected for a 200 ms pre-stimulus interval. Epochs containing artifacts were automatically removed based on the following criteria: amplitude changes exceeding 75 µV between consecutive data points, voltage differences exceeding 200 µV within a 200 ms interval, and voltage changes of less than 0.5 µV within a 100 ms interval. Afterwards, epochs were visually inspected for remaining artifacts. The average number of artifact-free trials was 22.9 (s.d. = 1.0) per condition and did not differ significantly between picture categories, regulation strategies or groups (all p's > 0.1). ERPs were averaged for each experimental condition. The LPP was evaluated as the average amplitude from 300 to 5000 ms collapsed across three centro-parietal electrodes (CPz, CP1, CP2; Thiruchselvam et al. Reference Thiruchselvam, Blechert, Sheppes, Rydstrom and Gross2011). Grand average ERPs were filtered at 12 Hz for visual presentation.
Statistical analyses
LPP amplitudes and arousal ratings were submitted to a repeated-measures analysis of variance (ANOVA) with regulation strategy (maintain, distraction, reappraisal) and picture category (OCD-related, aversive, neutral) as within-subjects factors and group (OCD, HC) as a between-subjects factor. Treatment and medication effects on LPP results were examined with two additional ANOVAs within the patient group with regulation strategy and picture category as within-subjects factors and status of medication (n = 9 v. n = 15) or therapy (n = 10 v. n = 14) as a between-subjects factor. For significant main effects and interactions, post-hoc tests were performed using Bonferroni correction. The Greenhouse–Geisser correction was used when appropriate.
Ethical standards
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the local ethics committee of the Humboldt-Universität zu Berlin.
Results
Dispositional emotion regulation
After correcting for multiple comparisons, OCD patients scored significantly lower in the CERQ subscale positive refocusing (t 46 = −4.18, p < 0.001) and in the reappraisal subscale of the ERQ (t 46 = −3.97, p < 0.001) than HCs. Significantly higher scores were found for OCD patients in the CERQ catastrophizing subscale (t 46 = 3.52, p = 0.001) (see online Supplementary Table S3).
LPP results
Fig. 1 illustrates the grand average ERPs for OCD-related, aversive and neutral pictures in the maintain condition for both groups as well as the scalp distributions for aversive and OCD-related minus neutral pictures. Mean LPP amplitudes are presented in Table 2. The LPP varied as a function of picture category (F 2,92 = 50.36, p < 0.001, ε = 0.99, η 2 P = 0.52). Overall, larger amplitudes were observed for aversive compared with neutral (t 47 = 9.43, p < 0.001) and OCD-related pictures (t 47 = 7.35, p < 0.001). OCD-related pictures elicited larger LPPs than neutral pictures at trend level (t 47 = 2.23, p = 0.07). At trend level, analyses revealed a picture category × group interaction (F 2,92 = 2.82, p = 0.07, ε = 0.99, η 2 P = 0.06). Follow-up ANOVAs for each group separately showed significant main effects for picture category (both p's < 0.001), but pairwise comparisons revealed that while both groups showed increased LPP amplitudes for aversive compared with neutral and OCD-related pictures (all p's < 0.001), OCD patients additionally showed a significant LPP enhancement for OCD-related relative to neutral pictures (t 23 = 3.75, p = 0.003) which was absent in HCs (p > 0.99).

Fig. 1. Mean late positive potential amplitudes for healthy controls (left) and patients with obsessive–compulsive (OC) disorder (OCD, right) in response to neutral, aversive and OCD-related pictures in the maintain condition. Event-related potentials were averaged at three centro-parietal recording sites (CP1, CPz, CP2). Scalp potential difference maps show aversive minus neutral and OCD-related minus neutral pictures in the maintain condition in the time window from 300 to 5000 ms.
Table 2. Arousal ratings and LPP amplitudes (from 300 to 5000 ms) in the maintain, distraction and reappraisal conditions for patients with OCD and HC subjects
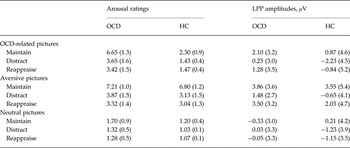
Data are given as mean (standard deviation).
LPP, Late positive potential; OCD, obsessive–compulsive disorder; HC, healthy control.
There was a significant main effect of regulation strategy (F 2,92 = 20.91, p < 0.001, ε = 0.91, η 2 P = 0.31), showing that relative to the maintain condition, the LPP was reduced during both distraction (t 47 = 5.60, p < 0.001) and reappraisal (t 47 = 2.64, p = 0.03), with greater LPP reductions for distraction relative to reappraisal (t 47 = 4.39, p < 0.001). Further, the ANOVA yielded a significant regulation strategy × picture category interaction (F 4,184 = 8.86, p < 0.001, ε = 0.91, η 2 P = 0.16). Follow-up ANOVAs for each picture category revealed that the regulation effect was present for aversive and OCD-related (both p's < 0.001), but not for neutral pictures (p = 0.32). As suggested by Fig. 2, the analysis identified a regulation strategy × group interaction (F 2,92 = 3.38, p = 0.04, ε = 0.91, η 2 P = 0.07). Follow-up ANOVAs for each group separately revealed a significant main effect of regulation for both groups (both p's ⩽ 0.03), but pairwise comparisons indicated that relative to the maintain condition, HCs showed significantly reduced LPP amplitudes during both distraction (t 23 = 6.08, p < 0.001) and reappraisal (t 23 = 3.14, p = 0.01), whereas OCD patients showed an LPP reduction during distraction at trend level (t 23 = 2.40, p = 0.08), but no significant LPP attenuation during reappraisal (p > 0.99). Results indicate that relative to OCD patients, HCs showed greater regulation effects in the LPP for both distraction (t 46 = 2.25, p = 0.03) and for reappraisal at trend level (t 46 = 1.81, p = 0.08). However, both comparisons were not significant after adjustment for multiple comparisons (i.e. Bonferroni-adjusted p value of 0.025).
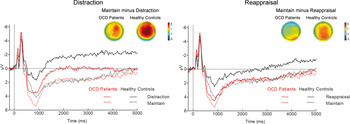
Fig. 2. Mean late positive potential (LPP) amplitudes averaged across picture categories during distraction (left) and reappraisal (right) relative to the maintain condition for healthy controls and patients with obsessive–compulsive disorder (OCD). The LPP was scored across three centro-parietal recording sites (CP1, CPz, CP2). Scalp potential difference maps for the regulation effects (maintain minus distraction and maintain minus reappraisal) are shown for each group.
Neither the main effect of group nor the three-way interaction of group × picture category × regulation strategy were significant (both p's > 0.17). Online Supplementary Fig. S1 shows mean LPP amplitudes to neutral, OCD-related and aversive pictures in each regulation condition for both groups. We found neither significant main effects of medication or therapy status, nor significant interactions with these variables (all p's > 0.1).
To investigate whether individual differences in habitual reappraisal relate to reappraisal success in the emotion-regulation task, we calculated a Pearson correlation between individual reappraisal subscale scores of the ERQ and LPP difference scores for maintain minus reappraisal trials collapsed across picture category. This post-hoc analysis revealed a positive correlation between the two variables (r = 0.39, p = 0.006).
Subjective ratings
Table 2 shows mean arousal ratings for each picture category and regulation instruction. Main effects of picture category (F 2,92 = 319.3, p < 0.001, η 2 P = 0.87) and group (F 1,46 = 43.67, p < 0.001, η 2 P = 0.49), and a significant picture category × group interaction (F 2,92 = 57.91, p < 0.001, η 2 P = 0.56) were found. Follow-up ANOVAs for each group separately revealed significant effects of picture category in both groups (both p's < 0.001). Pairwise comparisons showed that both groups rated neutral pictures as less arousing than aversive and OCD-related pictures (all p's < 0.001). While in HCs aversive pictures induced higher arousal than OCD-related pictures (t 23 = 12.33, p < 0.001), OCD patients rated OCD-related and aversive pictures as equally arousing (p = 0.55). Further significant effects were found for regulation strategy (F 2,92 = 194.49, p < 0.001, ε = 0.62, η 2 P = 0.81), regulation strategy × picture category (F 4,184 = 120.64, p < 0.001, ε = 0.55, η 2 P = 0.72) and regulation strategy × picture category × group (F 4,184 = 17.26, p < 0.001, ε = 0.55, η 2 P = 0.27). Post-hoc tests showed that, overall, pictures were rated as more arousing in the maintain condition than in both regulation conditions (distraction: t 47 = 12.80, p < 0.001; reappraisal: t 47 = 13.96, p < 0.001), with greater reductions in arousal for reappraisal compared with distraction (t 47 = 2.43, p = 0.04). Follow-up ANOVAs for each picture category and group revealed significant regulation effects for each picture category in both groups (all p's < 0.05), but pairwise comparisons showed that there were two exceptions to the direction of the general regulation effect (maintain > distraction > reappraisal). First, OCD patients rated aversive pictures as less arousing when using reappraisal compared with distraction (t 23 = 3.18, p = 0.01). This effect was absent in HCs (p = 0.99). Second, only distraction reduced arousal in response to neutral pictures in HC participants at trend level (t 23 = 2.53, p = 0.06), while reappraisal failed to reach significance (p = 0.17).
Discussion
This study investigated emotion regulation in patients with OCD. Down-regulating negative emotions using reappraisal yielded discrepant findings regarding the behavioural v. the electrocortical level. Whereas reappraisal successfully reduced self-reported arousal in both groups, OCD patients failed to show a corresponding LPP reduction. Emotional stimuli are important for survival and reproduction and therefore automatically attract attention, resulting in an LPP enhancement. Reducing the motivational significance of these stimuli through reappraisal should have reduced attention and thereby LPP amplitudes. However, only in HCs reappraised pictures attracted less attention, whereas ongoing elaborate processing was evident in OCD. Sustained attention to reappraised stimuli might result from patients’ inability to disengage from potentially meaningful events, once their content has been assessed. Ongoing processing might reflect the endeavour to make sure that no important information was missed or to verify that the stimulus is truly harmless. Additionally, OCD patients reported less frequent use of reappraisal in daily life, as revealed by emotion-regulation questionnaires. Further, increased habitual reappraisal was linked to greater LPP amplitude reduction during instructed reappraisal. This highlights the importance of reappraisal techniques as an integral part of CBT for OCD (Clark, Reference Clark2004).
While HCs showed slightly greater LPP reductions for both distraction and reappraisal compared with OCD patients, within the patient group, participants failed to show an LPP attenuation for reappraisal, but distraction successfully reduced LPP amplitudes. By contrast, in patients with specific phobia, visual distraction failed to reduce emotion-related neural activity in response to phobic stimuli (Straube et al. Reference Straube, Mentzel and Miltner2006, Reference Straube, Lipka, Sauer, Mothes-Lasch and Miltner2011; Norberg et al. Reference Norberg, Peira and Wiens2010; but see Alpers et al. Reference Alpers, Gerdes, Lagarie, Tabbert, Vaitl and Stark2009). Distraction is supposed to influence emotion generation before elaborate stimulus processing has taken place (Gross, Reference Gross1998). Thus, an earlier attenuation of the LPP has been demonstrated for distraction compared with reappraisal (Thiruchselvam et al. Reference Thiruchselvam, Blechert, Sheppes, Rydstrom and Gross2011; Paul et al. Reference Paul, Simon, Kniesche, Kathmann and Endrass2013; Schönfelder et al. Reference Schönfelder, Kanske, Heissler and Wessa2014). Whereas in specific phobia the sheer physical presence of feared stimuli triggers anxiety, anxiety in OCD is cognitively mediated by dysfunctional beliefs (Abramowitz & Jacoby, Reference Abramowitz and Jacoby2015). Withdrawing attention before elaborated stimulus processing might prevent the occurrence of dysfunctional thoughts resulting in reduced anxious apprehension following disorder-relevant stimuli.
Regarding the neural bases of those two emotion-regulation strategies, the orbitofrontal cortex (OFC) is selectively activated during cognitive reappraisal compared with distraction (Kanske et al. Reference Kanske, Heissler, Schönfelder, Bongers and Wessa2011; Dörfel et al. Reference Dörfel, Lamke, Hummel, Wagner, Erk and Walter2014). The OFC is also involved in value updating (Gottfried et al. Reference Gottfried, O'Doherty and Dolan2003; Rudebeck et al. Reference Rudebeck, Saunders, Prescott, Chau and Murray2013) and affective reversal learning (Fellows & Farah, Reference Fellows and Farah2003; Kringelbach & Rolls, Reference Kringelbach and Rolls2003; Remijnse et al. Reference Remijnse, Nielen, Uylings and Veltman2005). Thus, the OFC plays an important role in the evaluation and updating of the emotional value of encountered stimuli or events (Murray et al. Reference Murray, O'Doherty and Schoenbaum2007). Similarly, during reappraisal the affective value of a presented picture has to be replaced by a more neutral one, for example by modifying motivational relevance. Thus, an established response tendency (negative emotion experience) is changed in the face of a new outcome value. This process critically relies on OFC function (Roy et al. Reference Roy, Shohamy and Wager2012). Importantly, the OFC is central to neurobiological models of OCD (Saxena & Rauch, Reference Saxena and Rauch2000; Pauls et al. Reference Pauls, Abramovitch, Rauch and Geller2014). In reversal tasks, patients with OCD showed OFC hypoactivation during affective switching (Remijnse et al. Reference Remijnse, Nielen, van Balkom, Cath, van Oppen, Uylings and Veltman2006, Reference Remijnse, Nielen, van Balkom, Hendriks, Hoogendijk, Uylings and Veltman2009) while OFC hyperactivation was observed during rest (Saxena et al. Reference Saxena, Brody, Schwartz and Baxter1998; Beucke et al. Reference Beucke, Sepulcre, Talukdar, Linnman, Zschenderlein, Endrass, Kaufmann and Kathmann2013). Given that LPP modulations are related to neural activity in brain areas including the OFC (Liu et al. Reference Liu, Huang, McGinnis-Deweese, Keil and Ding2012), one could assume that OFC dysfunction might be associated with the sustained LPP response during reappraisal in OCD. However, this assumption needs further research.
In contrast to our expectation, the lack of LPP modulation was apparent not only for OCD-related but also for generally aversive pictures. This finding is difficult to reconcile with OCD symptomatology, which is characterized by anxiety to very specific stimuli and not to aversive stimuli in general. However, altered emotion regulation has been shown for a variety of psychological disorders (for a review, see Berking & Wupperman, Reference Berking and Wupperman2012). This abnormal emotion regulation may constitute a vulnerability factor for the development of psychological disorders (see Kret & Ploeger, Reference Kret and Ploeger2015), but the manifestation of specific symptom characteristics appears to be influenced by other factors.
In agreement with our hypothesis, facilitated processing of disorder-relevant stimuli was reflected in enhanced LPP amplitudes for OCD patients compared with HCs. An attentional bias toward disorder-relevant stimuli has also been shown for patients with specific phobia (e.g. Kolassa et al. Reference Kolassa, Musial, Mohr, Trippe and Miltner2005; Leutgeb et al. Reference Leutgeb, Schafer and Schienle2009, Reference Leutgeb, Schafer and Schienle2011; Michalowski et al. Reference Michalowski, Melzig, Weike, Stockburger, Schupp and Hamm2009; Schienle et al. Reference Schienle, Kochel and Leutgeb2011). However, while this clinical group showed LPP amplitudes to phobic stimuli that were comparable with (Michalowski et al. Reference Michalowski, Melzig, Weike, Stockburger, Schupp and Hamm2009) or higher than (Schienle et al. Reference Schienle, Schäfer and Naumann2008; Leutgeb et al. Reference Leutgeb, Schafer and Schienle2009) the LPP in response to aversive pictures, OCD patients showed larger LPPs to generally aversive compared with OCD-related stimuli. This could be related to differences regarding the nature of threatening stimuli. While symptom triggers in OCD constitute potentially threatening stimuli whose significance is cognitively mediated (Calkins et al. Reference Calkins, Berman and Wilhelm2013), phobic stimuli are mostly biologically prepared (e.g. spiders or snakes) and pose immediate threat to one's survival. Thus, these stimuli should draw more attention because quickly responding to them has proven to be critical for survival.
When interpreting the present findings, potential limitations have to be considered. First, we did not restrict the type of reappraisal that participants should use. Importantly, different reappraisal strategies (e.g. changing future consequences, reality challenge, or distancing) have been shown to be associated with different emotional outcomes (McRae et al. Reference McRae, Ciesielski and Gross2012). It is possible that altered emotion regulation in OCD resulted from differential use of preferred reappraisal strategy. Future studies should address this by comparing specific reappraisal techniques. Second, relative to HCs, patients with OCD reported generally aversive pictures to be less arousing following reappraisal compared with distraction, but did not show corresponding LPP reductions. As outlined above, the LPP predominantly reflects attentional processes instead of emotion per se, which might account for these apparently discrepant findings. Alternatively, self-reports can be influenced by social desirability, which might be more pronounced in individuals high in anxiety (Tanaka-Matsumi & Kameoka, Reference Tanaka-Matsumi and Kameoka1986). This might have led to increased self-reported reappraisal success in OCD patients. Future studies should include physiological measures that more directly measure emotional arousal such as skin conductance or heart rate.
To conclude, we demonstrate prioritized processing of disorder-specific stimuli in OCD. While cognitive distraction successfully reduced the LPP, OCD patients failed to show respective LPP modulations for cognitive reappraisal despite reductions in self-reported arousal. The obtained results highlight the importance of incorporating abnormal processes during emotion regulation into models of OCD.
Supplementary material
For Supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715001610
Acknowledgements
This work was supported by the German Research Foundation (grant number SI 1131/3-1/EN 906/2-1). S.P. received funding from Evangelisches Studienwerk e.V. Villigst.
We thank Ulrike Bunzenthal, Judith Süßenbach, Julia Preuß and Carolin Steglich for assisting in data collection and the team of the OCD out-patient clinic, especially Anja Thomanek, Dr Eva Kischkel and Dr Benedikt Reuter, for recruiting and diagnosing the patients.
Declaration of Interest
None.






