Introduction
Crustose Verrucariales are exceedingly poor in taxonomically useful characters. Furthermore, many of these characters are highly variable and largely dependent on environmental influences (Santesson Reference Santesson1939; Keller Reference Keller1996; Thüs Reference Thüs2002). The taxonomy of Verrucariales on all systematic levels, from species to genera, has often been the subject of debate, and nomenclatural changes have been frequent in the past. Molecular studies so far have mainly addressed the identification of the phylogenetic position of the Verrucariales among the Ascomycota (Lutzoni et al. Reference Lutzoni, Pagel and Reeb2001; Liu & Hall Reference Liu and Hall2004; Schmitt et al. Reference Schmitt, Mueller and Lumbsch2005) or the circumscription of generic boundaries within the order (Gueidan et al. Reference Gueidan, Roux and Lutzoni2007, Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss, Orange, Fröberg, Amtoft, Navarro-Rosinés, Krzewicka, Pykälä and Lutzoni2008; Savić Reference Savić2007; Savić & Tibell Reference Savić and Tibell2008; Savić et al. Reference Savić, Tibell, Gueidan and Luzoni2008). Molecular studies focusing on the significance of morphological characters in species delimitation have so far only been published for Atla (Savić & Tibell Reference Savić and Tibell2008), Dermatocarpon (Heiðmarsson Reference Heiðmarsson2003), and Sporodictyon (Savić Reference Savić2007).
Thelidium in its current circumscription differs from similar crustose genera, such as Verrucaria and Polyblastia, only by its transversally septate ascospores. However, ascospore septation has been shown to be an unreliable character in crustose Verrucariales and septation of the ascospores has evolved several times among different groups (Gueidan et al. Reference Gueidan, Roux and Lutzoni2007, Savić et al. Reference Savić, Tibell, Gueidan and Luzoni2008). Major changes in generic delimitations and the description of new genera are certainly to be expected, but at present no emendation of Thelidium has been suggested. The present study focuses on species delimitation but it may also help to identify species groups which may form distinct genera.
The ecology of species of the genus Thelidium as currently circumscribed covers a large range of environmental situations, ranging from dry exposed limestone to frequently submerged acidic siliceous rocks, and includes endolithic as well as epilithic crusts. In this paper we focus on the epilithic freshwater species from Central Europe as a first step towards the treatment of the genus Thelidium in the forthcoming lichen volume of the Freshwater Flora of Central Europe. Some of the Central European freshwater species are known only from their type localities or from herbarium specimens, and many species have rarely been collected since their first description. However, for some of these poorly known taxa, such as T. pertusatii (Garov.) Jatta, T. rehmii Zschacke, T. submethorium (Vain.) Zschacke and T. aethioboloides Zschacke non (Nyl.) Vain., fresh material became available for DNA extraction as a result of extensive fieldwork in Central Europe (including the Alps) during the past three years. Due to the poor knowledge of their distinctiveness, a statement on their threatened status for national Red Lists (e.g. Wirth et al. Reference Wirth, Schöller, Scholz, Ernst, Feuerer, Gnüchtel, Hauck, Jacobsen, John and Litterski1996) and their need for conservation have been open to debate. A better understanding of the genetic structure of freshwater Thelidium will help to discover whether these little known taxa are likely to represent monophyletic units or just morphological variants of widespread variable species.
Material and Methods
Taxon sampling
The sampling focused on representatives of two principal morphological groups within the epilithic Thelidium species which occur in Central European freshwater habitats, including the Alps: the Thelidium methorium group with large perithecia, a well-developed involucrellum and large ascospores, and the T. minutulum group with smaller perithecia and ascospores and absent or a weakly developed involucrellum. Sequences from GenBank of two species of Verrucula (V. granulosaria, V. inconnexaria,) were used as outgroup. The genus Verrucula has been shown to be the most basal group within the Verrucariales (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss, Orange, Fröberg, Amtoft, Navarro-Rosinés, Krzewicka, Pykälä and Lutzoni2008). Wherever possible, multiple accessions were used for each putative Thelidium species representing different geographic origins, morphological variants and ecology. The list of sequenced taxa together with their GenBank accession numbers is presented in Table 1. Each sequence represents an individual lichen thallus and multiple collections of the same species from identical geographical localities were sampled from different rocks or pebbles. For some rare species herbarium material or the protologues and line drawings of type specimens only were available. These taxa were included in the key and in the taxonomic treatment, but could not be included in the molecular studies.
Table 1. Voucher and GenBank accession numbers for sequences included in this study.
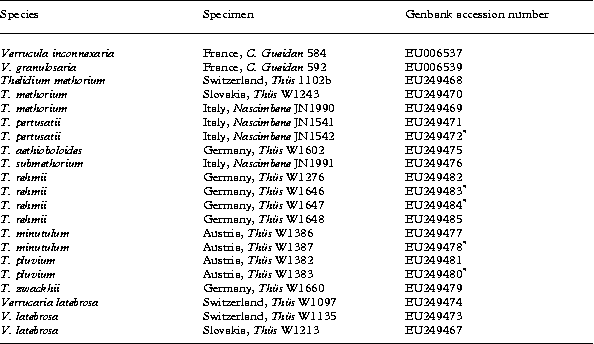
* Identical or near identical (≥ 0·5% similarity) sequences of duplicate specimens, collected at the same sampling site, but from different rocks. These sequences were excluded from the alignment prior to further analyses.
DNA extraction, PCR amplification and sequencing
DNA was extracted from freshly collected material within 12 months of collection. Four to ten mm2 of the thalli were scraped off their substrata, subsequently frozen in liquid nitrogen and ground to a fine powder. DNA extraction was performed with the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's protocol. From rock specimens with more than one taxon of crustose Verrucariales on the surface, direct PCR from hymenial tissue of selected perithecia within the area which was subsequently used for DNA extraction was also performed in order to test the correct assignment of species names to the DNA extracts. Undiluted DNA extracts were subjected to PCR using primers MY1700, ITS1 and ITS4 (Helms et al. Reference Helms, Friedl and Rambold2003, White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Snisky and White1990) in a reaction volume of 25 μl. Primer content in the Mastermix was raised to 4 pM μl−1 as lower contents did not give sufficient product yield for direct sequencing. The cycling profile for amplification was 95° (1 min) followed by 25 cycles at 95° (1 min), 51° (45 sec), 72° (2 min) and 15 cycles at 95° (1 min), 51° (45 sec) and 72° (2 min) with an additional increment of 5 sec and a final extension step at 72° for 10 min. For direct PCR small amounts of hymenial tissue were picked from within hydrated, fully swollen perithecia by means of a sterile needle, put in a PCR reaction tube, cooled in liquid nitrogen and ground with a glass pestle. The ground tissue was suspended in 20 μl of nuclease-free water and 5 μl of the diluted DNA was added to the PCR cocktail. The amplification products of this first PCR were subjected to a second nested PCR with primers 1800f and ITS4 using the following cycling profile: 95° (2 min) followed by 20 cycles at 95° (30 sec), 52° (30 sec with an additional increment of 2 sec), 72° (1 min). All PCR products were cleaned with the QIAquick Purification Kit (Qiagen, Hilden / Germany) following the manufacturer's protocol. PCR products were sequenced directly using the BigDye labelling method with primers 1800f and ITS4 (Friedl 1996; White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Snisky and White1990). Amplification products were sequenced bidirectionally on an ABI-Capillar-Sequencer. Electrophoresis and detection of fragments were performed on an ABI3100 (PE Biosystems, Foster City, CA). The sequences were assembled with ClustalW as implemented in BioEdit (Hall Reference Hall1999).
Sequence alignment and phylogenetic analyses
Sequences were aligned with ClustalX (Thompson et al. Reference Thompson, Higgins and Gibson1994) as implemented in BioEdit (Hall Reference Hall1999). Gaps were treated as missing data. The alignment was analysed with PAUP4.0b10a (Swofford Reference Swofford2002) for parsimony analyses and MrBayes3.1 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001) for Bayesian inference of phylogeny. Heuristic searches using parsimony as optimality criterion, TBR branch swapping, random taxon addition and 1000 replicates were performed with Multrees option on, steepest descent off and collapse zero length branches on. Fitting of tree topologies to the underlying dataset was evaluated by performing 1000 Bootstrap replicates on the original data and counting relative abundances of individual nodes in the resulting most parsimonious trees. A Bayesian approach (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001) was used to approximate posterior probabilities (PP) of nodes resulting from the calculation of topologies with Maximum Likelihood as optimality criterion. Settings for the best fitting model describing the dataset were estimated by Modeltest3.7 (Posada & Crandall Reference Posada and Crandall1998) and fixed to: revmatpr = fixed (1·0000, 3·1867, 1·0000, 1·0000, 5·7990, 1·0000), pinvarpr=fixed (0·5182), nst = 6 and rates = equal. MrBayes was run three times with 1 000 000 generations in five simultaneous chains without assuming a molecular clock for each run. Sampling frequency was set to every 100th tree. The log-likelihood values of the sampled trees were examined and 2500 trees generated before stationarity was achieved were discarded.
Assessment of morphological characters
Sections of thallus and perithecia were prepared by hand from freshly collected material within a week after collection using a razor blade or with a cryotome set at 10 μm. Observations of anatomical details were made at a magnification of ×1000 with a Zeiss Axiosskop compound microscope equipped with differential-interference-contrast (DIC). The presence of mucilaginous sheaths surrounding the ascospores was evaluated before and after application of a solution of Lugol's iodine and potassium hydroxide.
Results
PCR yielded 19 new sequences from nine putative species for the ingroup (Thelidium spp. and Verrucaria latebrosa, Table 1). Five of them were excluded from the alignment prior to further analyses since they are identical or near identical (≤0·5% differences) to those retrieved from duplicate specimens collected at the same site. Only sequences retrieved from one specimen for each site were therefore used in the analysis. The alignment of these remaining 14 sequences together with the ITS and 5.8S sequences of two outgroup taxa (Verrucula granulosaria and V. inconnexaria) consisted of 593 positions. It is available from the first author on request. Flanking areas of the nuLSU and the initial first 40 positions of the ITS1, which were obtained only for a subset of specimens, were excluded before further analysis. The final alignment of 483 positions contained 154 parsimony informative sites. All epilithic Thelidium species with small perithecia and a weak or absent involucrellum (Thelidium minutulum, T. pluvium, T. rehmii, T. zwackhii) fall within a single clade (Fig. 1). Branches of multiple accessions for taxa within this group are rather short; the number of ascospore septa varies among species from one to three. In the second clade of epilithic freshwater Thelidium branch lengths are generally much longer between species as well as within species. Ascospores in this group are either uniseptate or unicellular. ITS data confirm a close relationship of Verrucaria latebrosa to Thelidium methorium and T. pertusatii. Thelidium submethorium is distinct from the species of the T. methorium-group with large perithecia and ascospores and is positioned on a long branch together with T. aethioboloides. The reproductive structures of these two taxa are small and of a similar size to those of the T. minutulum-group, but differ in their constantly well-developed and often laterally spreading involucrellum. The genetic similarity of these two taxa is very high in the ITS and 5.8S regions (98%), indicating that their grouping on a joint branch reflects true genealogical affinity and is not an artifact resulting from long branch attraction.

Fig. 1. Phylogenetic tree based on ITS data of Thelidium spp. and Verrucaria latebrosa collected from freshwater habitats. Single most parsimonious tree with a length of 315 steps (CI 0·781, RI 0·862, RC 0·673, HI 0·219). Bayesian support values above 0·95 are indicated by thick branches; bootstrap values are shown below the branches. Verrucula granulosaria and V. inconnexariawere used as outgroup taxa.
Discussion
Diagnostic value of morphological characters
The dimensions of the reproductive structures appear to be generally good morphological characters for species delimitation in Thelidium. Even within species groups the dimensions of ascospores and perithecia are rather conservative. Qualitative characters such as the presence of an involucrellum or ascospore septation are valuable at the species level, while variability even between closely related species can be high.
Taxonomy
Within the Thelidium methorium clade two morphologically distinct types can be recognized. Specimens with a thick involucrellum and small excipulum diameter have been described under the name T. pertusatii while typical T. methorium has a rather thin involucrellum and larger exciple. No evidence was found for the separation of Thelidium methorium and T. aeneovinosum, thus T. aeneovinosum is a younger synonym of T. methorium. However, the genetic heterogeneity of T. methorium s. l. suggests that with a wider sampling further infraspecific taxa may be recognized. Thelidium methorium s. l. (often under its synonym T. aeneovinosum) is known from high altitudes of most parts of the Northern Hemisphere and appears to be widespread in the subarctic region (Thomson Reference Thomson1997; Zschacke Reference Zschacke1934). An extended sampling from non-European localities in order to explore the genetical structure of populations and distribution of morphological traits over the whole distribution range of T. methorium s.l. is needed. The genetic distance of Thelidium methorium and the closely related T. pertusatii, which both have 2-celled ascospores, to Verrucaria latebrosa with unicellular ascospores is smaller than to any other Thelidium species, indicating that the separation of the genera Thelidium and Verrucaria by ascospore septation is artificial; a result that also became evident in recent multigene studies with a wider taxon sampling (Gueidan et al. Reference Gueidan, Roux and Lutzoni2007; Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss, Orange, Fröberg, Amtoft, Navarro-Rosinés, Krzewicka, Pykälä and Lutzoni2008; Savić et al. Reference Savić, Tibell, Gueidan and Luzoni2008). Morphologically, Thelidium methorium, T. pertusatii, and Verrucaria latebrosa differ from all other taxa included in these multigene studies by the character combination of completely epilithic thalli, large (usually >25 μm) unicellular to 1-septate ascospores and perithecia which are covered by a distinct involucrellum, but lack a thalline mantle. Studies on the delimitation of this species group with a wider taxon sampling and more conservative genetic markers are currently underway (H. Thüs et al., unpublished).
The siliceous Thelidium submethorium with constantly 2-celled ascospores is closely related to T. aethioboloides Zschacke, a species from limestone with predominantly 4-celled ascospores. Thelidium submethorium and T. aethioboloides are a well supported monophylum within a larger clade including a second subclade with T. methorium, T. pertusatii, and Verrucaria latebrosa. Thelidium submethorium and T. aethioboloides differ from this second subclade by the smaller dimensions of their perithecia.
Small perithecia are also typical of a third species group of epilithic Thelidium species, but the involucrellum is only weak and thin or absent in this lineage, which includes the semi-aquatic species T. pluvium, the riparian T. rehmii as well as T. minutulum and T. zwackhii, which are both terrestrial species, but tolerant of short periods of inundation. Although having very similar morphology, T. minutulum and T. rehmii are not sister taxa. In the ITS phylogeny, T. rehmii is closer to T. pluvium than to T. minutulum. While morphological differences between T. rehmii and T. minutulum are small, niche separation of the two taxa appears to be distinct. Thelidium minutulum occurs on a wide range of substrata, but is usually restricted to well buffered habitats (carbonaceous rocks, basic silicates), tolerating both semi-aquatic (periodically inundated) and completely exposed (terrestrial) conditions in usually shaded but not necessarily very humid sites. Thelidium rehmii is restricted to substrata with little buffering capacity (pure sandstones) and is much more hygrophilic compared to T. minutulum. Typical habitats are small canyons or the borders of small creeks in shady ravines. Although it is restricted to sites with high air humidity and often found near small creeks, it apparently avoids sites with long periods of inundation. The preference for siliceous substrata and very humid sites is shared with its sister taxon T. pluvium, which is a highly specialized amphibious lichen. Like many other amphibious Verrucariales with prominent perithecia, it develops a distinct and spreading involucrellum and the thallus is paraplectenchymatous, while in T. rehmii and T. minutulum the thallus varies from proso- to paraplectenchymatous, often in parts of the same thallus.
Many species of freshwater Thelidium are very rare (T. aethioboloides, T. nigricans, T. pertusatii, T. rehmii, T. rivulicolum, T. submethorium) or are known only from the type locality (T. aquaticum, T. circumspersellum, T. klementii, T. suzaeanum) and no fresh material of them was available. The type specimens of these taxa, however, appear to be in good condition. The delimitation of these species is discussed in detail in the taxonomic treatment below. All of them are in need of reconsideration as soon as molecular data from freshly collected specimens becomes available.
Ecological remarks and conservation needs
Most freshwater Thelidium species require stable substrata and prefer neutral or only slightly acidic water (Gilbert & Giavarini Reference Gilbert1996; Gilbert Reference Gilbert and Giavarini1997; Thüs Reference Thüs2002). Sediment deposition is tolerated only by T. zwackhii and, to a certain degree, also by T. minutulum (Thüs Reference Thüs2002). Both species have rather wide ecological amplitudes and are not specialized for an amphibious life-style. Specialized amphibious species of Thelidium generally appear to be poor competitors, and are typically restricted to sites with little growth of free-living algae or mosses. These requirements are fulfilled in cool, unpolluted and more or less shaded creeks. It is remarkable that freshwater Thelidium taxa in general seem to prefer much more shaded habitats than other amphibious Verrucariales. Whether this niche separation is due to a lower light compensation point or reflects the avoidance of locations suitable for stronger competitors requires detailed physiological experiments. Sites with a well-developed freshwater Thelidium flora (except those of the pioneer species T. zwackhii and T. minutulum) are rare in Central Europe. Because of the much questioned taxonomy of Thelidium, their conservation status is largely unknown, although detailed investigations for T. methorium at least from the Black Forest indicate a strong decline of local populations (Thüs Reference Thüs2002), and T. submethorium is regarded as being extinct in this area (Wirth et al. Reference Wirth, Schöller, Scholz, Ernst, Feuerer, Gnüchtel, Hauck, Jacobsen, John and Litterski1996). However, for most species the status of their populations reported in the older literature (Servít Reference Servít1950, Reference Servít1954; Zschacke Reference Zschacke1934) needs checking. Certainly, the localities with recently discovered populations of rare species, such as T. aethioboloides, T. pluvium, T. rehmii or T. submethorium, are worthy of conservation.
Key to epilithic and semi-endolithic Central European freshwater species of Thelidium
1 Ascospores 2-celled 2
Ascospores 4-celled 11
2(1) On calcareous rocks 3
On siliceous rocks 5
3(2) Thallus epilithic, up to 100 μm thick, perithecia 100–300 μm diam.; usually a terrestrial species, tolerating only temporary submersion T. minutulum
Thallus usually semi-endolithic, epilithic forms > 100 μm thick, amphibious species, usually submersed 4
4(3) Exciple up to 200 μm diam., involucrellum apical or absent T. klementii
Exciple 300–450 μm diam., involucrellum absent T. rivulicolum
5(2) Involucrellum absent, or weak and indistinctly separated from the exciple 6
Involucrellum distinctly separated from the exciple 7
6(5) Photobiont cells irregularly arranged, ascospores 20–30 × 8–15 μm, involucrellum absent or fused with the exciple and visible as a slight thickening in the upper part of the perithecium; on sandstone T. rehmii
Photobiont cells arranged in small aggregated groups, ascospores 13–32 × 4–15 μm, involucrellum absent or thin, indistinctly separated from the exciple and reaching the thallus base; on various (usually well buffered) substrata T. minutulum
7(5) Ascospores 16–22 μm long T. lahmianum
Ascospores longer 8
8(7) Perithecia more than half covered by a thalline mantle, involucrellum usually weak (up to 30 μm thick) and often fading in lower parts T. pluvium
Perithecia naked, at least in upper two thirds, involucrellum thin to very thick (25–170 μm), evenly coloured throughout 9
9(8) Involucrellum very thick (115–170 μm in upper third), ratio of involucrellum thickness to exciple diameter >0·40 T. pertusatii
Involucrellum thinner (usually <90 μm in upper third), ratio of involucrellum thickness to exciple diameter <0·33 10
10(9) Perithecia (430–) 500 (–1600) μm diam., ascospores (26–) 34 (–46) μm long T. methorium
Perithecia (250–) 361 (–510) μm diam., ascospores (26–) 28 (–39) μm long T. submethorium
11(1) Involucrellum present 12
Involucrellum absent 16
12(11) Involucrellum thin, 10–30 (–35) μm, ascospores (22–) 30 (–36) μm long T. fontigenum
Involucrellum thick (> 40 μm), ascospores 23–52 μm long 13
13(12) Thallus light coloured, whitish, grey to brownish; perithecia 300–800 μm diam., (usually > 500 μm) 14
Thallus dark coloured, brown to brown-black or green-grey to olive green-black; perithecia 200–485 μm 15
14(13) Ascospores (13–) 17 (–21) μm wide, base of perithecia forming pits in the substratum, thallus thin and mostly endolithic to thick, with epilithic parts 10–80 μm, black basal layer absent T. papulare
Ascospores (16–) 19 (–23) μm wide, base of perithecia not forming pits in the substratum, thallus thick (> 100 μm), epilithic parts 50–100 μm, in parts with black basal layer T. suzaeanum
15(13) On siliceous rocks, thallus brown to brown-black, in parts with thin black basal layer, prothallus black T. nigricans
On calcareous rocks, thallus greenish, greyish olive green-black to brown-black, basal layer absent or orange to brown coloured, prothallus white or absent T. aethioboloides
16(11) Ascospores (20–) 29 (–36) μm long, asci 90–120 μm long T. zwackhii
Ascospores 16–27 μm long, asci 40–75 μm long 17
17(16) Exciple 200 μm diam., with black base, asci up to 75 μm long, thallus thin to semiendolithic; on calcareous substrata T. circumspersellum
Exciple 80–160 μm diam., with colourless base, asci 40–60 μm long, thallus thin to thick (20–60 μm); epilithic on siliceous substrata T. aquaticum
Taxonomic treatment of the species
Thelidium aethioboloides Zschacke
Hedw. 62: 144 (1920); type: [Switzerland, Grisons] Davos auf Kalkstein in einem Bache, nicht über Wasser, + Thelidium zwackhii A. Massal., 07.08.1916, Zschacke 3836 (B—syntype!).
Thallus black-brown, olive, dark greyish green, thin to thick (35–70 μm, up to 100 μm according to Zschacke Reference Zschacke1920), ± continuous with a few cracks. Prothallus whitish, little developed or absent. Cortex not differentiated, but uppermost parts of the thallus yellowish to brown, paraplectenchymatous, margins becoming ± transparent when wet (subgelatinous). Basal layer locally present, with orange-brown colour. Photobiont cells 4–9 μm diam., occasionally also with elongated cells 8–11 × 4–5 μm, dispersed in small aggregated groups.
Perithecia immersed in the ± flat thallus or embedded in flat thalline warts, exposing only the naked ostiolum, basal parts often immersed in the substratum. Exciple (145–) 202 ± 28·6 (–240) μm diam. (n=7, N=7). Periphyses up to 21 μm long. Involucrellum variable, apical to reaching the thallus base, (290–) 350 ± 64 (–465) μm diam. (n=7, N=7), laterally extended in the thallus. Asci 73–93 × 22–36 μm (n=4, N=4). Ascospores (1–) 2 (–3) septate, (25·2–) 31·6 ± 3·5 (–40) × (10–) 12·2 ± 1·3 (–15·4) μm (n= 67, N=7).
Habitat and distribution. Amphibious on limestone rocks, but usually not permanently submerged. Known only from a few localities in the Alps at upper montane to subalpine altitudes, always below the timber line and apparently avoiding sun exposed sites.
Nomenclatural notes. Thelidium aethioboloides Zschacke has priority over the heterotypic homonym T. aethioboloides (Nyl.) Vain. (= T. minutulum Körb.) based on Verrucaria intercedens var. aethioboloides Nyl. since this was published one year later than T. aethioboloides Zschacke. Thelidium aethioboloides Zschacke was regarded as a synonym of T. minutulum by Coppins (Reference Coppins2002), but the latter taxon clearly differs by its weakly developed or absent involucrellum, by mostly having ascospores with only one septum, by a greyish to greenish instead of blackish-olive thallus colour and at least in part by having a prosoplectenchymatous instead of strictly paraplectenchymatous thallus architecture. The distinction of both taxa at species rank is further corroborated by the results of our analysis of ITS sequences.
Similar species. Thelidium submethorium can be similar to T. aethioboloides, but differs by having 2-celled ascospores. Thelidium nigricans is also similar, but has a brown-black thallus, a black basal layer, and occasionally a black prothallus. Furthermore, it grows on siliceous rocks. The thalline colour in Thelidium is much dependent on habitat conditions, thus the distinction of both taxa is in need of confirmation by molecular characters. In all specimens of T. aethioboloides small numbers of ascospores with one or three septa were observed, while in all specimens of T. nigricans only 2-septate ascospores were seen. Whether this difference is a reliable character for the distinction of the two taxa requires further studies of more material.
Additional specimens examined. Germany: Bavaria: Allgäu, Oberstdorf, Faltenbachtobel, in splash water zone, 950–980 m, Thüs W0862, W1602 (BM), 900–1000 m, inundated in a small creek, July 1918, Lettau (B); Garmisch-Partenkirchen, Lindertal, “7-Quellen”, 1100 m, periodically inundated, M. Schultz 08155c (hb. M. Schultz, Hamburg).—Italy: Trentino-Alto Adige (Bolzano): Sciliar Natural Park, Altopiano dello Sciliar, 2400 m, Nascimbene JN2027 (hb. J. Nascimbene). Veneto (Belluno): Val Tovanella, La Stua, 950 m, Nascimbene JN1996 (hb. J. Nascimbene).
Thelidium aquaticum Servít
Rozpravy Československė Akademie Věd 63 (7): 11, fig. 9 (1935); type: [Czech Republic:] Montes Rudohoří, Hradištĕ prope Kadaň, 600 m, silic. In rivulo, 1950, Servít 842845 (PRM—holotype!).
Thallus dark olive greenish to brownish, continuous or with small cracks, 20–75 μm thick, paraplectenchymatous, margins not becoming transparent when wet. Cortex absent, but uppermost part of the thallus (–10 μm) in parts yellowish brown. Basal layer (?) developed in parts of the thallus (maybe remnants of other lichens which have been overgrown), light brownish, up to 20 μm high. Photobiont cells 6–9 μm diam., cells densely packed and homogenously distributed, only in parts agglomerated.
Perithecia immersed to semi-immersed, exposed parts naked. Exciple 80–160 μm diam. Periphyses up to 12 μm long. Involucrellum absent or very thin and apical, covering exposed parts of the exciple up to 150 μm diam. Asci 40–60 × 9·6–16 μm. Ascospores (1–) 3 (–4)-septate, slightly constricted at the septa, (16–) 19·7 ± 4·2 (–27) × (4·8–) 6·1 ± 1·3 (–8) μm (n=7, N=3).
Habitat and distribution. The species is known only from the type locality in the Czech Republic where it grew in an amphibious situation in a river on siliceous substrata. The type specimen is associated with the aquatic Verrucaria cf. funckii.
Similar species. Thelidium aethioboloides has a similar thallus, but the exciple is protected by a distinct involucrellum and the ascospores are larger.
Taxonomic notes. The type material consists of three pieces of rock mounted on the same card. Most of Servít's annotations refer to the large piece at the top of the card. A section of the thallus from all three pieces of rock revealed perithecia of similar morphology, but those from the smaller pieces had a slightly larger diameter compared to those on the largest piece of rock (145–160 vs. 80–120 μm). Ascospores were traced only on the large piece from the top of the card. Alan Orange (unpublished annotation and sections in the herbarium capsule of the type specimen) indicated the presence of a lichenicolous fungus with perithecia and ascospores of the same size as T. aquaticum but with 6-septate instead of 1–4-septate ascospores and with a distinct constriction in the median septum on the type material. The perithecia in his sections appear to be more prominent and almost sessile on the thallus whereas the majority of the perithecia in all parts of the type specimen are completely immersed or only the upper third exposed. The documented section most probably represents either an unidentified lichenicolous fungus on the Verrucaria cf. funckii thalli on one of the smaller pieces of rock or an over- mature perithecium from a specimen of T. aquaticum. In the majority of the Thelidium thalli on all three pieces of rock, however, the perithecia are immersed in the thallus or exposed only in their uppermost part, the ascospores are 1–3-septate with only slight constrictions, the thallus shows a dense arrangement of paraplectenchymatous hyphal cells and contains densely packed algae without any signs of reduced vitality, indicating that Thelidium aquaticum is a lichenized and not a lichenicolous fungus.
Thelidium circumspersellum (Nyl.) Zschacke
Verrucaria circumspersella Nyl., Flora 64: 536 (1881); type: [Romania] in rivulo prope Ponor-Ohaba, com. Hunyad in Transsylvania, Lojka: Lich. Hung. 114 (not seen). –Thelidium circumspersellum var. lojkeanum (Szatala) Szatala, Folia Cryptog. I (5): 371 (1927).–Thelidium lojkanum Szatala, Magy. Bot. Lap. 24: 29 (1925); type: [Romania] ad saxa calcarea in rivulo ex antro minore oriundo prope Ponor-Ohaba, com. Hunyad in Transsylvania, 1874, Lojka 3384, (W—holotype!).
Thallus inconspicuous, with orange tinge in var. lojkeanum (due to the presence of Trentepohlia cells), semi-endolithic, very thin, of loosely interwoven, mostly vertically orientated hyphae, surface powdery, in parts minutely rimose. Cortex absent. Basal layer absent. Photobiont cells very sparse, irregularly dispersed or in small groups, 3–6 μm diam.; additional cells of Trentepohlia mostly on and near the surface; cyanobacteria scattered throughout the thallus but also completely endolithic below the thallus.
Perithecia sessile to semi-immersed, exposed parts naked. Exciple black-brown, 150–218 μm diam. Periphyses soon vanishing. Involucrellum absent. Asci (64–) 70·3 ± 3·8 (–75) × (17·4–) 19·6 ± 2·9 (–25) μm (n=6, N=2). Ascospores 3-septate, (18–) 23·1 ± 3·1 (–27) × (7·3–) 8·2 ± 0·9 (–9·6) μm (n=6, N=2) (data for var. circumspersellum adapted from Zschacke 1922).
Habitat and distribution. The species is known only from the type locality in Romania where it was collected on periodically inundated limestone.
Similar species. Thelidium zwackhii has larger ascospores and asci, a distinct thallus with a tight hyphal arrangement and the exciple is transparent in its lower parts. It is necessary to study fresh material and molecular characters in order to determine if Thelidium circumspersellum is more than a depauperate form of T. zwackhii. The co-occurrence of cyanobacteria, Trentepohlia and coccoid green algal cells among thin and little connected fungal hyphae may also indicate that T. circumspersellum is an algicolous fungus but not a lichenized species.
Thelidium fontigenum A. Massal
Miscell. Lich.: 31 (1856); type: [Gemany, Bavaria] ad ligna irrigata in Franconia media (Dietenhofen), Anzi: Lich Rar. Venet. 171, leg. Rehm, ex Hb. Mass. (W—isotype!, FR 0059100—syntype!).
Sagedia cataractarum Hepp, Lich. Europ. 442 (1857); type: [Switzerland?] an Sandsteinfelsen bei Wasserfällen, Hepp 442 (FR—syntype!).
Sagedia zwackhii ß toficola Hepp, Lich. Europ. 443 (1857); type: [Switzerland?] auf Kalktufffelsen, häufig in Gesellschaft von Scytonema heerianum Näegeli und Scytonema ambigua Ktz., Hepp 443 (FR—syntype!).
?Sagedia riparia Hepp, Lich. Europ. 96 (1857); type: [Switzerland] Zürich, an entblößten Wurzeln alter Weidenstämme, Hepp 96 (not seen, see taxonomic notes).
?Thelidium rodnense Zschacke, Mag. Bot. Lap.: 299–298 (1912); type: [Romania] Rodnaborborek am Isvorul rosii auf Kalkgestein, Zschacke (not seen, see taxonomic notes).
Thallus white, whitish grey to greyish green, on wood occasionally with red, K+ purple patches, epilithic to semi-endolithic, 45–145 μm thick, proso- to paraplectenchmatous. Cortex not differentiated, but uppermost 10–15 μm of the thallus occasionally yellowish brown. Basal layer absent. Photobiont a coccoid green alga, cells ± clustered, 4–7 μm diam.
Perithecia immersed to semi-immersed in the thallus, epilithic or basal part to one third immersed in the substratum, naked. Exciple 200–320 μm diam., black-brown. Periphyses up to 30 μm. Involucrellum apical to almost reaching the thallus base, thin (10–35 μm), adjacent to the exciple or laterally extending in the thallus, 200–360 μm diam. (n=7, N=7). Asci 58–113 × 13·4–50 μm (n=8, N=4). Ascospores eventually with three septa (1–2 septa in young ascospores), (22–) 30 ± 4·9 (–36) × (9·5–) 11·7 ± 1·8 (–14) μm (n=28, N=7).
Habitat and distribution. This species occurs in damp to amphibious sites on base-rich substrata (limestone, lime-containing sandstone, on silt-impregnated wood, also on mica schist). It is widely distributed but rare, occurring from low mountain ranges to (sub-) alpine altitudes.
Similar species. Thelidium zwackhii does not have an involucrellum and its perithecia are usually largely exposed to almost sessile on the thallus surface.
Taxonomic notes. The isotypes of Thelidium fontigenum differ from those of T. cataractarum only in the development of the thallus (very thin in T. cataractarum, thicker in T. fontigenum), the reddish colour and the I+ blue reaction of the hymenium (I+ red in T. cataractarum). However, the reddish colour in the type specimens of T. fontigenum is not restricted to the lichen thalli but is also present within the wood and maybe caused by co-occurring saprobic fungi or bacteria. Thalloid features in Thelidium species from calcareous substrata are in general notoriously variable, often even within a single collection, and the iodine reaction of the hymenium alone appears insufficient for species delimitation in this genus. Thelidium riparium (Hepp) Zschacke, from submerged willow bark, differs from the type material of T. fontigenum only by the I + red reaction instead of a blue reaction as in the type specimens of T. fontigenum (according to the description given in Zschacke Reference Zschacke1920). As in the case of T. cataractarum (from which it differs only by its substratum) this character alone is not sufficient for species delimitation. Thelidium rodnense was separated from T. zwackhii by the presence of an involucrellum. All anatomical details as given in the protologue are within the range of T. fontigenum. It is most probable that Zschacke did not consider T. fontigenum (nor its synonym T. cataractarum) when he described T. rodnense. He corrected this error in a subsequent paper (Zschacke Reference Zschacke1920), where he placed T. rodnense in synonymy under T. cataractarum without further comment.
Additional specimens examined. Germany: Northrhine-Westphalia: in den Quellen der Alme, May 1865, Lahm, sub. T. cataractarum Hepp (B).—Austria: Tyrol: Matrei, bei der Waldrast, 5000 ft., Glimmer im Bach, Arnold, sub. T. cataractarum Hepp (B).—Switzerland: Bernese Oberland: Schattenhalb, Rosenlaui, 1400 m, amphibious in a small spring fed creek, Thüs W1749 (BM).
Thelidium klementii Servít
Mitt. Bot. Staatssamml. München 11: 41–43 (1954); [Gemany, Bavaria] Lechdruchbruch am Illasberg bei Roßhaupten, ad saxa temporaria inundata, calcarea-arenaria, 1953, Klement (M—holotype!).
Thallus whitish grey, semi-endolithic, thick (120–230 μm), finely rimose to areolate. Cortex 10–25 μm high, colourless. Basal layer absent. Photobiont cells rounded, 4–9 μm, dispersed.
Perithecia semi-immersed in the thallus, naked. Exciple 200 μm diam. Periphyses up to 40 μm. Involucrellum absent or apical and much varying in thickness. Asci 70–80 × 25–28 μm. Ascospores mostly uniseptate, occasionally unicellular, one apex rounded, the other acute, (17–) 22·7 ± 4·5 (–30) × (10–) 11·7 ± 1·9 (–15) μm (n=6, N=1).
Habitat and distribution. Known only from the type locality from temporarily inundated calcareous rocks with high content of silica. The type locality is now destroyed following the construction of a large reservoir and the species is now possibly extinct.
Similar species. Thelidium rivulicolum can develop a similar thick thallus, but this species has much larger perithecia and asci and the ascospores are slightly larger.
Taxonomic note. Servít (Reference Servít1954) did not mention any involucrellum in his original description, but new sections from the type specimen revealed that at least in some perithecia a distinct apical involucrellum is present.
Thelidium lahmianum Lojka ex Zschacke
Hedwigia 62: 131 (1920); type: [Poland, Tatra], an überfluteten Granitblöcken im Koprovatal, Lojka (W—holotype!).
Thallus black-brown, 50–85 μm thick, rimose, entirely paraplectenchymatous. Cortex not differentiated, but in parts of the thallus the uppermost layer is without algae. Basal layer black-brown, up to 50 μm thick. Photobiont cells sparse, rounded, 4–7 μm diam.
Perithecia semi-immersed to sessile. Exciple 200–300 μm diam. Periphyses up to 20 μm. Involucrellum adpressed to the exciple, thin and hardly discernable, extending to the thallus base, 290–350 μm diam. Asci 66–70 × 22–25 μm, 8-spored. Ascospores uniseptate, (16–) 18·4 ± 1·9 (21·7) × (7·3–) 9·0 ± 1·0 (10·1) μm (n=7).
Habitat and distribution. Known only from the type locality in the Koprova Valley (Slovakia) from inundated granite boulders.
Taxonomic note. This poorly known taxon is in need of revision. It may be conspecific with the terrestrial Thelidium tiroliense from calcareous rocks. Based on the information given by Zschacke (Reference Zschacke1920), the two taxa differ only in their substratum and thallus colour.
Thelidium methorium (Nyl.) Hellb
Verrucaria methoria Nyl., Öfvers. K. Vetensk-Akad. Förhandl. 17: 296 (1860); type: [Norway, Sør-Trøndelag], Dronthjem, W. P. Schimper (H-NYL 2121—holotype, H-NYL 6933—isotype!).
Sagedia aeneovinosa Anzi, Comment. Soc. Crittogam. Ital. 2 (1): 25 (1864); type: [Italy] ad rupes et saxa granitica humida, vet saepe aqua adspersa in monitem alpius que vallis Tellinae alpe di Bodrio, Valle di Mello, Anzi: Lich. Langob. 342A (FR 0059094—syntype!).
Thelidium aeneovinosum var. kutakii Servít, Beih. Bot. Centralbl. 55 Abt B: 263 (1936); type: [Czechia] Krkonoše (Riesengebirge): in convalle Obři důl (Riesengrund) ad saxa schistosa, 1000 m, Kuták: Lich. Bohemiae 452, 1917, leg. V. Kuták, hb. Servít 858024 (PRM—holotype!; 858026, PRM—isotype!), Suza: Lich. Bohemoslov. Exs. 271, leg. V. Kuták (W—isotype!).
Thallus (black-) brown to grey, thin to thick, (10–) 67 ± 30 (–130) μm (n=29, N=19), continuous or finely rimose, paraplectenchymatous, ± subgelatinous and with transparent margins when wet. Cortex indistinct or composed of an algal-free layer, uppermost part of thalli from sunny locations often brown. Basal layer absent. Photobiont cells dispersed, 5–7 (–12) μm diam.
Perithecia prominent, naked, at least in upper half. Exciple (228–) 361 ± 70 (–465) μm (n=29, N=19) diam. Periphyses up to 60 μm. Involucrellum almost reaching to the thallus base, (430–) 668 ± 264 (–1600) μm (n=29, N=19) diam., (40–) 64 ± 16 (–100) μm thick (n=29, N=19), ratio of involcrellum thickness to exciple diameter <0·33 (0·1–0·32). Asci (69–) 91 ±11 (–100) μm (n= 7, N=7). Ascospores uniseptate, (26–) 33·8 ± 4·3 (–46) × (10–) 14·4 ± 1·7 (–18·8) μm (n=195, N=19) (up to 21 μm wide according to Wirth Reference Wirth1995).
Habitat and distribution. This species was found on siliceous rocks that were frequently wetted but usually not submerged for more than a few months in a year (Keller & Scheidegger Reference Keller and Scheidegger1994). Terrestrial populations exist in regions with high precipitation. Mostly on well-lit to semi-shaded sites in upland areas. Widely distributed at sub-alpine to alpine altitudes, very rare in high mountain ranges (e.g. Black Forest) and here probably at high risk of extinction owing to climatic changes and reforestation of open meadows.
Similar species. Thelidium submethorium differs by its smaller perithecia and ascospores. Thelidium pluvium has perithecia which are covered by a thalline mantle. Thelidium pyrenophorum Mudd is a predominantly terrestrial species, but amphibious populations are reported from Great Britain and may also be expected in upland regions of Central Europe. Zschacke (Reference Zschacke1920, Reference Zschacke1934), however, did not regard this species as hydrophytic. It differs from T. methorium by its whitish to greyish colours and smaller ascospores (19–32 μm). Thelidium schibleri Zschacke is a non-amphibious species from calcareous rock with similar sized ascospores but smaller perithecia (240–480 μm, A. Orange pers. comm.). Further studies are required to determine whether it is conspecific with T. methorium.
Taxonomic and nomenclatural notes. There has been some controversy on the use of the names T. methorium and T. aeneovinosum (A. Massal.) Arn. The slight nuances in thallus colour as well as the occasional occurrence of uniseptate ascospores motivated Zschake (Reference Zschacke1934) to regard them as separate taxa, but this was not followed by authors of recent flora projects, and it is also rejected by our analysis of ITS-data which placed specimens with combinations of both character states in different nested clades within T. methorium s. l. The isotype specimen seen from FR is characterized by a rather thin and spreading involucrellum compared to most other specimens of T. methorium, but forms intergrading to a more adpressed involucrellum do exist in the same specimen. Nimis (Reference Nimis2003) regarded T. methorium as a synonym of T. aeneovinosum while Coppins (Reference Coppins2002) considered T. methorium to be the correct name for this lichen. As Thelidium methorium is based on Verrucaria methoria from 1860 it has priority over Thelidium aeneovinosum which is based on the younger basionym Sagedia aeneovinosa from 1864. Servít separated Thelidium kutakii from T. methorium by the occasional presence of 2-septate rather than uniseptate ascospores. Some perithecia in the syntypes of Thelidium kutakii contain a minority of ascospores with up to three septa among a majority of 2-celled ascospores. As there are also perithecia from the same thallus with exclusively uniseptate ascospores, the occasional occurrence of single 3-septate ascospores, without any other supporting characters is not a sufficient basis for species recognition.
Selected additional specimens examined. Sweden: Jämtland: Undersåker, Vällista, Malme: Lich. Exsic. 673 (W).—Germany: Baden-Württemberg: Black Forest, Zastler Bach, 1320 m NN, Thüs W0367 (FR).—Switzerland: Grisons: Davos, Dorfbach, 2000 m, splashwater zone, Thüs & Keller W1089 (BM); Flüela-river, 1965 m, Thüs & Keller W1131 (BM); Karlimatten, small ephemeral creek contributing to the Flüela-river, 2040 m, Thüs & Keller W1087 (BM); small ephemeral creek contributing to the Flüela-river, 2250 m, Thüs & Keller W1088, W1091, W1138, W1142, W1143 (BM); Schottensee, 2390 m, Thüs & Keller W1090 (BM).—Italy: South Tyrol: on porphyric rocks in a forest brook between Predazzo and Val Maor, 1882, [sub. Thelidium diaboli var. aeneovinosum], Arnold 952 (FR). Sondrio: Bormio, on mica-schist, Anzi [?, sub Sagedia aeneo-vinosa f. depauperata] (FR 0059093). Trentino Alto Adige (Trento): Paneveggio Nat. Park, Valbona, 1800 m, subalpine spruce forest, periodically submerged, Nascimbene JN1990 (hb. J. Nascimbene).—Austria: Salzburg: Preberkessel, rapids of a stream, 2010 m, Thüs W1804 (BM); ephemeral small creek in larch forest, Thüs W1822b (BM). Tyrol: Brenner, Griesberg valley, on wet mica-schist, 1524 m, Arnold 475b, (FR 0059096).—Slovakia: High Tatra: Bielovodska dolina, Litvorova Kotlina, 1500 m, Thüs W1243 (BM).
Thelidium minutulum Körb
Parerga Lichenol.: 351 (1863); syntypes: [Germany, Northrhine-Westphalia] auf Kalksteinen im Bagno bei Burgsteinfurth in Westphalen, leg. Nitschke, in hb. A. Metzler 15713 (FR 0059146–syntype!); Switzerland, auf schiefrigem Gestein bei Bern, leg. Kemmler (not seen).
Verrucaria intercedens var. aethioboloides Nyl. (type not seen).
Thelidium acrotellum Arnold; type: Herbarium Lichenum Nr. 10389, leg. Arnold (M—lectotype!).
Thelidium parvulum Arnold; type: [Germany, Northrhine-Westphalia] auf Steinen einer Straße zu Münster in Westphalen, 1868, Lahm 390, (M—isotype!).
Thelidium eitneri Zahlbr. = Thelidium viride Eitner (non Deakin); type: [Czech Republic, Chrudim] Chrast, auf überrieseltem Kalksandstein, Eitner, 07.1907, Acqu.-Nr. 1923-4141 (W—isotype!); Acqu.-Nr. 1929-12729 (W—isotype!).
Thallus thin to thick (30–100 μm), grey, brownish, greenish, very variable in appearance, from almost continuous to tartareous-rimose or granular. Hyphal arrangement proso- to paraplectenchymatous. Photobiont cells usually aggregated in small groups, 4–9 μm diam.
Perithecia usually prominent and naked, occasionally semi-immersed or basal parts covered by a thin layer of algae containing thallus. Exciple (100–) 194·2 ± 49·2 (–300) μm diam. (n=20, N=15), brownish in upper half. Periphyses up to 30 μm. Involucrellum absent, rarely thin (up to 30 μm) and covering the upper, exposed half of the exciple, 245–300 μm diam. Asci up to 90 × 23 μm. Ascospores uniseptate, (13·0–) 22·7 ± 4·0 (–31·9) × (4·0–) 10·3 ± 2·1 (–15·3) μm (n= 111, N=15).
Habitat and distribution. A terrestrial species, but occasionally in the splash water zone of creeks and rivers on limestone or basic siliceous rocks (especially metamorphic rocks). Widely distributed, but almost absent from regions with poorly buffered bedrock (here occasionally on man-made substrata, e.g. concrete). According to Cezanne et al. (Reference Cezanne, Eichler, Hohman and Wirth2008) this species has clearly declined in the Odenwald region, Germany.
Similar species. Thelidium minutulum is a variable species, which in some forms can be very similar to T. rehmii, but the thallus is usually more granular compared to the rather even crusts formed by T. rehmii. In some morphs (including the type specimen) of T. minutulum the perithecia contain some small mature ascospores (16–18 μm) among larger ones, but morphs with consistently large ascospores exist. Such forms of T. minutulum (including the specimens which we sequenced in this study) differ from T. rehmii mainly by the arrangement of the photobionts in small aggregated groups, while in T. rehmii the algal cells are irregularly dispersed in the thallus. Whether this character is a reliable morphological marker for the separation of the two species requires further study. In some specimens of T. minutulum a weakly developed involucrellum is found. It can reach down to the exciple base and stretch slightly laterally in the thallus. In T. rehmii an involucrellum is usually absent, but the upper part of the exciple can be slightly thickened and may be indistinguishable from a weakly developed apical involucrellum. Thelidium minutulum as understood here is restricted to well-buffered, basic substrata (limestone, metamorphic silicates), whereas T. rehmii is restricted to pure sandstones with low buffer capacity.
Taxonomic notes. Thelidium eitneri was separated only by its amphibious habitat from terrestrial forms of T. minutulum. Thelidium minutulum is interpreted here as a species with a wide ecological amplitude, including amphibious habitats and T. eitneri has thus to be regarded as a synonym. Thelidium parvulum was distinguished from T. minutulum by the presence of 3-septate ascospores among uniseptate ones. In his re-examination of the type specimen Zschacke (Reference Zschacke1934) found only uniseptate ascospores. Obviously there is a high variability in ascospore septation even among the perithecia of the type specimen, indicating that this character alone is not suitable for the separation of this morph from T. minutulum. Thelidium viride differs only in its greenish colour from T. minutulum. The colour of T. minutulum depends largely on the degree of insolation and many intermediate forms between grey and green can be observed. The intense green colour in the type specimens of T. viride is not caused by a specific pigment, but by the lack of any pigmentation in the cortex and a rather thin thallus.
Additional specimens examined. Estonia: Raplamaa, Kädva stream (58°54′N 25°05′E), on inundated stones, Suija 56c (TU).—Germany: Northrhine-Westphalia: Hösel, 95 m, spring contributing to the creek Dickelsbach, periodically immersed, Thüs W1840 (BM); Bensberg, 127 m, banks of the creek ‘Kleiner Wahlbach’, Thüs W0668 (FR); Bielsteinhöhle bei Lippspringe, Beckhaus, sub T. parvulum (B); Münster, on pebbles on a street, 1868, Lahm 390 (FR 0059145). Hesse: Taunus, Wisper, 155 m, on boulders bordering the river, Thüs W0060 (FR); Frankfurter Wald, 110 m, 1864, A. Metzler 2614 (FR 0059137). Rhineland-Palatinate: Pfauengrund, 190m, ephemeric creek, periodically inundated, Thüs W0265 (FR). Saarland: Mettlacher Saarschleife, Steinbach, on temporarily inundated sandstone, Thüs W1711 (cited in Thüs Reference Thüs2006 as T. cf. parvulum). Baden-Württemberg: Odenwald, Zwingenberg / Neckar, Wolfschlucht, on carbonaceous gravel on a path, Thüs W1654 (BM); north of Schlossau, west of Bönigfeld, small stone on the slope of a path, 1998. Cezanne & Eichler 4876a/b (hb. Cezanne & Eichler); Wachenberg, east of Weinheim, on porphyric pebbles on the ground, 390 m NN, 2004, Cezanne & Eichler 6778 (hb. Cezanne & Eichler). Bavaria: Allgäu, Oberstdorf, Faltenbach, 980 m, on boulders in the splashwater zone, Thüs W1613 (BM); Garmisch-Partenkirchen, Lindertal, ‘7-Quellen’, 1100m, periodically inundated, M. Schultz 08155c (hb. M. Schultz).—Austria: Steiermark: small creek contributing to the River Teigitsch, 430–480 m, Thüs W1386, W1387 (BM).
Thelidium nigricans Zschacke
Magy. Bot. Lap.: 300 (1912); type: Romania, Südkarpathen, Bach unterhalb des Bullea Sees, July 1910, Zschacke 2843 (B—holotype!).
Thallus black-brown, thin (30–35 μm); according to Zschacke (1912) with single photobiont cells protruding up to 100 μm below the thallus surface (chasmoendolithic), finely rimose, paraplectenchymatous. Cortex absent, but uppermost layer of the thallus yellowish to brownish. Black basal layer very thin and developed only in parts of the thallus, partially reduced to isolated black patches. Photobiont cells 5–8 μm diam., mostly arranged in small groups.
Perithecia at first immersed in the thallus, but finally upper half exposed. Exciple 190–230 μm diam. Periphyses up to 30 μm. Involucrellum 200–485 μm diam., reaching the base of the thallus and in some perithecia connected with the black basal layer. Asci 70 × 30 μm. Ascospores 3-septate, (21·9–) 32·3 ± 3·4 (–40) × (10·0–) 12·2 ± 1·1 (–14·5) μm (n=35, N=3).
Habitat and distribution. This species is known only from two widely separated collections from Romania and Switzerland, but is possibly overlooked elsewhere. It grows on siliceous rocks in the splash water zone of small creeks.
Similar species. Thelidium nigricans is very similar to T. aethioboloides and molecular data are needed in order to clarify if they are genetically distinct species. In addition to their different substratum preferences they may be distinguished by the colour of the thallus, prothallus and basal layer.
Additional specimen examined. Switzerland: Berner Oberland, Schattenhalb, Rosenlaui, 1400 m, small spring fed creek, Thüs W1747 (BM).
Thelidium papulare (Fr.) Arnold
Verrucaria papularis Fr., Lichenogr. Europ. Reform.: 434 (1831); type: ad rupes calcareas silvae nigrae in regno Württembergiae, leg. Hochstetter; not seen.
Thallus grey, brown, occasionally with whitish pruina, thin and ± semi-endolithic to thick and epilithic (epilithic parts 10–80μm). Black basal layer absent. Photobiont cells 6–9 μm, aggregated in small groups.
Perithecia half-immersed, often with the basal part in small pits in the rock. Exciple (370–) 410 ± 39·4 (–485) μm diam. Periphyses up to 70 μm. Involucrellum thick (60–85 μm), (570–) 636·7 ± 48·7 (–685) μm diam. (n=5, N=6), 300–800 μm in terrestrial specimens according to Wirth (Reference Wirth1995); apical to reaching the base of the exciple, often extending laterally in the thallus. Asci 90–110 × 36–43 μm. Ascospores (1–) 3-septate, (34·8–) 47·3 ± 4·5 (–56·6) × (13–) 17·1 ± 1·8 (–21·2) μm (n=73, N=6); 30–65 × 12–20 μm in terrestrial specimens according to Wirth (Reference Wirth1995).
Habitat and distribution. This is a common terrestrial species but in upland regions it also colonizes temporarily submerged sites, especially in limestone areas with high amplitudes of water level changes. It is frequent on calcareous rocks but rarely also found on siliceous substrata, often in shaded locations. It is a widespread species, but amphibious populations are ± restricted to cool (micro-) climates.
Similar species. Thelidium suzaeanum differs mainly in its slightly wider ascospores, which are in the range of (16–) 18–23 μm. In T. papulare such wide ascospores may occur in small numbers among a majority of narrower spores; most spores are less than 18 μm wide. The thallus in T. suzaeanum is usually thicker than in T. papulare, i.e. semi-endolithic, but mostly developed above the substratum surface, and the bases of the perithecia never form pits in the substratum. The two species are sometimes difficult to distinguish and further studies including molecular characters are needed in order to verify their status.
Selected specimens examined. Germany: Hessen: Lahntal, Villmar, Bodensteinerlei, on rarely inundated dolomite, Thüs W0944 (FR). Bavaria: Allgäu, Oberstdorf, Breitach, on periodically immersed boulders, Thüs W0824, W0830, (FR), W1604 (BM); Nebelhorn, ephemeric spring of the creek ‘Wengenbach’ Thüs W0859 (FR).—Austria: Upper Austria: Roßleiten, creek close to ‘Pießlingursprung’, on moist rocks, 750 m, F. Berger 21401 (hb. F. Berger); ibid., on rocks wetted by splash water, F.Berger 21416 (hb. F. Berger).
Thelidium pertusatii (Garov.) Jatta
Verrucaria pertusatii Garov., Tent. disp. Lich. Langob. 61: plate 4, fig. 4 (1865); type: ad rupes graniticas in Alpibus Valsesiae, leg. Garovaglio (PAV—lectotype, hic designatus).
Thallus thin to rather thick (30–145 μm), grey, brown to brown-black, continuous to minutely rimose, paraplectenchymatous. Photobionts scattered, up to 6 μm diam.
Perithecia prominent, naked. Exciple 230–295 μm diam. Periphyses up to 45 μm. Involucrellum reaching the thallus base, 514–570 μm diam., very thick (115–170 μm), ratio of involucrellum thickness to exciple diameter >0·4. Ascospores uniseptate, (26·1–) 32·1 ± 4·2 (–40) × (11·6–) 13·5 ± 1·4 (–16) μm (n=16, N=3), fresh ascospores in part halonate.
Habitat and distribution. An amphibious species on frequently wetted rocks in alpine rivers and irrigated rocks in the Central and Western Alps, possibly endemic.
Similar taxa. Thelidium pertusatii differs from the closely related T. methorium by the small diameter of the exciple which is covered by a massive involucrellum.
Taxonomic note. The drawing in the protologue (also reproduced in Zschacke Reference Zschacke1934) is schematic and does not represent the correct proportions of exciple and involucrellum in the type material.
Additional specimens examined. Italy: Trentino Alto Adige (Trento): Stelvio National Park, Val de la Mare, 46°25′16, 1″N, 010°41′04,3″E, 2200 m, alpine environment, well-lit site above the tree-line, Nascimbene JN1541, JN1542 (hb. J. Nascimbene).
Thelidium pluvium Orange
Lichenologist 23: 101 (1991); type: Anglia, Westmoreland, prope Newby Bridge, Yew Barrow, Ellerside, 54°16′N, 2°59′W (34/355867), 120 m, on acidic stone in small shaded stream in woodland, 19 February 1988, A. Orange 6647 (NMW–holotype!).
Thallus greyish, brown, greenish, continuous or with small cracks, 35–85 μm high, paraplectenchymatous. Photobiont rounded, up to 8 μm diam. or elongated 5·5 × 9 μm, irregularly arranged.
Perithecia in prominent thalline warts. Periphyses up to 40 μm long. Exciple 100–320 μm diam. (up to 350 μm, Orange Reference Orange1991). Involcurellum apical, to reaching the thallus base, 150–500 μm diam., thin (up to 30 μm) but distinct, often spreading ventrolaterally, leaving a transparent area between exciple and involucrellum, upper part dark coloured, lower part fading, covered by a thin thallus mantle, involucrellum pigment K−. Asci 75–100 × 25–30 μm (Orange Reference Orange1991). Ascospores uniseptate, (19·4–) 27·6 ± 4·7 (–36·3) μm × (8·1–) 11·6 ± 2·1 (–17·4) μm (n=27, N=4).
Habitat and distribution. This species grows in the splash zone of small rivers and creeks on siliceous rocks and pebbles, in usually shaded situations. In Central Europe it is recorded from the Alps (Berger & Priemetzhofer Reference Berger and Priemetzhofer2000; Keller Reference Keller2000), Black Forest (Wirth Reference Wirth1999) and the Odenwald (Cezanne et al. Reference Cezanne, Eichler, Hohman and Wirth2008), but should be looked for in other mountain regions. It is also known from the British Isles, Norway (Orange Reference Orange1991) and Tasmania (McCarthy Reference McCarthy1994).
Similar species. No other freshwater Thelidium with small uniseptate ascospores has perithecia with a thalline mantle. The terrestrial T. fumidum (B—isotype!) has a dark brown thallus, a well-developed involucrellum reaching the exciple base and a black basal layer. The involucrellum has a reddish brown pigment, which turns grey after application of K. The representatives of the T. methorium group have larger perithecia and a well-developed and thicker involucrellum.
Specimens examined: Germany: Baden-Württemberg: Reichenbach, small creek contributing to the ‘Seebach’, 320 m, on temporarily inundated sandstone together with T. rehmii, Thüs W1647 (BM).—Austria: Teigitsch, 430–480 m, on frequently inundated mica schist, Thüs W1382, W1383 (BM), Keller 2098 (teste A.Orange, hb. Christine Keller).
Thelidium rehmii Zschacke
Hedw. 62: 116 (1921); type: [Germany, Bavaria] Deutenheim in Mittelfranken im Keupersandsteinbruche, Rabenhorst: Lichenes Europaei 594, leg. Rehm, sub Thelidium nylanderi (Hepp) Kremp. (B—syntype!, W—syntype!).
?Thelidium margaceum Zschacke Hedwigia 62: 121 (1921); type: England, Yorkshire, in a creek near Ayton, sub Verrucaria margacea var., in hb. Lahm, leg. Leighton (W—isotype!).
Thallus a whitish grey to green-grey, thin tartareous crust (15–110 μm) with fine cracks, proso- to paraplectenchymatous. Photobionts dispersed, not aggregated in small groups, ± globose, 3–10 μm diam.
Perithecia semi-immersed to almost sessile. Exciple 200–320 μm (n=19, N=15) diam., dark brown in upper parts, hyaline below. Periphyses up to 30 μm. Involucrellum absent or indistinguishable from the exciple, forming a slight apical thickening of the perithecium. Asci (67–) 80·4 ± 6·5 (–93) × (17–) 25·8 ± 5·4 (–40) μm (n=16, N=14). Ascospores uniseptate, (20–) 25·4 ± 2·3 (–29·9) × (7·8–) 10·1 ± 1·4(–14·5) μm (n=104, N=15).
Habitat and distribution. This species is restricted to sandstone rocks in humid and more or less shaded places, often in the vicinity of little creeks but usually restricted to micro-sites without or with only short submersion periods. Thelidium rehmii is known only from a few regions in southern Germany (Frankonia, Odenwald, Black Forest). A distribution map for the Odenwald is given in Cezanne et al. (Reference Cezanne, Eichler, Hohman and Wirth2008).
Similar species. Thelidium minutulum is a variable species, which in some forms can be very similar to T. rehmii. The thallus is usually more granular compared to the rather even crusts formed by T. rehmii. In some morphs (including the type specimen) of T. minutulum the perithecia contain some small mature ascospores (16–18 μm) among larger ones, but morphs with consistently large ascospores also exist. Such forms of T. minutulum (including the specimens which we sequenced in this study) differ from T. rehmii only by the arrangement of the photobionts in small aggregated groups, while in T. rehmii the algal cells are irregularly dispersed in the thallus. Whether this character is a reliable morphological marker for the separation of the two species requires further study. Thelidium minutulum as understood here is restricted to well buffered, basic substrata (limestone, metamorphic silicates), while T. rehmii is restricted to pure sandstones with low buffer capacity.
Taxonomic note. Thelidium margaceum Zschacke differs from T. rehmii s. str. mainly in its entirely paraplectenchymatous thallus which becomes gelatinous when wet and growing at temporarily inundated sites. This species is known from England and Scandinavia and a single collection from the Czech Republic (Servít Reference Servít1954). Zschacke (Reference Zschacke1920, Reference Zschacke1934) mentioned a distinct involucrellum closely attached to the exciple in T. margaceum. Re-examination of the isotype specimen from Vienna made it clear that there is no separate involucrellum but only a slight apical thickening of the exciple, very similar to the perithecia of the isotype specimens of T. rehmii. The basal part of the exciple is colourless in both forms. Whether the two taxa should be separated at species rank requires further studies, including the collection of molecular data for T. margaceum.
Selected specimens examined. Germany: Hessen: Würzberg, Römerbad, shaded walls, on bricks, 500 m, O. Behr 3626 (B). Baden-Württemberg: SE Mittelberg, Eichelbachtal, on temporarily inudated sanstone in a creek, Cezanne & Eichler 5258 (hb. Cezanne & Eichler); NNE of Ünglert, Ünglertstal, on sandstone at the bank of the creek Mudbach, 320 m NN, Cezanne & Eichler 2215 (hb. Cezanne & Eichler); small valley NE Märzenbrunnen, NW Waldbrunn Trienzbachtal, on rarely inundated sandstone, Cezanne & Eichler 762 (hb. Cezanne & Eichler); Heuweg NE Sattelbach, on rocks in a creek, Cezanne & Eichler 3415 (hb. Cezanne & Eichler); W Wohlfahrtsmühle, NO Höpfingen, on temporarily inundated sandstone in a creek Cezanne & Eichler 5264 (hb. Cezanne & Eichler); SE Hirschhorn/Neckar, “Morsklinge”, on temporarily inundated sandstone Cezanne & Eichler 2725 (hb. Cezanne & Eichler); Reichenbach, small creek contributing to the ‘Seebach’, 320m, Thüs W1646–1648 (BM); Zwingenberg (Neckar), Wolfschlucht, 200 m, Thüs, W1652 (BM); Reisenbacher Grund, Antonslust, 360 m, Thüs W1276 (BM); Black-Forest, Vorbergzone, Schirnburg b. Maulburg (Wiesental), Arnold, in herb. Zschacke 3821 (B); Black-Forest?, on sandstone, Lettau 187 (B). Bavaria: Odenwald, Kaltenbrunn, Kaltenbach, on sandstone boulders above the splashwater zone, Thüs W0921 (FR), on temporarily inundated sandstone Cezanne & Eichler 5187 (hb. Cezanne & Eichler).
Thelidium rivulicolum (Nyl.) Migula
Verrucaria rivulicola Nyl., Flora de Ratisbonne 25: 13 (1875); type: supra lapides cretaceos (saepius submersus) rivuli prope Lenharrée in regione Catalaunensi (Marne), M. Brisson (W—syntype!).
Thallus epilithic or semi-endolithic, thick (100–200 μm), continuous to rimose, with powdery surface, whitish to greenish, with colourless cortex or cortex absent.
Perithecia semi-immersed to sessile, naked. Exciple 300–450 μm diam. Periphyses not seen (soon vanishing?). Involucrellum absent. Asci 90 × 35 μm. Ascospores uniseptate, 23–42 × 7–15 μm.
Habitat and distribution. Known only from the type locality in France and an old collection from Bavaria, both on temporarily inundated carbonaceous rocks.
Similar species: Thelidium klementii has much smaller perithecia, which often have an apical involucrellum and a semi-endolithic thallus. Thelidium zwackhii has 3-septate ascospores.
Taxonomic note. All the available herbarium specimens are in very poor condition and are either sterile or with only one or a few perithecia. A good illustration of the anatomy of the Bavarian material however, is given in Migula (Reference Migula and Thomes1931) and detailed anatomical data for the type material are given in the annotation to the syntype in the Vienna herbarium.
Additional specimens examined. Germany: Bavaria: on pebbles in the headwater of a creek near Kofel in the vicinity of Oberammergau, leg. Schnabl = Arnold, Lich. Exs. 1633 (B, FR, W).
Thelidium submethorium (Vain.) Zahlbr
Verrucaria aeneovinosa subsp. submethoria Vain., Medd. Soc. Fauna et Flora Fenn. 19: 170 (1883); type: [Russia, Karelian ASSR] Kuusamo, Paanajärvi, Kiekkipuro [ad saxa gneissacea in rivulo Kiekkipuro], 1877, E. A. Vainio (TUR-V 3116—holotype; H—isotype!).
Thallus thin to thick (10–130 μm), continuous or finely rimose, paraplectenchymatous, ± subgelatinous and with transparent margins when wet. With olive, brown or brown-grey colours.
Perithecia prominent. Exciple (150–) 222·6 ± 41 (–275) μm (n=11, N=5) diam. Periphyses up to 30 μm. Involucrellum variable, even within a single thallus from apical and adpressed to the exciple to spreading and reaching the thallus base, (250–) 361·1 ± 87·5 (–510) μm (n=11, N=5) diam., thin to thick (25–85 μm); ratio of involucrellum thickness to exciple diameter < 0·33. Ascospores uniseptate, rarely unicellular, (26·1–) 28·1 ± 3·7 (–39) × (8·7–) 13·1 ± 1·8 (–18) μm (n=54, N=5), without perispore.
Habitat and distribution. This species occurs on siliceous substrata in clean creeks and rivers of high mountain ranges. Apart from the Finnish type locality it is known only from historical records from the Black Forest and the Italian region of Trentino-Alto Adige (Southern Alps). Scandinavian records of T. aethioboloides from siliceous substrata (e.g. Nordin Reference Nordin2002) may also refer to this species, but are in need of confirmation. Due to its scarce recent records and its need for unpolluted, cool creeks, T. submethorium is a species with high risk of extinction in Central Europe.
Similar species. Thelidium pertusatii is distinguished by its very thick involucrellum, reaching 30% or more of the diameter of the excipulum. Thelidium methorium is easily distinguished from T. submethorium in Central Europe by its distinctly larger perithecia and ascospores. On the same piece of rock from which T. submethorium JN1991 was sampled, we also found T. methorium, which differed clearly in its perithecium and ascospore size. The thalli of both species from this piece of rock were sequenced. Thelidium methorium JN1991 clusters together with sequences of T. methorium from other regions while T. submethorium JN1991 is a sister taxon to T. aethioboloides (Fig.1). The type specimens of T. submethorium and T. methorium from northern Karelia and Norway respectively, are much more similar to each other, with slightly overlapping extreme sizes of ascospores and perithecia. The possibility cannot be excluded at present that the type specimen of T. submethorium is a depauperate form of T. methorium and that the Central European specimens represent a separate taxon. Molecular data for Nordic specimens of T. submethorium may be necessary to verify the identity of our specimens and the nordic T. submethorium.
Specimens examined. Germany: Baden-Württemberg, Black Forest, Zastler Bach, 1953, Ried 140(FR), Zell im Wiesenttal, Hohe Möhr, 700 m, at and in a small creek in forested area, 25 v 1922, Lettau (B).—Italy: Trentino Alto Adige (Trento): Paneveggio Nat. Park, Valbona, subalpine spruce forest, periodically submerged, 1800 m, 2005, Nascimbene JN1991 (hb. J. Nascimbene).
Thelidium suzaeanum (Servít) Servít
Involucrothele suzaeana Servít, Rozpravy Československė Akademie Věd. 63 (7): 23 (1953); type: [Slovakia] Tatry Bielské, ad saxa calcarea inundata in rivo Havran, 950 m, Servít 858027 (PRM—holotype!), Servít 858028 (PRM—isotype!).
Thallus dark olive to light brownish, thick (100–180 μm), semi-endolithic, epilithic part 50–100 μm, surface smooth, continuous to partially rimose-areolate, proso- to paraplectenchymatous. Cortex brownish. Black basal layer locally present, 20–120 μm. Prothallus dark or indistinct. Photobiont cells up to 8 μm diam., arranged in small groups. Perithecia semi-immersed in the thallus, not forming pits in the substratum. Exciple 300–510 μm diam. Periphyses up to 70 μm. Involucrellum thick (50–80 μm), apical to dimidiate but protruding a little laterally, up to 630 μm diam. Asci 70–85 × 22–25 μm. Ascospores eventually 3-septate, (31·7–) 33·4 ± (–55·1) × (16–) 18·6 (–23·2) μm (n=15, N=2).
Habitat and distribution. The species is known only from the type locality in the Tatry Bielské (Slovakia) from inundated limestone rocks.
Similar species. The majority of ascospores in the perithecia of Thelidium papulare are less than 18 μm wide, the thallus is thinner, always without a black basal layer, and the bases of the perithecia form pits in the substratum.
Thelidium zwackhii (Hepp) A.Massal
Sagedia zwackhii Hepp., Lich. exs.: 96 (1853); type: an Nagelfluhblöcken des Zürichsees, Hepp, Lich. exs. 96 (FR—syntype!).
Verrucaria xylospila Nyl. (type not seen, see taxonomic notes).
?Thelidium subgelatinosum Zschacke; type: [Great Britain] Cleveland, in a stream near Ayton, Mudd 281 (W—syntype!).
Thallus thin, 35–65 μm; greyish, whitish to brownish, proso- to paraplectenchymatous, occasionally with a pale, algal free basal layer. Photobiont cells arranged in small groups.
Perithecia at first more or less immersed in the thallus, finally usually almost sessile on the thallus. Exciple 180–260 μm diam. Periphyses up to 20 μm. Involucrellum absent or indistinct, occasionally the exciple is slightly swollen (up to 23 μm) in the uppermost third. Asci 90–120 × 29–40 μm (according to Servít Reference Servít1954). Ascospores 3-septate, (20·3–) 29·1 ± 3·9 (–34·8) μm × (8·7–) 12·9 ± 4·1 (–15·4) μm, up to 36 μm long according to Wirth (Reference Wirth1995).
Habitat and distribution. This species is most frequently found at terrestrial sites, but amphibious populations exist although it does not occur in permanently submerged sites. It is found on a wide diversity of substrata, often as a pioneer species on silt incrusted limestone, lime impregnated sandstone or hard wood, but generally does not occur on acidic substrata. Although little recorded it is presumably widely distributed but often overlooked.
Similar species. Among the 3-septate species, Thelidium fontigenum is most similar, differing only by the presence of a thin involucrellum. Thelidium aethioboloides has an olive-black to dark green-grey thallus and a well-developed involucrellum. Thelidium circumspersellum and T. aquaticum have smaller asci and ascospores.
Taxonomic notes. The separation of Thelidium xylospilum (Nyl.) Zschacke is based only on the substratum type (lignum instead of rock or soil) and Zschacke (Reference Zschacke1920) commented that Thelidium xylospilum has to be regarded as a ‘form’ of T. zwackhii, thus contradicting his own combination. The substratum alone should never be used as the only basis for separating a taxon at specific level, if there are no other characters supporting this view. According to Zschacke (1933), Thelidium subgelatinosum differs mainly by the paraplectenchymatous instead of a prosoplectenchymatous thallus, but intergrading forms do exist, indicating that this character alone is not sufficient for recognizing this form at the species level. The syntype specimen in the Vienna collection (W), however, has mainly 2-celled ascospores. This lichen is most probably an over-mature specimen of T. minutulum. As other syntype specimens have not been checked so far, it is still possible that Mudd 281 is a heterogeneous exsiccate and different parts may belong to either T. minutulum and T. zwackhii.
Additional specimens examined. Germany: Northrhine-Westphalia: Teutoburger Wald, Lienen, spring in a beech forest, on calcareous pebbles above water level, Thüs W0954 (FR). Rheinland-Pfalz: Kaiserslautern, Botanical Garden of the Technical University, sandstone in the spray zone of an artificial waterfall, Thüs W1627, W1649, W1660 (BM); creek, close to the clay mine at the village Nohfelden-Eisen, on rocks at the river bank, Thüs W0028 (FR). Saarland: Orscholz, Saarschleife, Steinbach, on lime-impregnated sandstone close by the creek, Thüs W1702 (BM).—Italy: Veneto (Belluno): Pedavena, Val Porcilla 600 m, N 46°03′39″, E 011°50′32″, deciduous forest, in shaded conditions and periodically inundated rocks, Nascimbene JN1992 (hb. J. Nascimbene).
Excluded Taxa
Thelidium epomphalum (Nyl.) Zahlbr
Verrucaria epomphala Nyl.; Flora 64: 536 (1881): [Romania] Super lapides calacareos subinundatos prope pagum Ponor Chába [=Ohába] in Transsylvania, Lojka Lich. Hung. Fasc.II Nr. 99, B600140923, B600140845, B600140847, B600140889 (B—syntypes!).—Polyblastia epomphala (Nyl.) Zschacke.
Thelidium epomphalum (Nyl.) Zahlbr is very similar to T. papulare. This taxon was described from temporarily irrigated rocks in Romania and no other Central European records from creeks or rivers are known. It was first placed in Verrucaria and then transferred to Thelidium by Zschacke (Reference Zschacke1920), who observed exclusively 3-septate ascospores in a collection of specimens from the type locality. Nylander (Reference Nylander1881) mentioned only a single ascospore with one additional longitudinal septum in the protologue, and Zschacke (Reference Zschacke1934) transferred the taxon to Polyblastia in his comprehensive treatment of Verrucariaceae. This generic affiliation is certainly not settled and future changes are to be expected (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss, Orange, Fröberg, Amtoft, Navarro-Rosinés, Krzewicka, Pykälä and Lutzoni2008; Savić et al. Reference Savić, Tibell, Gueidan and Luzoni2008). Apart from the occasional occurrence of longitudinal septae, T. epomphalum differs from T. papulare mainly by its thin and mostly endolithic thallus with short chains of oil-cells 15 μm diam. contrasting with the majority of hyphal cells that do not exceed a diameter of 3·6 μm. Zschacke (Reference Zschacke1920) also mentioned the flattened perithecia with depressed ostiolum as a diagnostic character, but this does not hold true, as there are populations of T. papulare that share this character, but lack oil cells and longitudinal septae (e.g. Thüs W0859).
Thelidium jizerae (Servít) H. Riedl
Involucrothele jizerae Servít; Československé Lisejniky Čeledi: 187–188 (1954); type: [Czech Republic] Bohemia, saxa inundata schistosa flum. Jizerae pr. Urb. Semilyi 300 m, 1925, Servít 858029, 858030, 858031, 858032 (PRM—syntypes!).—Verrucaria jizerae (Servít) J. Nowak & Tobolewski.
Servít (Reference Servít1954) based his placement of this taxon in the genus Involucrothele on the observation of a single two-celled ascospore among a majority of unicellular ascospores. Nowak & Tobolewski (Reference Nowak and Tobolewski1975) regarded this placement as too schematic and proposed the new combination Verrucaria jizerae (Servít) J. Nowak & Tobol. This view is supported by the results of our re-examination of the syntypes from the collection at PRM, which revealed exclusively unicellular ascospores. The thalli are blackish brown with a rough and uneven surface which is continuous in parts but mostly cracked to almost areolate. Large areas appear to be eroded. Many perithecia are largely exposed and have the same roughened surface as the thallus. Most ascospores have collapsed cytoplasm, but all of the ascospores (n=20) seen were unicellular. However, the size and form of the ascospores (17–28 μm × 7–12 μm), the dimidiate involucrellum with the triangle between the involucrellum and the exciple base being dark coloured, as well as the vertical arrangement of the photobiont cells in short chains, make it most probable that the material represents an over-mature or damaged thallus of Verrucaria funckii (Spreng.) Zahlbr. and not a separate species.
Thelidium spadanum B. de Lesdain
Bull. Soc. Bot. Belg. 47: 43 (1910); type: [Belgium], Theux bei Spa auf Schiefer in einem Graben, leg. Bouly; Torfondry, auf Kieselsteinen in einem Bache, leg. Lochenies (material presumably lost during the second world war).
Thallus grey-brown, thin, fissured; perithecia 0·3 mm, semi-immersed in a thalline wart. Ascospores 24–30 μm × 12 μm (leg. Bouly) & 27–31 μm ×15–16 μm (leg. Lochenies). In the original description there is no information on the structure of the involucrellum, and T. spadanum remains a nomem dubium.
Thelidium subcontinuum (Nyl.) Servít
Verrucaria delita f. subcontinua Nyl., Flora 64: 189 (1881); type: Romania, Transsilvania, lacum Tenoga, saxa garanit. inund. 1872, leg. Lojka (W—holotype!).— Verrucaria zenogensis Zschacke.
According to Servít (Reference Servít1954) there is a small number of 2-celled ascospores among a majority of unicellular spores in the type specimen. Re-examination of the holotype revealed only unicellular ascospores and the older name Verrucaria subcontinua (Nyl.) Zschacke should therefore be applied instead of Thelidium subcontinuum. For the same type specimen, Zschacke (1933) replaced the name V. subcontinua (Nyl.) Zschacke with the superfluous new name V. zenogensis without further comments explaining the reasons of his decision. The ascospores of the type specimen are 23–38 × 11–15 μm (up to 40 × 17 μm according to Servít Reference Servít1954), the involucrellum is dimidiate and laterally spreading, leaving a colourless triangle between the involucrellum and the base of exciple. With these characters, this taxon is apparently conspecific with Verrucaria applanata Hepp. It differs only by transparent exciple bases, while in V. applanata the exciple base is reported to be dark coloured in some (!) perithecia (Zschacke 1933). Since in several syntype specimens of V. applanata (FR, W) only transparent exciples were found, a separation of the two taxa based on this character is not justified.
Thelidium sublacteum Eitner
Jahresber. schles. Gesellsch. vaterl. Kultur 88: 60 (1910); type: Bohemia, Chrudim, infra ruinam Rabštejn in rivulo, schist., 1908, leg. Kalenský (FR, W, PRM—syntypes!).
Re-examination of several syntype specimens from different herbaria showed that most of the perithecia have distinct and persistent periphyses. Furthermore, the photobiont was identified as Trentepohlia. This lichen can thus no longer be considered a representative of the Verrucariales, but has to be transferred to Anisomeridium. It is very similar to Anisomeridium carinthiacum (J. Steiner) R. C. Harris and the relation between both taxa will be discussed in more detail elsewhere.
We thank the curators of B, BM, FR, PAV, PRM, TU and W for loan of material and Franz Berger (Kopfing), Rainer Cezanne (Darmstadt), Marion Eichler (Darmstadt), Christine Keller (Birmensdorf) and Matthias Schultz (Hamburg) for loans from their private herbaria, help and stimulating discussions during collecting trips. We thank Alan Orange for correcting the English in the final version of the manuscript and for important additional information on synonyms and type specimens. Leif Tibell and Christine Keller contributed many helpful suggestions on the manuscript. P. L. Nimis (Trieste) and R. Duque-Thüs (Frankfurt) made suggestions for improvements of the English in a previous version of this manuscript. Financial support to the senior author was given by the German Research Foundation (TH –840/2-1). This research received further support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP6 “Structuring the European Research Area” Programme. The contribution of the junior author is part of the CRENODAT Project coordinated by the Museo Tridentino di Scienze Naturali (Trento) and funded by the Provincia Autonoma di Trento.




