Significant outcomes
• In this first nationwide study on neurosyphilis in the Netherlands, a mean annual incidence of 0.47 per 100 000 adults was found, with predominantly male subjects.
• The highest incidence of neurosyphilis was found in the most urbanised part of the Netherlands (0.6 per 100 000 adults).
• Tabes dorsalis is the most frequently registered subclassification of neurosyphilis, comparable to observations in the pre-antibiotic era.
Limitations
• Given the sometimes very long latent period, the increased incidence of syphilis could still be followed by an enhanced incidence of neurosyphilis in the near future.
• Underdiagnosis and underrepresentation of neurosyphilis and HIV should still be taken into account.
Introduction
Neurosyphilis is defined as any involvement of the central nervous system (CNS) at any stage of syphilitic infection, caused by Treponema pallidum subspecies pallidum (Reference Ghanem1). On the basis of clinical manifestations, it is classified in early, late and asymptomatic neurosyphilis. Early neurosyphilis is characterised by meningitis, cranial nerve abnormalities and cerebrospinal accidents. The most common clinical syndromes of late neurosyphilis are tabes dorsalis and general paralysis of the insane, of which the former was the most prevalent form in the pre-antibiotic era (Reference Ho and Lukehart2).
After the introduction of penicillin therapy in the 1940s and the expansion of screening, treatment and prevention programmes, the incidence of both syphilis and neurosyphilis decreased significantly in high-income countries (Reference Hook3,Reference Fenton, Breban and Vardavas4). Given the subsequently rapid decrease of syphilis-associated dementia, the American Academy of Neurology omitted standard screening for syphilis in the diagnostic workup of dementia, except for the high-incidence regions (Reference Knopman, DeKosky and Cummings5). It should be stressed, however, that, despite the post-antibiotic reduction of syphilis incidence, re-emergence of the disease occurred at several time points in the United States, Australia and many European countries (Reference Fenton, Breban and Vardavas4,Reference Read and Donovan6). In the latter, such as the Netherlands, the increase of syphilis incidence was most pronounced in major urban centres, particularly in populations of men who have sex with men (Reference Fenton, Breban and Vardavas4,Reference Van de Laar, van Veen, Gotz, Nuradini, van der Meijden and Thio7,8). Other factors contributing to this increase are HIV infection, intravenous use of illegal drugs and immigration into western European countries of people from countries with endemic syphilis (Reference Golden, Marra and Holmes9–Reference Fenton and Lowndes11). The number of hospitalisations because of syphilis increased in large urban areas in Spain between 1997 and 2006 (Reference García-García, Ariza Megía, Álvaro, Gil de Miguel and Gil-Prieto12), whereas in the United Kingdom resurgence was initially observed in the larger cities but later progressed to the suburban and rural settings (Reference Simms, Fenton and Ashton13).
In the western European countries, there is an ongoing debate about the utility of routine serological screening for syphilis in the workup of neurological and psychiatric conditions (Reference Van de Ree, Stam, Hische and Van Ketel14–Reference Friedrich, Geusau, Friedrich, Vyssoki, Pfleger and Aigner16). Consequently, clinicians are increasingly unaware of syphilis – a disease referred to as ‘the great imitator’ by Sir William Osler (1849–1919) because of its varied presentations – and less experienced in the interpretation of serological values. This relative unfamiliarity with neurosyphilis may lead to a marked delay in case detection, as had happened with other infectious diseases that were assumed to be under control (Reference Paolo and Nosanchuk17). In the Netherlands, 11 cases of neurosyphilis with neurological and/or psychiatric phenomena have been recently published (Table 1). Analysis of these reports showed that neurosyphilis was often not included in the differential diagnosis but incidentally detected by routine blood screening, followed by examination of the cerebrospinal fluid.
Table 1 Case reports on neurosyphilis in the Netherlands over the last decade
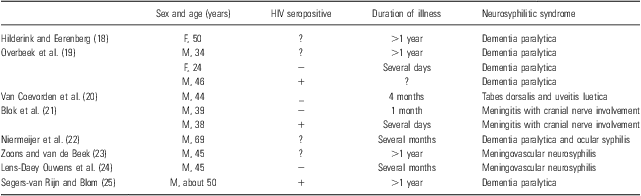
F, female; M, male; ?, unknown.
As the efficacy of antibiotic treatment depends on the stage of neurosyphilis, early diagnosis is highly important (Reference Ghanem1). In general, all symptoms completely resolve after antibiotic treatment in patients with early, meningeal neurosyphilis, with the exception of HIV-infected patients who may have persistent signs and symptoms for more than a year after such a therapy (Reference Ghanem, Moore, Rompalo, Erbelding, Zenilman and Gebo26,Reference Chahine, Khoriaty, Tomford and Hussain27). However, owing to parenchymal brain damage, complete remission of symptoms does not always occur in patients with late neurosyphilis, albeit that disease progression may be prevented (Reference Hahn, Webster and Weickhardt28–Reference Hotson30).
In Europe, a yearly neurosyphilis incidence varying from 0.16 to 2.1 per 100 000 inhabitants is documented (Reference Nordenbo and Sørensen31–Reference Conde-Sendín, Amela-Peris, Aladro-Benito and Maroto33). As the incidence of syphilis is probably related to demographic parameters such as the urban–rural balance, a small country with a high population density such as the Netherlands is suitable for investigating the epidemiology of syphilis and neurosyphilis.
Aims of the study
In the absence of a nationwide surveillance programme for neurosyphilis, data on syphilis and neurosyphilis in the Netherlands over a 12-year period (1999–2010) were analysed in the present study, by their epidemiological and clinical characteristics.
Materials and methods
Hospital data
In this retrospective cohort study, hospital data of neurosyphilis cases admitted to general hospitals were collected from the Dutch National Medical Registration over a 12-year period. In 1999–2004, this register had a coverage of 99%, whereas in the period 2005–2010 this figure was about 80–90% (population 16 million). All the patients included were aged 20 years or above, with a primary or secondary discharge diagnosis of neurosyphilis. Neurosyphilis was defined according to the International Classification of Diseases, ninth revision Clinical Modification (ICD9-CM) using the ICD9-CM code 094 and subcategory codes for all manifestations of neurosyphilis and 91.81 for acute syphilitic meningitis. The hospital data comprised age, gender, date of discharge and a four-digit zip code. These data combined with patient identifiers were used to exclude duplicate hospitalisations.
Incidence was defined as the number of cases with a discharge diagnosis (primary or secondary) of neurosyphilis per 100 000 of the Dutch population, aged 20 years or above in the period from 1999 to 2010. Demographic parameters were analysed for all neurosyphilitic patients. Comorbidity with HIV infection was recorded according to ICD9-CM codes for HIV seropositivity (042 to 044 and 795.8). Zip codes were used for geographic classification.
Data from centres for sexually transmitted infections (STI)
Data from the Dutch STI centres were used to analyse the number of syphilis cases diagnosed between 1999 and 2010. Until 2002, STI surveillance was based on voluntary registration by these centres. In 2003, a surveillance system comprising the most important centres became operational, yielding an 80% coverage. One year later, all existing STI centres were connected, providing national coverage. These clinics offer STI and HIV testing and treatment, free of charge, for high-risk groups and people who want to be tested anonymously. All new STI consultations and corresponding diagnoses are reported anonymously to the Centre for Infectious Disease Control for surveillance purposes. Attendees of a STI clinic are offered standard testing for chlamydia, gonorrhoea, syphilis and HIV. Other STI tests are conducted if necessary.
Data analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Although the number of syphilis cases diagnosed in the STI clinics increased to 700 cases in 2004, followed by a decrease to 500 new cases in 2010 (Fig. 1), no time trend for incidence of neurosyphilis could be found. In the period from January 1999 to December 2010, 695 discharge diagnoses of neurosyphilis were registered in the Dutch National Medical Registration, varying from 41 to 72 cases per year. A primary discharge diagnosis of neurosyphilis was recorded in 560 cases (81%) and a secondary in 135 cases (19%).

Fig. 1 Number of syphilis diagnoses, number of hospitalisations because of neurosyphilis (left axis) and incidence of neurosyphilis (right axis) in the Netherlands (1999–2010).
The annual incidence of neurosyphilis varied from 0.34 to 0.59 per 100 000 of the population aged 20 years or above. Of all the patients recorded with syphilis (n = 5311) in the STI clinics or neurosyphilis (527) in the Dutch National Medical Registration (n = 695), the majority were men (91% and 76%, respectively). With respect to neurosyphilis, the mean annual incidence per 100 000 was 0.7 in men and 0.2 in women. Median age for men was 47 years (oldest 86 years) and 54 years for women (oldest 92 years) (Fig. 2).

Fig. 2 Mean incidence of hospitalisations because of neurosyphilis in the Netherlands by age group and sex (1999–2010).
As presented in Table 2, the incidence of neurosyphilis differed per age group and gender, with the highest incidence for men in the age range of 30–50 years, and for women in the age range of 70 years and above. The mean annual incidence of hospitalisation for neurosyphilis was significantly higher in the western, most urbanised part of the Netherlands (0.6 per 100 000 of the population). In the rest of the Netherlands, the incidence was 0.4 per 100 000 of the population.
Table 2 Overall data on syphilis and neurosyphilis in the Netherlands (1999–2010)

Hospital data of all patients revealed a diagnosis of late neurosyphilis in 204 cases (29%), asymptomatic neurosyphilis in 38 (5%), early neurosyphilis in 11 (2%) and other forms of neurosyphilis in 15 (2%) cases. Clinical characteristics are summarised in Table 2.
Clinical manifestation was not specified (neurosyphilis NEC or NOS) in 427 cases (61%). Tabes dorsalis (170 cases) was the most frequent registered form of neurosyphilis. Median age did not differ significantly between patients with general paresis of the insane (59.5 years) and patients with tabes dorsalis (59.0 years). Patients with early neurosyphilis were significantly younger (median age 42 years) than those with late neurosyphilis (median age 59 years). Gender was not correlated with any clinical manifestation. An additional HIV-positive status was reported in 101 patients (15%) who had a median age of 40 years (range 27–70) and were mostly men (93%).
Discussion
This is the first nationwide study on neurosyphilis in the Netherlands, covering a recent period of 12 years, in which a mean annual incidence of 0.47 per 100 000 adults was found from a retrospective analysis of hospital data, with predominantly male subjects. The mean annual incidence of hospitalisation for neurosyphilis was significantly higher in the western, most urbanised part of the Netherlands (0.6 per 100 000 of the population). In the rest of the Netherlands, the incidence was 0.4 per 100 000 of the population.
The yearly incidence found in this study is higher than that observed in the 1970s in Leicester (0.18 per 100 000 inhabitants) (Reference Alani and Millac32) and in the 1980s in Greater Copenhagen (0.16–0.46) (Reference Nordenbo and Sørensen31), but lower than in the northern area of the island of Gran Canaria and the whole island of Lanzarote in the 1990s (0.2–2.1 per 100 000) (Reference Conde-Sendín, Amela-Peris, Aladro-Benito and Maroto33). As for the sex ratio, it was found that the higher syphilis infection rate in men is comparable to that reported in most European countries (8). This also holds true for neurosyphilis (Reference Nordenbo and Sørensen31–Reference Danielsen, Weismann, Jørgensen, Heidenheim and Fugleholm36). Similarly, neurosyphilis affected men twice as often as women (10% and 5%, respectively) in the pre-antibiotic era (Reference Clark and Danbolt37). However, comparison of the incidence rates of neurosyphilis in the various European countries is difficult because of the considerable variatiability in case definitions.
Interestingly, although a marked increase in syphilis is documented nationwide in STI clinics in the Netherlands up to 2004, no change in neurosyphilis incidence is observed over the past 12 years. This might be the result of continued efforts towards earlier and improved syphilis screening and treatment in STI clinics, and routine screening of pregnant women and blood donors (Reference Op de Coul, Hahné and van Weert38,Reference Koedijk, Vriend and Broek van den39). As standard screening for syphilis in psychiatric and neurological patients is no longer routinely included in laboratory testing, there is a risk for underdiagnosis and subsequent underregistration of neurosyphilis. It should be stressed, however, that because of the long latency between syphilis and neurosyphilis, a rise in the documented incidence of neurosyphilis may still occur in the decades to come.
Although tabes dorsalis is the most frequently registered subclassification of neurosyphilis in the present study, comparable to observations in the pre-antibiotic era, a correct interpretation of this finding cannot be given as 54% of cases was classified as neurosyphilis NOS. In contrast, other studies have reported that tabes dorsalis has become increasingly rare in the antibiotic era (Reference Nordenbo and Sørensen31,Reference Wolters35,Reference Burke and Schaberg40). In an earlier Dutch clinical cohort study, the overall figures of early and late neurosyphilitic syndromes were more or less the same in the pre- and post-antibiotic eras, with the exception of an increased incidence of asymptomatic neurosyphilis (1930–1940: n = 518; 1970–1984: n = 121). The rise in incidence of tabo paralysis and decline in tabes dorsalis in the post-antibiotic era group was interpreted as the result of a more accurate neuropsychological patient investigation (Reference Wolters35).
As can be inferred from this study on a relatively large group of patients, neurosyphilis in the Netherlands is not restricted to HIV-positive patients, a finding that corroborates results from a smaller sample Danish study (Reference Danielsen, Weismann, Jørgensen, Heidenheim and Fugleholm36). In some studies, HIV-positive patients are reported to not only have a higher incidence of early rather than late neurosyphilis, but also a shorter period before the manifestation of late forms of neurosyphilis (Reference Chahine, Khoriaty, Tomford and Hussain27,Reference Flood, Weinstock, Guroy, Bayne, Simon and Bolan41,Reference Zellan and Augenbraun42). This may be explained by their compromised immune system. In the pre-antibiotic era, the majority of cases with acute syphilitic meningitis occurred after an unsuccessful therapy for early syphilis. It was hypothesised that exposure to drugs that did not reach treponemacidal levels in the CNS resulted in diminished immune response and clinically manifest neurosyphilis (Reference Ghanem1).
In line with these observations, the median age of HIV-seropositive neurosyphilitic patients in the present study was significantly lower than the overall median age. As subclassification of neurosyphilis was registered in only 24% of the HIV-positive neurosyphilitic patients, the relationship between early versus late neurosyphilis and HIV seropositivity cannot be fully elucidated. The high number of cases with an ICD9-CM neurosyphilis NOS/NEC code may result from atypical clinical manifestations of the disease. In contrast, in a recent Danish study, only 13% of the cases were reported with the classification of neurosyphilis NOS (Reference Danielsen, Weismann, Jørgensen, Heidenheim and Fugleholm36).
In the present study, cases with early neurosyphilis were significantly younger than those with late forms of the disease that could have been expected, assuming that manifestations of neurosyphilis occur in a consecutive order. In contrast, however, no significant difference in median age in patients with tabes dorsalis and general paralysis of the insane (considered the last stage of neurosyphilis) was found in the present study. Moreover, previous studies in the Netherlands and Denmark did not disclose any differences in age between cases classified with ‘early’ and ‘late’ neurosyphilis (Reference Wolters35,Reference Danielsen, Weismann, Jørgensen, Heidenheim and Fugleholm36). Although the traditional classification is based on the idea that syphilitic meningitis, meningovascular syphilis, tabes dorsalis and general paresis of the insane occur in a sequential order, these forms rarely exist in pure form at autopsy (Reference Carr43). Classifying the clinical manifestations of neurosyphilis might be complicated by the overlap between the clinical syndromes (Reference Yao, Huang, Xie and Cheng44), thus explaining the high rate of cases without clinical classification (neurosyphilis NOS and NEC) in this study.
Although it seems unlikely that new cases of neurosyphilis are missed out as all patients with a new diagnosis of neurosyphilis are treated with intravenous antibiotics in a general hospital, underdiagnosis and therefore underregistration of neurosyphilis and HIV should still be taken into account. As stated before, the low incidence of neurosyphilis co-occurs with less familiarity of clinicians with the asymptomatic and atypical manifestations of the disease. Reintroduction of screening for neurosyphilis should therefore be considered seriously (Reference Seña, White and Sparling45). Further research on the frequent atypical clinical symptomatology is needed to improve both diagnosis and management of patients with neurosyphilis.
Summarising, over the past 12 years, neurosyphilis was diagnosed in about 60 Dutch hospitalised adult patients per year, indicating that neurosyphilis is yet to be considered in the differential diagnosis. The incidence of neurosyphilis is highest in young, urban men, although tabes dorsalis is the most frequently reported clinical manifestation.
Acknowledgement
The authors are indebted to the staff members of the Centre for Infection Disease Control for their kind cooperativeness in collecting the data.
Authors’ contributions
I.M. Daey Ouwens contributed substantially to the conception and design of the study and the interpretation of the data and the draft of the article. F.D.H. Koedijk contributed substantially to the acquisition, analysis and interpretation of data and the draft of the article. A.T.L. Fiolet contributed substantially to the design of the study, the interpretation of the data and the draft of the article. M.G. Van Veen and C.C. Van den Wijngaard contributed substantially to the conception and design of the study, the acquisition, analysis and interpretation of the data. W.M.A. Verhoeven, J.I.M. Egger and M.A.B. van der Sande contributed substantially to the conception and design of the study and the interpretation of the data and revised the manuscript critically for intellectual content. All authors have seen and approved the final version of the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.





