Introduction
There are 791 Anguilliform species including the moray eel Muraena helena, family Muraenidae (Nelson, Reference Nelson2006). Muraena helena is broadly spread, commonly occurring through the Eastern Atlantic and the Mediterranean Sea, and recorded in the Red and Adriatic Seas (Smith & Böhlke, Reference Smith, Böhlke, Quero, Hureau, Karrer, Post and Saldanha1990; Randall & Golani, Reference Randall and Golani1995; Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011). The species maintains high fidelity to cryptic habitats (Reece et al., Reference Reece, Bowen, Smith and Larson2010). Throughout the Mediterranean Sea, M. helena is reported as the most common moray eel. It is mainly caught in northern Tunisian waters, where rocky bottoms are more frequent, as this species prefers to live concealed in crevices (Fischer et al., Reference Fischer, Bauchot and Schneider1987). Until now, very little was known about this species, despite its wide geographic distribution and its important ecological role as a top predator in Mediterranean benthic communities (Matić-Skoko et al., Reference Matić-Skoko, Tutman, Bojanic Varezic, Skaramuca, Đikic, Lisičić and Skaramuca2014). Studies on M. helena remain fragmentary and concern morphological, ecological and molecular aspects of the species from the Canary Islands (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007), their biology in the southern Adriatic Sea (Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011, Reference Matić-Skoko, Tutman, Bojanic Varezic, Skaramuca, Đikic, Lisičić and Skaramuca2014), and their feeding habits, age and growth on the northern Tunisian coast (Sallami et al., Reference Sallami, Ben Salem, Reynaud and Capapé2014, Reference Sallami, Béarez and Ben Salem2016). The purpose of this study, therefore, is to expand the available knowledge on this species, giving insights on its meristic, morphometric and biological characteristics in northern Tunisian waters. Data were compared, when possible, with results from other marine areas, in order to detect the existence of spatial variations of life parameters among different populations.
Materials and methods
For this study, 310 specimens of Muraena helena were sampled using longlines at a 50–100 m depth from inshore waters off Bizerte and El Haouaria, on the north coast of Tunisia, from October 2011 to November 2013 (Figure 1). The sampling effort was temporally spread across a year (Froese et al., Reference Froese, Tsikliras and Stergiou2011).

Fig. 1. Geographic location of the sampling sites of M. helena from northern Tunisian waters.
All specimens were sexed, after dissection, by a macroscopic examination of the gonads. To test the sex ratio, a simple χ2 test was used.
To establish the vertebral formula, a subsample of fish were selected (N = 142). The abdominal, caudal and total vertebrae for each specimen were enumerated according to the approach adopted by Aboussouan (Reference Aboussouan1994), and the parameters of distribution (average) and the parameters of dispersion (minimum, maximum, standard error) of each meristic character were calculated.
Measurements and weights were performed on freshly captured material. All specimens (N = 310) were measured for total length (TL), pre-anal length (paL), pre-dorsal length (pdL) and head length (hL) to the nearest millimetre (mm) (Figure 2). Individual weights were recorded to the nearest 0.1 g.
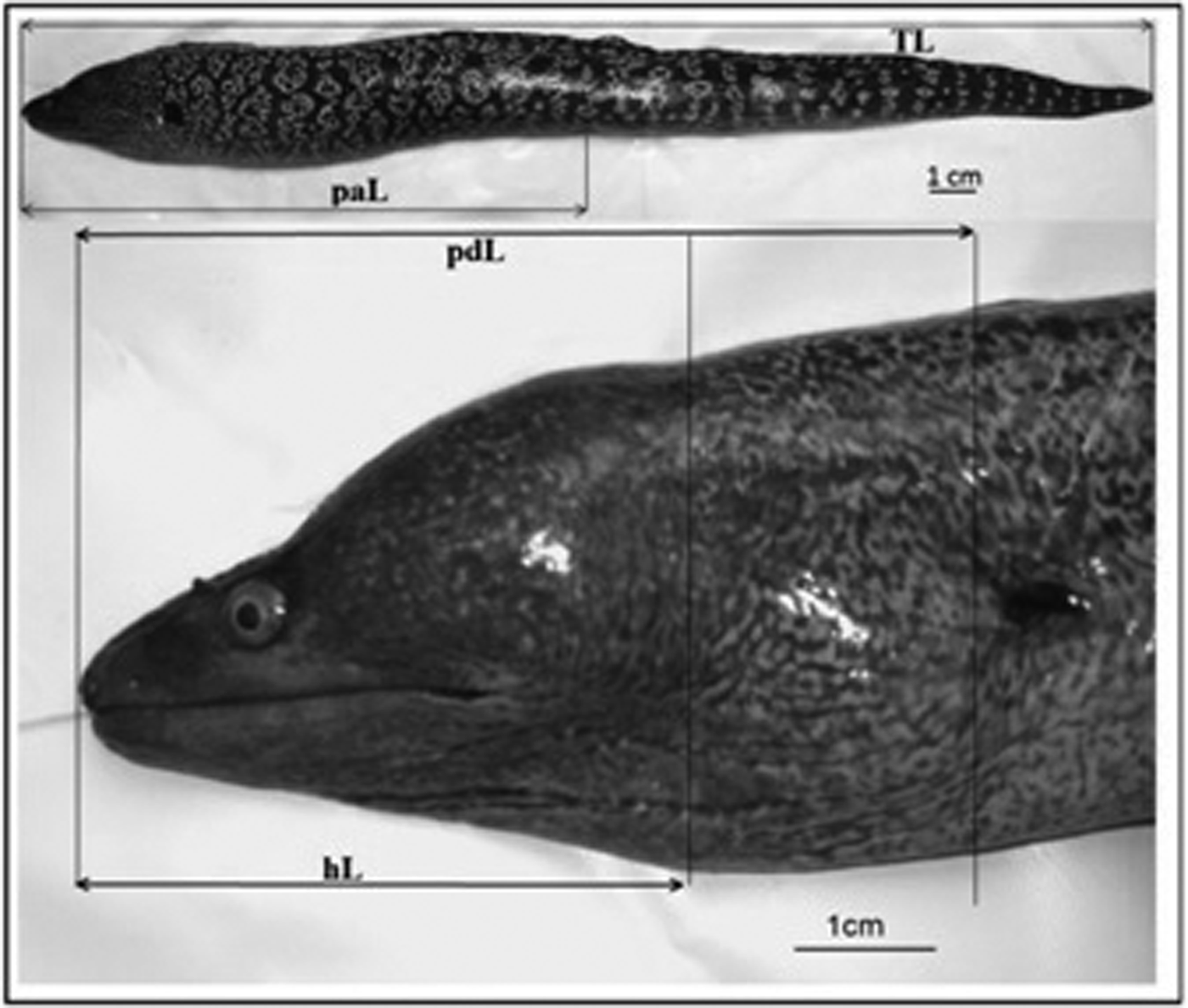
Fig. 2. Morphometric measurements of M. helena. (TL) total length; (paL) pre-anal length; (pdL) pre-dorsal length; (hL) head length.
Weight-length relationships (WLR) are generally expressed by the equation TW = a TLb (Le Cren, Reference Le Cren1951; Ricker, Reference Ricker1975), where TW is the total body weight (g), TL is the total length (cm), a is the intercept and b is the slope of the regression tracing fish somatic growth rate (Beverton & Holt, Reference Beverton and Holt1996). The WLR were converted into their logarithmic (base 10) expression log TW = log a + b log TL for the statistical analysis of the slope toward the theoretical value 3. Parameters a and b were calculated by least-squares regression, as well as the coefficient of determination (r 2). When the ‘b’ value was significantly (P < 0.05) larger or smaller than 3.0, the somatic growth presented a positive or negative allometry; however, when the ‘b’ value was not significantly different from 3.0 the somatic growth was considered to be isometric. Additionally, the parameters a and b of the WLR between sexes were compared with the analysis of covariance (ANCOVA).
For the morphometric study, the log-transformed data were plotted and obvious outliers were excluded from analyses following the recommendations of Froese (Reference Froese2006) and Froese et al. (Reference Froese, Tsikliras and Stergiou2011).
The seasonal relative condition factor (CF) was estimated from the equation: log TW = b log TL + log a (Ricker, Reference Ricker1975). TW is the total weight (g), TL the total length (cm), b the slope of the regression and a the constant. The CF was calculated for each individual using the formula: CF = TW/a TLb (Le Cren, Reference Le Cren1951).
The gonads and liver for each specimen were weighed to the nearest 0.1 g. The seasonal gonadosomatic index and the hepatosomatic index were calculated as GSI = (Wg/TW) × 100 and HSI = (LW/TW) × 100, where Wg is the gonad weight, LW is the liver weight and TW is the total weight. This GSI formula was chosen in order to make valuable comparisons of our results with those from other marine sectors.
Seasonal variations in condition factor (CF), gonadosomatic index (GSI) and hepatosomatic index (HSI) were determined, data were tested for homogeneity of variances. Significant differences were evaluated with one-way ANOVA, followed by the Tukey test. For analyses, the established level of significance was 0.05. All data exploration, statistical analyses and comparisons were estimated using IBM SPSS Statistics 22.
Results
Structure of sampling
The total sample comprised 188 males (60.64%) and 122 females (39.35%). The female-to-male sex ratio was 1:1.5, with males significantly outnumbering females (χ2 = 14.05; N = 310). The total length (TL) ranged from 47.20–109.20 cm for all specimens: from 48.50–109.20 cm for males and from 47.20–97.60 cm for females. The average TL of the total sample was 70.46 ± 0.59. There was a significant difference in the mean TL between males (TL = 71.85 ± 0.82) and females (TL = 68.32 ± 0.78) (Mann–Whitney U test; N = 310, P = 0.0126). The total weight varied between a minimum of 161.08 g and a maximum of 3000 g with an average of 731.29 ± 24.39. The male weights ranged from 161.08–3000 g with an average of 786.52 ± 35.09 g, and females ranged from 187.39–2084.65 g with an average weight of 646.18 ± 28.80 g. There was a significant difference in the mean weight between males and females (Mann–Whitney U test; N = 310, P = 0.0357).
Meristic characteristic
The range, mean values, dispersion and t-test values of the vertebra, and the numerical character studied of the M. helena males and females are given in Table 1.
Table 1. Vertebral counts of the M. helena specimens from northern Tunisian waters

N, sample size; SE, standard error; P, P-values.
Results showed that there were no significant differences between male and female vertebral counts (P > 0.05).
Length-length relationships
The estimated parameters for the three length-length relationships considered and the related statistics are displayed in Table 2.
Table 2. Parameters of the length-length relationships for males, females and the total sample of M. helena from northern Tunisian waters

M, males; F, females; T, whole sample; N, sample size; a, intercept; b, slope; CI, confidence interval; r 2, coefficient of determination; A+, positive allometry.
All the coefficients of determination (r 2) have values that vary from 0.83–0.97 and all the variables are well correlated (P = 0.0001). The three metric characters studied, pre-anal length, pre-dorsal length and head length, presented a positive allometric growth for males, females and mixed sexes.
Statistical analysis of the regressions between males and females indicated that there were no significant differences in the slopes of the three length-length relationships (one-way ANCOVA, P > 0.05) or in the intercepts (ANCOVA, P > 0.05).
Weight-length relationships
Parameters of the weight-length relationships for males, females and the total sample of M. helena are presented in Table 3.
Table 3. Parameters of weight-length relationships of M. helena from northern Tunisian waters

M, males; F, females; T, whole sample; N, samples size; min., minimum; max., maximum; Cl, confidence intervals; a, intercept; b, slope, A+, positive allometry; r 2, coefficient of determination.
All coefficients of determination (r 2) have values >0.95 which revealed the existence of a strong relationship between weight and length (P = 0.0001). The values of the somatic growth coefficient ranged from 3.46–3.60, with statistical analysis indicating a positive allometry (hyperallometry) between the two variables for males, females and combined sexes.
The slopes (b) values did not present significant differences between males and females (one-way ANCOVA, N = 310, P > 0.05); nevertheless, the intercepts were significantly different (ANCOVA, N = 310, P < 0.05).
Biophysiological indices
The seasonal relative condition factor (CF) fluctuates from 0.83–1.23 for females (N = 122) and from 0.77–1.48 for males (N = 188). Although seasonal variation of CF is more noticeable in males than in females (Figure 3), no significant mean difference between seasons was observed for either sex (males: one-way ANOVA, P > 0.05; females: one-way ANOVA, P > 0.05). The average relative condition factor of females is slightly higher than the males, except in summer.
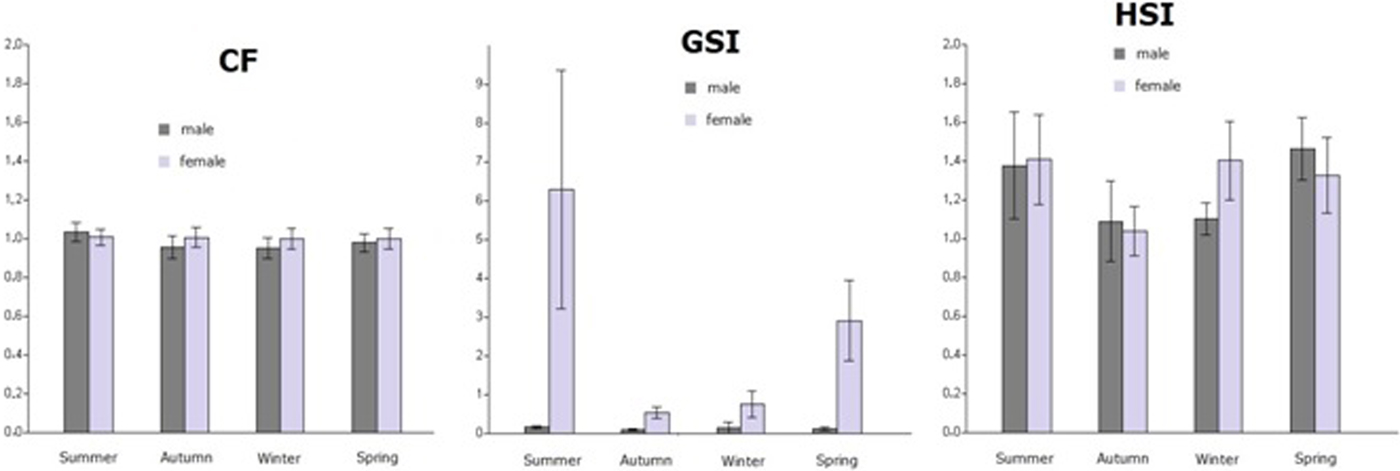
Fig. 3. Seasonal variation of the relative condition factor (CF), gonadosomatic index (GSI) and hepatosomatic index (HSI) for males and females of M. helena.
The seasonal GSI varied from 0.14–16.22 for females (N = 122) with the highest values observed during the summer. This index ranged from 0.01–1.24 for males (N = 188). Seasonal fluctuations of GSI were more marked in females than in males. Significant differences in the mean value of GSI were found among seasons for females (one-way ANOVA, P < 0.05), with the GSI being significantly higher in summer than during the other seasons (Tukey test, P < 0.05). There was no significant mean difference between seasons detected for males (one-way ANOVA, P > 0.05) (Figure 3); however, a significant difference in GSI between the sexes was observed for each season (one-way ANOVA, P < 0.05).
The seasonal HSI ranged from 0.66–2.62 for females (N = 122) and 0.19–3.04 for males (N = 188). Significant seasonal mean differences were recorded for females (one-way ANOVA, P < 0.05), especially between summer and autumn (Tukey test, P < 0.05), and for males (one-way ANOVA, P < 0.05), namely between autumn and spring (Tukey test, P < 0.05) (Figure 3). The HSI varied significantly between females and males only during winter (one-way ANOVA, P < 0.05).
Discussion
This study is the first investigation of Muraena helena inhabiting northern Tunisian waters. We initially adopted a morphometric approach based on the study of meristic and metric characters, then subsequently performed statistical analysis of seasonal variations in biological indices.
The maximum length of M. helena caught on the northern coasts of Tunisia (TL = 109.2 cm) was smaller than the maximum length recorded elsewhere. This includes the Adriatic Sea (121 cm; Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011, Reference Matić-Skoko, Tutman, Bojanic Varezic, Skaramuca, Đikic, Lisičić and Skaramuca2014) and the Canary Islands (134 cm; Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007), which has the largest individual on record. These differences could be due to the trophic conditions in the Mediterranean Sea, which is considered one of the most oligotrophic regions in the world in terms of both primary productivity and chlorophyll a concentrations (Azov, Reference Azov1991). Lower productivity in northern Tunisian waters probably reduces nutritional value to higher order carnivores/scavengers such as moray eels, through trophic pathways.
The overall female-to-male ratio (1:1.5) was clearly in favour of males for this studied sample. A similar finding has already been reported in the same area, with a female-to-male ratio of 1:1.12 (Sallami et al., Reference Sallami, Béarez and Ben Salem2016). However, this differs from the southern Adriatic Sea, where the sex ratio was 1:0.35 (Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011), and from the Canary Islands, where the sex ratio was 1.38:1 (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007). These differences could be attributed to the different bathymetrical distribution of sexes with depth. Indeed, Matić-Skoko et al. (Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011) indicates that the majority of males are localized at depths greater than 30 m. Moreover, the predominance of males in deeper waters has been observed in other Anguilliform species, such as C. conger in the Sardinian Channel (Cau & Manconi, Reference Cau and Manconi1983) and the Atlantic Iberian waters (Correia et al., Reference Correia, Manso and Coimbra2009), and the Muraenesox cinereus in the Seto Inland Sea of Japan (Kobayashi et al., Reference Kobayashi, Mototani, Murayama and Sakamoto2015).
Muraena helena is rarely found in water shallower than 50 m (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007); therefore longline fishing (at depth of between 50–100 m) was the only way that the fishermen could collect a sufficient sample for our biological study.
In this study, the total number of M. helena vertebrae ranged from between 138 and 146. Comparison with the vertebrae totals from different marine areas (Table 4) shows that they are similar to those recorded in the Mediterranean Sea (Fischer et al., Reference Fischer, Bauchot and Schneider1987; Aboussouan, Reference Aboussouan1994) and the Eastern Atlantic (Blache, Reference Blache1967; Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007). The amplitude variation of vertebrae numbers agrees with the limits mentioned by these authors. Knowledge of vertebral formula made it possible to understand the normal number of vertebrae as well as variations and anomalies; in many cases this variation testifies to the existence of subspecies or even ecological populations (Aboussouan, Reference Aboussouan1994).
Table 4. Comparison of the numerical counts of M. helena vertebra reported from different marine areas

N, sample size; min., minimum number; max., maximum number.
Length-length relationships are important in fisheries management for comparative growth studies (Moutopoulos & Stergiou, Reference Moutopoulos and Stergiou2002), but until now none have been reported for the moray eel. Our results revealed a positive allometric growth for the pre-anal, pre-dorsal and head lengths of M. helena in northern Tunisian waters, and that somatic growth presented a positive allometry for females, males and combined sexes.
Table 5 shows comparisons of the length–weight relationship parameters of M. helena obtained from various areas. The weight-length relationship (WLR) allowed comparisons of somatic growth between fish populations from different habitats or regions (Gonçalves et al., Reference Gonçalves, Bentes, Lino, Ribeiro, Canário and Erzini1997). Biogeographic comparison of M. helena WLR (Table 5) showed that the b value in this study is higher than those found in other parts of the Mediterranean Sea (Morey et al., Reference Morey, Moranta, Massuti, Grau, Linde, Riera and Morales-Nin2003; Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011) and the Atlantic Ocean (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007); although this does not include the work of Ferreira et al. (Reference Ferreira, Sousa, Delgado, Carvalho and Chada2008) because their sampling period did not cover every season. In this study, the WLR analysis also showed a positive allometry similar to the results obtained from the western Mediterranean (Morey et al., Reference Morey, Moranta, Massuti, Grau, Linde, Riera and Morales-Nin2003) and the Canary Islands (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007). However, in other parts of the Mediterranean Sea, M. helena exhibit negative allometric somatic growth (Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011). These differences can be explained by the different size ranges of the sampled fishes (Table 5) and the proper environmental condition of each locality (Bolger & Connolly, Reference Bolger and Connolly1989; Froese, Reference Froese2006).
Table 5. Weight-length relationship parameters of M. helena (mixed sex) reported from different marine areas

N, sample size; min., minimum; max., maximum; a, intercept; b, slope of regression line; r 2, coefficient of determination.
The relative condition factor (CF) was lower in winter and highest in summer. However, Matić-Skoko et al. (Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011) noted that this index (CF) was lowest in winter and highest in spring for M. helena collected in the Adriatic Sea. This difference may be due to sampling as the CF varies according to several factors, such as the sex and sample size (Froese, Reference Froese2006).
The GSI values of females notably exceed those of males. Generally, the tests account for a lower proportion of the total fish weight than ovaries (Tsikliras et al., Reference Tsikliras, Antonopoulou and Stergiou2010) because female investment in reproduction is higher. Only for males was the variation of GSI not significant. The GSI of M. helena females off the northern Tunisian coasts reached a peak in summer and declined in autumn. The seasonal variations of GSI are exactly similar to those recorded by Matić-Skoko et al. (Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011) in the Adriatic Sea, and the GSI maxima occurs earlier (May) in the Canary Islands (Jiménez et al., Reference Jiménez, Schônhuth, Lozano, Gonzalez, Sevilla, Diez and Bautista2007), which may be influenced by regional differences in sea temperature (Table 6). The lowest value, in autumn, can be explained by the emission of mature germ cells during the spawning season (summer) (Matić-Skoko et al., Reference Matić-Skoko, Tutman, Petric, Skaramuca, Dikic, Lisičić and Skaramuca2011). In the same season, we recorded a decline in the value of HSI that may have resulted from the investment of somatic energy for reproduction. This study presents the first information on the seasonal variations of M. helena HSI.
Table 6. Biogeographic comparison of variation of the M. helena gonadosomatic index (GSI)

N, total sample size; M, male; F, female; S, significant difference; NS, no significant difference.
Acknowledgements
We would like to thank the anonymous reviewers whose comments helped to improve the manuscript.











