Introduction
The invasive red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Curculionidae), is one of the most destructive pests of palms in the world. It is widely distributed in Oceania, Asia, Africa and Europe (EPPO, 2008) and was found in the Caribbean in December 2008 (EPPO, 2009). Rhynchophorus ferrugineus has been reported as a serious pest of coconut, oil palm, sago palm and date palm (EPPO, 2008).
In the Mediterranean Basin, this pest was first detected in the early 1990s. It spread slowly until 2004; but nowadays it can be found in almost all Mediterranean countries, where it has become the major pest of palms, mainly Phoenix canariensis Hort. ex Chabaud. This palm is endemic to the Canary Islands (Barrow, Reference Barrow1998) and is widely used as an ornamental plant worldwide (Morici, Reference Morici1998). In the Autonomous Community of Valencia (eastern Spain), where the pest was first detected in 2004 (Tejedo, Reference Tejedo2006), 19,677 palms, mostly P. canariensis, have been documented as killed by R. ferrugineus from 2004 to 2009. Actual figures are certainly higher. The mean cost of these palms has been estimated at 800€ per palm (Favà, Reference Favà2009). Some monumental specimens had much higher values, especially those at historic sites, some of them included in the UNESCO World Heritage Site list (UNESCO, 2010). Besides the ornamental value of these palms, control measures taken against the weevil (mainly an unsuccessful eradication program) had a cost of about 11 million € (P. Baraja, Valencian Department of Agriculture, personal communication). Therefore, R. ferrugineus cost this Autonomous Community around 27 million € in this period. A similar situation can be found all around the Mediterranean Basin.
Adult R. ferrugineus are large reddish brown beetles about 3 cm long with a characteristic long curved rostrum. They have strong wings which enable them to undertake long flights. Female weevils lay their eggs singly at the base of the fronds in separate holes made with their rostrum. Neonate larvae bore into the palm core making tunnels and feeding on its inner contents. As larvae moult, their feeding rate increases and they tend to damage the soft tissues surrounding the apical meristem. Mature grubs return to the periphery of the stem and prepare a cocoon made of palm fibres. After protecting themselves with the cocoon, larvae enter a prepupal stage followed by the pupal stage. The new generation adults that emerge remain within the host where they feed and reproduce. These activities result in the destruction of the meristem and eventual death of the palm. Subsequently, adults will fly away and look for new hosts. Rhynchophorus ferrugineus has been reported on 19 palm species belonging to 15 different genera (EPPO, 2008; Dembilio et al., Reference Dembilio, Jacas and Llácer2009). Since R. ferrugineus remains inside the palms, the detection of early infestations is very difficult; and the inevitable delay in detection may lead to permanent collapse of the palms, especially for P. canariensis, which is extremely susceptible to R. ferrugineus attack (Dembilio et al., Reference Dembilio, Jacas and Llácer2009). This cryptic life cycle makes control of the pest very difficult (Llácer et al., Reference Llácer, Martínez and Jacas2009, Reference Llácer, Dembilio and Jacas2010; Dembilio et al., Reference Dembilio, Llácer, Martínez de Altube and Jacas2010). There is a need to better understand the biology and ecology of R. ferrugineus for effective pest management measures to be developed.
The life cycle of R. ferrugineus has been studied by several authors in different countries, either on artificial substrates or plant pieces under controlled environmental conditions (table 1). However, no results on the life cycle of R. ferrugineus in any of its hosts under natural conditions are available. According to published information, R. ferrugineus eggs can take from 1 to 6 days to hatch in palm lumps (Avand-Faghih, Reference Avand-Faghih1996). The various studies (table 1) indicate a great variation in the development period and the number of larval instars. Larval development has been reported to last from 24 (Butani, Reference Butani1975) to 128 days (Salama et al., Reference Salama, Zaki and Abdel-Razek2009), depending on temperature and feeding substrate. Pupal development times reported range from 11 (Viado & Bigornia, Reference Viado and Bigornia1949) to 45 days (Esteban-Durán et al., Reference Esteban-Durán, Yela, Beitia-Crespo and Jiménez-Álvarez1998); and the life cycle of R. ferrugineus may vary from just 44 days (Butani, Reference Butani1975) to 210 days (Kalshoven, Reference Kalshoven1981), depending on the feeding substrate and environmental conditions (table 1). Nirula (Reference Nirula1956) estimated that R. ferrugineus had three instars, whereas Martín & Cabello (Reference Martín and Cabello2006) described 17. Rahalkar et al. (Reference Rahalkar, Harwalkar and Rananavare1972) reported the occurrence of 3 to 4 generations per year in India in sugarcane. However, Salama et al. (Reference Salama, Hamdy and Magd El-Din2002) estimated that it had 21 generations annually in Egypt. The extremely low lower temperature threshold (LTT) for pupae estimated by Salama et al. (Reference Salama, Hamdy and Magd El-Din2002) (LTT=−2.3°C) may have lead to unrealistic results. Martín & Cabello (Reference Martín and Cabello2006) set the LTT at 13° and 15°C for pupae and larvae, respectively. However, no LTT has been established for eggs yet.
Table 1. Development time and number of instars reported by different authors for R. ferrugineus feeding on different substrates.

NA, not available.
Because of the aforementioned variability on some basic bio-ecological parameters of R. ferrugineus and the importance that this invasive pest has gained in the northern Mediterranean Basin countries as a pest of P. canariensis, the objective of the present study was to determine the thermal constant of R. ferrugineus and the number of instars when feeding in living P. canariensis palms under natural conditions in a Mediterranean climate.
Material and methods
Assays dealing with eggs were carried out in the laboratory. Those dealing with larvae and pupae took place in a mesh enclosure located at the Institut Valencià d'Investigacions Agràries, Montcada, Spain (latitude: 39°35′ 19.73″ N; longitude: 0°23′ 43.09″ W; altitude: 33 m) from June 2008 to July 2009. The mesh enclosure contained 24 independent screened cages (4×3×3 m) under natural light and temperature conditions. During this period, temperature data were collected hourly with a data logger Testo® 175-T1 (Testo AG, Germany).
Experimental insects
Adult weevils collected in the province of Valencia in traps baited with ferrugineol (the male R. ferrugineus aggregation pheromone) and plant kairomones (ethyl acetate and pieces of palm fronds) were used to initiate the stock colonies. These colonies were established in 2007 and have been periodically supplemented with the introduction of additional wild specimens. Adult weevils were reared in a controlled environment cabinet at 25±1°C, 75±5% RH and a 16L:8D in perspex cages (30×30×45 cm depth) with a density of 120–150 weevils per cage. These cages had a hole (8 cm in diameter) on the upper side covered by a mesh used for manipulation of the specimens, and its bottom side consisted of a 2-mm metal mesh used by females for oviposition. Cages were set on top of a tray containing a folded piece of moistened filter paper containing thin apple slices used by female weevils as oviposition substrate and as food by weevils. Apple slices were replaced three times per week (Dembilio et al., Reference Dembilio, Jacas and Llácer2009). Eggs obtained from the stock colonies were either used to determine egg development time or further kept on apple slices under the same conditions until hatching. Less than 24-h-old neonate larvae were used in the infestation assays.
Egg lower temperature threshold and development time
Three replicates of ten eggs less than 2-h old were kept on apple slices at either 10, 15, 20 and 25°C in a climatic chamber for up to 20 days. Eggs were examined twice daily (at 8 am and 3 pm) and hatched eggs recorded. Based on these observations, mean development times (y) for the egg stage at the experimental temperatures were established. Developmental rates (r(T)=y −1) were plotted against temperatures and fitted with a linear regression to estimate LTT (Logan et al., Reference Logan, Wallkind, Hoyt and Tanigoshi1976). Based on the LTT obtained, the thermal constant required for egg hatching was calculated using the following equation (Varley et al., Reference Varley, Gradwell and Hassell1974): K=Σ [y i (t i−x)]/n; where: K, thermal constant; y i, development time; t i, temperature; x, LTT and n, replicates.
Larval and pupal development in palm
Larval and pupal development studies were conducted using 7-year-old potted P. canariensis palms, which are a suitable host for R. ferrugineus. The stipe of these palms was around 75 cm high and 50 cm wide. They were planted in 50-l containers and were watered every other day. Groups of 12 palms enclosed together in separate cages were infested as described below at one-month intervals from June 2008 to May 2009. Each palm was artificially infested with R. ferrugineus neonate larvae. Sixteen holes 30 mm deep and 4 mm in diameter were uniformly drilled along a ring 10 cm above the palm apex. Subsequently, one neonate larvae was introduced into each hole. Previously removed plant material was used to seal the holes (Dembilio et al., Reference Dembilio, Jacas and Llácer2009). Infested palms were dissected at different time intervals depending on mean temperatures registered (from four days in summer to one month in winter). Palms were cut in pieces with a chain saw in the mesh enclosure, and these pieces were taken to the laboratory for further processing. In the laboratory, all larvae, pupae and adults were carefully extracted from the palm pieces. Larvae were weighed and then submerged in hot water (100°C) for 30 to 60 s depending on larval size to fix the head capsule (Martín-Molina, Reference Martín-Molina2004). Subsequently, larvae were preserved in 125-ml vials containing 70% of ethanol until further processing. Head capsules were separated and captured with a high-resolution digital camera (Leica® DFC360 FX, Leica Microsystems) in a binocular microscope. Each photo was processed and measured with the ImageJ® program (National Institute of Health, USA). Head capsule lengths and widths and labrum, clypeus and mandible lengths were measured. The relationships between head capsule lengths and the rest of head and mouthparts measurements were studied using regression analysis. Head capsule widths were further analyzed using the Hcap program developed by Logan et al. (Reference Logan, Bentz, Vandygriff and Turner1998). This program elaborates a graphic of the frequency distribution of head capsule widths and, following the method of analysis described by McClellan & Logan (Reference McClellan and Logan1994), determines optimum instar separation points, mean and SD of head capsule widths for each instar, number in each instar and probabilities of misclassification. In addition, the method of Gaines & Campbell (Reference Gaines and Campbell1935), based on the Dyar's rule, was used to analyze the fit between instar number (indicated by the Hcap program) and the natural log of mean head capsule width per instar. The relationship between the latter and larval weight was also studied.
The cumulated degree days (DD) required for each specimen recovered from the infested palms to develop from neonate larvae to its last moult was calculated. We assumed that moulting had taken place at the midpoint between two successive dissections of palms from the same month-group where different stages had been found. Daily temperatures from palm infestation until that point were integrated, taking into account the LTTs determined by Martín & Cabello (Reference Martín and Cabello2006). Subsequently, actual cumulated DD for larvae were plotted against their instar mean head capsule widths (obtained from Hcap). Mean thermal constants per instar were estimated from this regression.
Mean monthly temperatures recorded during the period of the study were plotted against mean monthly mortality of immature R. ferrugineus to study the relationship between temperature and immature survival.
Mean monthly temperatures from 1971 to 2000 from 46 climatic stations in the Iberian Peninsula were obtained from the Spanish Agencia Estatal de Meteorología (http://www.aemet.es/). Estimation of number of generations per year at these sites was obtained by dividing the available DD above 14°C (mean of egg-larval and pupal LTT values) at each station by the thermal constant of R. ferrugineus. These values were plotted against mean, mean maximum and mean minimum annual temperatures at the selected stations to study the relationship between temperature and the number of generations per year.
The software package, Statgraphics Plus 4.1 (Manugistics Group Inc., Rockville, MD, USA), was used to perform the aforementioned analyses.
Results
Egg lower temperature threshold (LTT) and development time
Egg hatching occurred between 15 and 25°C and, as expected, increased with temperature, from 40 to 70%, respectively (fig. 1). LTT was 13.1°C. Based on this estimation, a thermal constant of 40.4±2.0 DD was obtained for the egg stage.

Fig. 1. Development rate (line) and egg hatching (bars) of R. ferrugineus eggs exposed to different temperatures. The line represents the linear regression used to estimate LTT (y=0.0269×x–0.3512; LTT=13.1°C). Each bar is the mean of three groups of ten eggs less than two hours old.
Larval and pupal development in palm
Totals of 555 larvae, 135 pupae and 87 adults could be satisfactorily recovered from the 2304 neonate larvae introduced into the 144 palms used in this study. Therefore, mean immature mortality was 66.7%. Maximum mortality rates were observed for palms infested either in December or January (100%), whereas those infested from April through September showed maximum survival rates. Intermediate rates were observed for the remaining months (fig. 2). When considering mortality according to the month when palms were dissected, February showed the highest record (100% mortality), whereas palms dissected from April though September showed again maximum survival rates (fig. 3). Mortality values in both cases could be satisfactorily related to mean monthly temperatures recorded (fig. 4, table 2). From these regressions, it was inferred that mean monthly temperatures below 10.3°C were lethal for neonate larvae and 4.5°C for older immature stages.

Fig. 2. Survivorship of R. ferrugineus neonate larvae artificially introduced into P. canariensis palms. Each bar is the mean of 12 palms infested with 16 neonate larvae per month. Bars with the same letter are not statistically different (Kruskal-Wallis K=165.913; P<0.0001).

Fig. 3. Survivorship of R. ferrugineus immature stages in P. canariensis palms. Each bar is the mean of 7–25 palms (see number in parenthesis below each month) dissected each month. Palms were originally infested with 16 neonate larvae. Bars with the same letter are not statistically different (Kruskal-Wallis K=70.266; P<0.0001).

Fig. 4. Mean, maximum and minimum temperatures registered in the mesh enclosure where infestations took place. ![]() , Mean;
, Mean; ![]() ;
; ![]() Min.
Min.
Table 2. Relationship between mean monthly mortality of R. ferrugineus larvae (y) and mean monthly temperatures (x=maximum, Tmax; minimum, Tmin; and mean, Tmean) registered during the assay (2008–2009). 2a. Mortality of neonate larvae (see Fig. 2). 2b. Mortality of immature stages (see fig. 3).
a. Neonate mortality
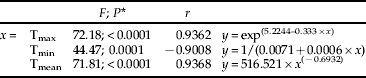
* df were 1, 11 in all cases.
b. Immature mortality
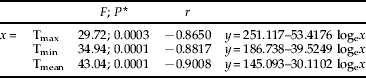
* df were 1, 11 in all cases.
Head capsule measurements
The width of the head capsule of R. ferrugineus larvae ranged from 0.51 to 8.24 mm (table 3). The frequency distribution of this measure showed 13 peaks (fig. 5), which most likely represent 13 larval instars. Probabilities of misclassification calculated by Hcap are shown in table 3. These results were also supported by Dyar's rule (Dyar, Reference Dyar1890) (fig. 6). Other measurements of the head capsule taken are shown in table 4. All of them were strongly correlated to the head capsule width (table 5). This value was also positively correlated to larval weight (fig. 7).

Fig. 5. Head capsule width distribution of R. ferrugineus larvae reared in P. canariensis. The lines are the individual instar distributions. Graphs produced by Hcap (Logan et al., Reference Logan, Bentz, Vandygriff and Turner1998).

Fig. 6. Linear regression between the natural logarithm (loge) of mean larval head capsule width (y) and instar number (x) of R. ferrugineus larvae reared in P. canariensis.

Fig. 7. Linear regression between head capsule width and larval weight of R. ferrugineus larvae reared in P. canariensis.
Table 3. Number of instars of R. ferrugineus, including the mean, size and probability of misclassifying of head capsule width generated by Hcap program (Logan et al., Reference Logan, Bentz, Vandygriff and Turner1998).

Table 4. Mean values and standard deviation of lengths and widths of head capsule, labrum and clypeus and mandible length (mm) of R. ferrugineus larvae reared in P. canariensis.

Table 5. Relationship between larval head capsule width and other larval head measurements of R. ferrugineus larvae reared in P. canariensis.

Preimaginal development
The cumulated degree day (CDD) values calculated for each larvae recovered from the artificially infested palms were plotted against the mean head capsule width of the corresponding larval instar based on the Hcap program (fig. 8). Although CDD-values (y) were highly variable for each head capsule width (x), a strong relationship could be obtained between these variables (y=exp[6.79977−(2.35256/x)]; r=−0.9029; P<0.0001). From this equation, it was estimated that 666.5 DD were necessary for complete larval development. Pupal develoment required an additional 282.5 DD. Therefore, K (thermal constant) of R. ferrugineus (egg to adult) feeding in P. canariensis was 989.3 DD. The relationships between K and day length (short or long), day photoperiod (increasing or decreasing) and season (spring, summer, autumn or winter) were determined for the 135 individuals recovered as pupae from our palms (those that were used to estimate L13 CDD) to check whether these factors could affect R. ferrugineus development. Most of these specimens corresponded to insects developing under long day (n=128), decreasing photoperiod (n=127) and summer (n=120). Therefore, comparisons were not possible. However, we could conclude that, under these particular conditions, variation was as large as for the whole data set.

Fig. 8. Relationship between mean head capsule width per instar and cumulated heat units (DD) above lower temperature threshold (15°: Martín & Cabello, Reference Martín and Cabello2006) for R. ferrugineus larvae reared in P. canariensis.
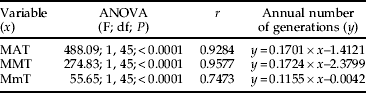
Based on K and on the temperatures from the selected 46 climatic stations in Spain and Portugal, an estimation of the number of annual generations in these points was obtained (fig. 9). Strong relationships between these values and annual mean, annual mean maximum and annual mean minimum temperatures were obtained (table 6).

Fig. 9. Map of the Iberian Peninsula showing mean annual temperatures (MAT) (Ninyerola et al., Reference Ninyerola, Pons and Roure2005). Dots represent the 46 climatic stations where the number of generations of R. ferrugineus per year was estimated based on the regression shown in table 6, which uses MAT as independent variable.
Table 6. Linear relationships between the number of annual generations of R. ferrugineus estimated in 46 climatic stations in the Iberian Peninsula (see fig. 9) and mean annual temperatures (MAT), mean maximum temperatures (MMT) and mean minimum temperatures (MmT).
Discussion
Temperature is the main abiotic factor influencing the biology, ecology and population dynamics of poikilothermic organisms as insects. Martín & Cabello (Reference Martín and Cabello2006) established under laboratory conditions the lower lethal temperature (LLT) for eggs and larvae at 10°C and 5°C, respectively. In our assays, no hatching was observed at 10°C (fig. 1); and, as expected for values above LLT, developmental time and temperature were negatively related – egg hatching could be completed in two days at 25°C but took 18 days at 15°C. Our results are also indicative that neonate larvae are more sensitive to lower air temperatures than what Martín & Cabello (Reference Martín and Cabello2006) reported, and 10.3°C were enough to kill all neonate larvae infesting P. canariensis (table 2a). However, the value found by these authors roughly coincides with what we found for older instars within the palm (4.5°C; table 2b). Larval development could be completed in 40 days in summer and 160 days in winter-spring. Pupal development could be completed in 13 days in the summer, but it took several months for those specimens reaching this stage from autumn to spring. These results can partly explain differences reported by other authors (table 1). All larvae recovered from our palms were successfully classified according to one of 13 instars with a probability of error below 0.05, based on their head capsule width (table 3). These results were also supported by Dyar's rule (Dyar, Reference Dyar1890; Gaines & Campbell, Reference Gaines and Campbell1935), which hypothesizes a geometric head capsule growth (fig. 6). The excellent fit obtained between head capsule width and instar number indicates that no instar was overlooked. Furthermore, our results demonstrate that any of the head measurements considered (table 4) could be satisfactorily used for determining larval instar stages (table 5). All previous reports on the number of larval instars of R. ferrugineus gave values below 13 except for Martín-Molina (Reference Martín-Molina2004) (table 1) who, under laboratory conditions, found up to 17 instars in sugarcane lumps and up to 15 in palm lumps. The number and nature of the moults can be frequently altered by external factors, chiefly temperature, diet and their interaction (Wigglesworth, Reference Wigglesworth1954; Stamp, Reference Stamp1990). Therefore, the differences reported could be at least partially attributed to the feeding substrate used, e.g. fruit slices or sugarcane lumps (table 1), the environmental conditions and their interaction. In the case of the supernumerary instars found by Martín-Molina (Reference Martín-Molina2004) in sugarcane and palm lumps, moulting was reported to occur without variation of size and weight. In our case, larval weight significantly changed along with head capsule width for the whole set of data obtained (fig. 7).
Both temperature and diet can also affect development (Stamp, Reference Stamp1990). Our results indicate that R. ferrugineus requires 40.4 DD for egg hatching under laboratory conditions, 666.5 DD for complete larval development in P. canariensis and another 282.5 DD to reach adulthood. These values are less than what Martín & Cabello (Reference Martín and Cabello2006) found for larvae fed in an artificial diet under laboratory conditions (1,106 DD) and pupae (328 DD). As a consequence, the thermal constant of R. ferrugineus found by these authors is 1.5 times higher that what we found (1436 versus 989.3 DD). Differences are certainly higher because Martín & Cabello (Reference Martín and Cabello2006) did not consider the egg thermal constant.
According to different authors (Rahalkar et al., Reference Rahalkar, Harwalkar and Rananavare1972; Abe et al., Reference Abe, Ohkusu, Kubo, Kawamoto, Sone and Hata2010), environmental conditions do not necessarily preclude the growth and development of the immature stages of R. ferrugineus. Salama et al. (Reference Salama, Zaki and Abdel-Razek2009) established a difference of 2° to 6°C between the outer atmospheric temperature and that inside infested palms in Egypt in winter and summer, respectively. Higher temperatures inside infested palms are the result of the fermentation process occurring when relatively high populations of larvae coincide within one single palm. These differences could account for the wide variation in cumulative heat units required for each instar to moult recorded in our assays (fig. 8). However, the 16 neonate larvae inoculated per palm in our study never led to the occurrence of high populations inside the palm; and, therefore, differences between air and palm temperatures are not likely to be the cause of the variation observed. Other factors could have a more dramatic effect on this variation.
Our study took place under natural conditions, and temperature varied throughout the year (fig. 4). The effect of food quality on growth rate is a function of the thermal conditions (Stamp, Reference Stamp1990); and, therefore, although the feeding substrate was the same during the assay (living P. canariensis palms), the performance of R. ferrugineus could change with the season. The influence of photoperiod, day length and season on R. ferrugineus development could not be proved in our assays. However, our results are indicative of large variations of K within the most favourable season, the summer. These variations could be related to either the quality of the feeding substrate or to insect characteristics. In our study, when dissecting the palms, we usually observed larger instars feeding in the palm core and smaller ones in the periphery. In fact, the palm periphery is more fibrous than the core, which is more juicy, and, therefore, could have a different nutritional value, leading to different growth rates under the same climatic conditions. Additionally, because the Canary palm is dioecious, we can not ignore the existence of nutritional differences linked to palm sex which could contribute to the variation found in our study. Furthermore, sexual dimorphism is common among insects (Snodgrass & Eickwort, Reference Snodgrass and Eickwort1993), and females are usually larger than males. In R. ferrugineus, adult females are significantly bigger than males (1.15±0.04 and 0.91±0.06, respectively: O. Dembilio and J.A. Jacas, unpublished results). Because larvae were not sexed in our assay, we cannot exclude the existence of different growth rates for either sex, which could lead to the occurrence of either protandry or protogyny. Moreover, genetic variation among the individuals used in our assays could also contribute to the differences observed. Finally, the method used, which was destructive and did not allow for a continuous monitoring of the insects within the palms, did not favour precision when estimating the occurrence of a moult and, therefore, may have contributed too to the high variation obtained when estimating K.
Based on the strong relationship found between mean annual temperature (MAT) and the number of generations of R. ferrugineus in the Iberian Peninsula (table 6), less than one generation per year can be expected in areas with MAT below 15°C and more than two where MAT is above 19°C. This is important because we have observed that usually a minimum of two weevil generations are necessary to kill an adult Canary palm, and this means that at least two years are necessary for R. ferrugineus to kill a palm in the Iberian Peninsula. Should these results apply to other areas, a complete plus a partial generation per year would be expected to occur in most of the northern Mediterranean basin, whereas at least two complete generations would be expected in most of the southern shore of this area. Similarly, in America, less than two generations per year would be expected in California, southern Brazil and Argentina, but more than two in Florida, the Caribbean and most of central and South America.
Acknowledgements
The authors thank A. Urbaneja (IVIA) for critically reviewing the manuscript, L. Bellver, M. Piquer and E. Llácer (IVIA) for their help during the assays and the palm nurserymen association ASFPLANT for providing the palms used in our assays. This research was partially funded by the Spanish Ministerio de Ciencia e Innovación (project TRT2006-00016-C07-05) and the Valencian Conselleria d'Agricultura, Pesca i Alimentació (project IVIA-5611). O. Dembilio was recipient of a predoctoral grant from IVIA.


















