Introduction
More than a decade of neuroimaging studies point towards functional abnormalities of the mesocorticolimbic reward system in substance use disorders (Redish et al., Reference Redish, Jensen and Johnson2008; Volkow et al., Reference Volkow, Fowler, Wang, Baler and Telang2009). Overall, the data imply that chronic drug use can lead to increased neuronal activation in response to drug-associated cues, and reduced response to natural rewards, a maladaptive process thought to facilitate the progression towards excessive drug choice (Volkow et al., Reference Volkow, Fowler, Wang, Baler and Telang2009). While much attention in the field has focused on identifying addiction-related endophenotypes contributing to pre-existing abnormalities in reward circuit function, the search has been plagued by questions of causality: do the observed reward-related abnormalities stem from chronic drug use itself, or from factors related to genetics that facilitate a progression towards excessive drug use, or some combination of both? Of importance, the longitudinal path from a potential genetic vulnerability to substance misuse outcomes later in life have not been investigated from such a neurodevelopmental perspective, due, in part, to the lack of sufficiently powered longitudinal genetic-neuroimaging studies (Conrod and Nikolaou, Reference Conrod and Nikolaou2016).
Using a uniquely large genetic-neuroimaging dataset, the IMAGEN study (Schumann et al., Reference Schumann, Loth, Banaschewski, Barbot, Barker, Buchel, Conrod, Dalley, Flor, Gallinat, Garavan, Heinz, Itterman, Lathrop, Mallik, Mann, Martinot, Paus, Poline, Robbins, Rietschel, Reed, Smolka, Spanagel, Speiser, Stephens, Ströhle and Struve2010), we addressed this unsolved issue by applying structural equation modeling (SEM) to examine whether the selective modulation of key components of the reward circuitry – ventral striatum (VS) and orbital frontal cortex (OFC) – by dopaminergic functional polymorphisms contribute to the degree of perilous alcohol use behavior observed at 14 years of age and again at 16 years of age. In particular, functional magnetic resonance imaging (fMRI) data collected from 14-year-old adolescence participants performing the monetary incentive delay (MID) task were used to quantify the blood-oxygen-level-dependent (BOLD) response of the VS and OFC during the anticipation of large and small rewards. The MID task has been used extensively to investigate changes in neural activity in response to the processing of different stages of reward processing (e.g. reward prediction, anticipation of obtaining rewards of different magnitude or avoiding punishment, outcome processing) in typical and atypical populations, with findings that converge with both animal and human studies emphasizing the essential role of the VS and OFC in processing reward-related information (for review, see Lutz and Widmer, Reference Lutz and Widmer2014; Balodis and Potenza, Reference Balodis and Potenza2015; Knutson and Heinz, Reference Knutson and Heinz2015). However, both hypo-responsiveness and hyper-responsiveness of reward-related brain regions (e.g. VS) have been reported during anticipation of reward in the MID task in substance dependent adults (for review, see Balodis and Potenza, Reference Balodis and Potenza2015), so it remains uncertain what functional state (hyper v. hypo) of the reward system may actually precipitate a substance use disorder.
Nevertheless, the relevant reward signal (i.e. positive and negative reward prediction error signals) critical to the functioning of the VS and OFC are thought to originate in the midbrain dopamine system (Schultz et al., Reference Schultz, Tremblay and Hollerman2000; Schultz, Reference Schultz2001). These reward signals are conveyed to the neural targets of the dopamine system where their impact reorganizes synaptic connectivity in a way that drives learning and motivation (Schultz, Reference Schultz2001, Reference Schultz2010). For this reason, we focused on functional polymorphisms that would appear to alter dopaminergic signaling in the VS and OFC during reward valuation and prediction. To be specific, we selected the 7-SNP haplotype of the PPP1R1B gene − mRNA expression highest for G alleles of the rs87694 SNP (Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Straub, Lipska, Verchinski, Goldberg, Callicott, Egan, Huffaker, Mattay, Kolachana, Kleinman and Weinberger2007) – because of its critical function in integrating dopaminergic and glutaminergic signaling (Svenningsson et al., Reference Svenningsson, Nishi, Fisone, Girault, Nairn and Greengard2004), and its association with reward learning (Frank et al., Reference Frank, Moustafa, Haughey, Curran and Hutchison2007) and cognitive performance (Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Straub, Lipska, Verchinski, Goldberg, Callicott, Egan, Huffaker, Mattay, Kolachana, Kleinman and Weinberger2007). The rs686 SNP of the D1 dopamine receptor (DRD1) − the G allele linked to increases in DRD1 expression (Huang and Li, Reference Huang and Li2009) – selected because of the role D1 has in reward signaling (Ikemoto et al., Reference Ikemoto, Glazier, Murphy and McBride1997; Suhara and Miyoshi, Reference Suhara and Miyoshi2007) and addiction (Comings et al., Reference Comings, Gade, Wu, Chiu, Dietz, Muhleman, Saucier, Ferry, Rosenthal, Lesieur, Rugle and MacMurray1997; Batel et al., Reference Batel, Houchi, Daoust, Ramoz, Naassila and Gorwood2008; Huang et al., Reference Huang, Ma, Payne, Beuten, Dupont and Li2008; Zhu et al., Reference Zhu, Yan, Wen, Wang, Bi, Zhao, Wei, Gao, Jia and Li2013). To date, the rs686 SNP of the DRD1 has yet to be investigated in the context of human reward-related learning or behavior. Further, we selected the promoter rs12364283 SNP of the D2 dopamine receptor (DRD2) gene – the C allele has been shown to confer higher transcriptional activity (Zhang et al., Reference Zhang, Bertolino, Fazio, Blasi, Rampino, Romano, Lee, Xiao, Papp, Wang and Sadée2007) − because of the association D2 has with reward signaling (Suhara and Miyoshi, Reference Suhara and Miyoshi2007; Assadi et al., Reference Assadi, Yucel and Pantelis2009), reinforcement learning (Frank and Hutchison, Reference Frank and Hutchison2009; Baker et al., Reference Baker, Stockwell and Holroyd2013), and addiction (Noble, Reference Noble1994, Reference Noble2000). Likewise, the Taq1A polymorphism (rs 1800497) of the ANKK1 gene was also selected because of its association with striatal D2 receptor function (Thompson et al., Reference Thompson, Thomas, Singleton, Piggott, Lloyd, Perry, Morris, Perry, Ferrier and Court1997) but see Laruelle et al. (Reference Laruelle, Gelernter and Innis1998), altered activation of OFC (Cohen et al., Reference Cohen, Young, Baek, Kessler and Ranganath2005), and VS (Nymberg et al., Reference Nymberg, Banaschewski, Bokde, Buchel, Conrod, Flor, Frouin, Garavan, Gowland, Heinz, Ittermann, Mann, Martinot, Nees, Paus, Pausova, Rietschel, Robbins, Smolka, Ströhle, Schumann and Klingberg2014), impaired reinforcement learning (Klein et al., Reference Klein, Neumann, Reuter, Hennig, von Cramon and Ullsperger2007), and addiction (Noble et al., Reference Noble, Syndulko, Fitch, Ritchie, Bohlman, Guth, Sheridan, Montgomery, Heinzmann and Sparkes1994; Noble, Reference Noble1998, Reference Noble2000, Reference Noble2003; Abi-Dargham, Reference Abi-Dargham2004; Munafo et al., Reference Munafo, Matheson and Flint2007).
Taken together, we hypothesized that these specific dopaminergic functional polymorphisms − DRD1rs686, DRD2rs12364283, ANKK1rs1800497, and PPP1R1Brs87694 – may selectively modulate the VS and OFC BOLD signal (hyper v. hypo) during reward anticipation. In turn, we predicted that the relationship between these SNPs and alcohol-related behavior at 14 years and 16 years of age would be indirect and be mediated by their effect on the reward response in these selected brain regions. Although less explored, because both the VS and OFC have been proposed to play an important role in reward learning (Frank and Claus, Reference Frank and Claus2006), adolescent risk-taking behaviors (Galvan et al., Reference Galvan, Hare, Parra, Penn, Voss, Glover and Casey2006; Conrod and Nikolaou, Reference Conrod and Nikolaou2016) and the development of addiction (Pujara and Koenigs, Reference Pujara and Koenigs2014), we used an interaction term to investigate the influence of the balance of activity between these two regions during reward anticipation as a variable of interest in our SEM. Our proposed imaging genetics approach constitutes a natural application of SEM, which provides a means for modeling such complex interrelationships.
Methods
Participants and procedure
A community-based sample of young adolescents (N = 2463) was recruited for the IMAGEN study (for details on the IMAGEN project, see Schumann et al., Reference Schumann, Loth, Banaschewski, Barbot, Barker, Buchel, Conrod, Dalley, Flor, Gallinat, Garavan, Heinz, Itterman, Lathrop, Mallik, Mann, Martinot, Paus, Poline, Robbins, Rietschel, Reed, Smolka, Spanagel, Speiser, Stephens, Ströhle and Struve2010). Individuals who provided assent, and whose parents provided informed written consent, completed an extensive battery of neuropsychological, clinical, personality and drug use assessments online, and at the testing centers. Participants were excluded if, among other criteria, they had contra-indications for MRI (for example, metal implants, claustrophobia). After data quality control, complete and reliable datasets were available for 1840 participants at Time 1 (1666 participants at Time 2). Of these volunteers at Time 2, 1639 had complete neuroimaging data. The demographic information of the participants at time 1 was: mean age = 14.55 ± 0.447 years, 51.7% female, 88.80% right-handed, verbal IQ = 110.67 ± 14.85, performance IQ = 107.57 ± 14.77.
Alcohol use disorders identification test (AUDIT)
Problematic alcohol use behaviors were assessed twice, at 14 and 16 years of age, using the total score of the AUDIT (Bohn et al., Reference Bohn, Babor and Kranzler1995) via the online computer Psytools® (Delosis Ltd, London, UK) platforms at the participant's home. Of the 1840 adolescents in Time 1 (AUDIT mean = 1.56 ± 0.06), 877 scored 0 on the AUDIT and thus had never used alcohol, whereas 963 adolescents reported the use of alcohol at some degree (score >0) (Table 1). Of the 1666 adolescents in Time 2 (AUDIT mean = 3.7 ± 0.08), 288 scored 0 on the AUDIT and thus had never used alcohol, whereas 1378 adolescents reported the use of alcohol (score >0) (for an overview of these data, see Table 1). To note, participants AUDIT score were significantly larger at Time 2 compared with Time 1, t (1461) = −25.8, p < 0.001.
Table 1. Overview of alcohol use of all adolescents at time 1 (N = 1840) and Time 2 (N = 1666)

Zone 0 (scored 0) = never tried alcohol; Zone I (scores 1–7) = low level of alcohol problems; Zone II (scores 8–15) = medium level of alcohol problems; Zone III (scores 16–19); Zone IV (scores 20–40) = high level of alcohol problems; AUDIT = Alcohol Use Disorders Identification Test.
fMRI task, acquisition, and analysis
MID task
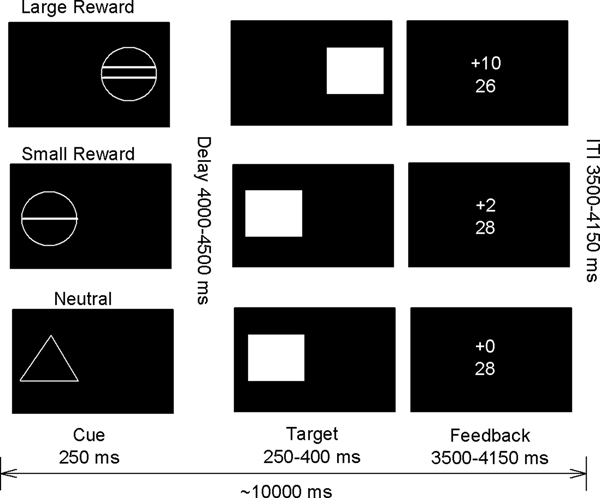
In order to assess reward processing during fMRI in an adolescence population, a modified version of the MID task was used (Fig. 1). In brief, each trial consisted of anticipation, response, and feedback related cues. Before the anticipation phase, a cue signaled the position of the target as well as the type of reward that could be attained by a correct response. Different cues distinguished between large reward (10 points), small reward (2 points), and neutral (zero points) conditions. After a random anticipation interval of 4000–4500 ms length, the target appeared. Participants were instructed to respond to the target as quickly as possible via button press and informed that the points they earned would be converted into chocolate treats after scanning [i.e. 1 candy (M&Ms) for every 5 points scored]. The duration of the target was continuously adapted to the performance of the subject, ensuring a successful performance on approximately 66% of all the trials. Immediately following the response, feedback indicated the number of points attained in the recent trial as well as the total points earned during the task. The inter-trial interval varied so that each trial took approximately 10 000 ms (Fig. 1). Large, small, and neutral conditions were randomized throughout the task (22 trials each, summing up to 66 trials in total). Task presentation and recording of the behavioral responses were performed using Visual Basic 2005 and NET Framework Version 2.0, as well as the visual and response grip system from Nordic Neuro Lab (NordicNeuroLab AS, Bergen, Norway).

Fig. 1. Monetary incentive delay (MID) task, adapted from Knutson et al. (Reference Knutson, Westdorp, Kaiser and Hommer2000).
Imaging parameters
All scanning was performed with a 3T whole-body MRI system made by several manufacturers (Siemens, Philips, General Electric, Bruker) at the eight IMAGEN assessment sites (London, Nottingham, Dublin, Mannheim, Dresden, Berlin, Hamburg, and Paris). To ensure a comparison of MRI data acquired on these different scanners, we implemented image-acquisition techniques using a set of parameters compatible with all scanners that were held constant across sites (cf., Schumann et al., Reference Schumann, Loth, Banaschewski, Barbot, Barker, Buchel, Conrod, Dalley, Flor, Gallinat, Garavan, Heinz, Itterman, Lathrop, Mallik, Mann, Martinot, Paus, Poline, Robbins, Rietschel, Reed, Smolka, Spanagel, Speiser, Stephens, Ströhle and Struve2010). We acquired 40 slices in descending order (2.4 mm, 1 mm gap) using a gradient-echo T2*-weighted sequence (EPI) with the following image parameters: TR = 2200 ms, TE = 30 ms, and an in-plane matrix size of 64 × 64 pixels. We used a plane of acquisition tilted to the anterior–posterior commissure line (rostral > caudal). For anatomical reference, a 3D magnetization prepared gradient-echo sequence (MPRAGE) based on the ADNI protocol (http://www.loni.ucla.edu/ADNI/Cores/index.shtml) with TR = 6.8 ms and TE = 3.2 ms over the whole brain was carried out.
Functional preprocessing and analysis
The fMRI data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, London, UK). All individual data were slice-time corrected using the first slice as a reference, then spatially realigned to correct for head movement, and non-linearly warped on the MNI space using custom EPI template based on an average of mean images of 400 adolescents. This custom template image (53 × 63 × 46 voxels) was subsequently applied to all functional T2* data and voxels were resampled at a resolution of 3 × 3 × 3 mm3. The functional data were smoothed using an isotropic Gaussian kernel for group analysis (5 mm full-width at half-maximum). First level statistics were performed by modeling reward anticipation and reward feedback as predictor variables within the context of the GLM on a voxel-by-voxel basis, with AR noise model against a design matrix. The estimated movement was added to the design matrix in the form of 18 additional columns (3 translational, 3 rotations, 3 quadratic, and 3 cubic translations, 3 translations shifted 1 TR before, and 3 translations shifted 1 TR later). A movement threshold of 2 mm was employed. Furthermore, each individual fMRI time series underwent an automatic spike detection method.
For anticipation cues of neutral, small reward, and large reward, as well as information on feedback [hit (response within the correct time window) v. missed (response outside the correct time window)] trials, were entered in a parametric design, and study center was included as a covariate. The regressors modeling the experimental conditions (e.g. cues predicting large reward, small reward, and neutral reward trials) were convolved using SPM's default hemodynamic response function. The individual contrast images were entered in a second-level random-effects analysis (full flexible procedure of SPM8), and a non-sphericity correction was performed. A one-sample t test was conducted, testing activity on large reward trials (and separately on small reward trials) against the implicit baseline of the neutral condition, removing variance associated with the other regressors in the design matrix. A significance level of p < 0.05 was selected (Family-Wise Error-corrected), with a minimum cluster size of 10 voxels.
Based on previous IMAGEN studies (cf., Nees et al., Reference Nees, Tzschoppe, Patrick, Vollstädt-Klein, Steiner, Poustka, Banaschewski, Barker, Büchel, Conrod, Garavan, Heinz, Gallinat, Lathrop, Mann, Artiges, Paus, Poline, Robbins, Rietschel, Smolka, Spanagel, Struve, Loth, Schumann and Flor2012, Whelan et al., Reference Whelan, Conrod, Poline, Lourdusamy, Banaschewski, Barker, Bellgrove, Büchel, Byrne, Cummins, Fauth-Bühler, Flor, Gallinat, Heinz, Ittermann, Mann, Martinot, Lalor, Lathrop, Loth, Nees, Paus, Rietschel, Smolka, Spanagel, Stephens, Struve, Thyreau, Vollstaedt-Klein, Robbins, Schumann and Garavan2012), the analyses focused on weighted mean BOLD signal change of the designated regions of interest (ROIs) (OFC and VS) over both hemispheres for anticipation of large reward v. neutral (large reward condition), and anticipation of small reward v. neutral (small reward conditions). Furthermore, we analyzed two distinct regions in OFC (medial OFC and lateral OFC) based on evidence suggesting dissociable functions in reward processing (Elliott et al., Reference Elliott, Dolan and Frith2000, Reference Elliott, Agnew and Deakin2008; Frank and Claus, Reference Frank and Claus2006) (O'Doherty et al., Reference O'Doherty, Kringelbach, Rolls, Hornak and Andrews2001; Diekhof et al., Reference Diekhof, Kaps, Falkai and Gruber2012). The ROI masks were taken from the Wake Forest University Pick-Atlas (Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003) using various atlases [medial OFC (aal atlas), lateral OFC (Broadman's area 47), VS (nucleus accumbens)], and the mean contrast value for each ROI was calculated for each subject for both large reward and small reward contrastsFootnote 1. To note, only trials that subjects made a successful response were included in this analysis and our analysis focused on the reward anticipation period of the task.
Genetic data
After quality control, genome-wide data were available for N = 1839 of the participants. Details of quality control procedures are available in the online Supplementary material. We investigated 4 SNPs, which were selected from each member of the full set of autosomal catecholamine genes; namely, those that have empirical support for variation in the degradation and receptor signaling of dopamine D1 and D2 receptors (Table 2). In brief, we focused on two functional polymorphisms related to D1 receptors (DRD1rs686, PPP1R1Brs87694), and two genetic polymorphisms that affect D2 expression (DRD2rs12364283, ANKK1rs1800497).
Table 2. Overview of genotype data

SNP, Single Nucleotide Polymorphism.
↑↓, denotes an increase or decrease in dopaminergic function.
Statistical analysis strategy
We performed two main sets of analyses using SPSS 17.0.1 and MPlus version 6.12. First, a simple regression analysis was performed to identify unique relationships between genetic data (DRD1rs686, DRD2rs12364283, ANKK1rs1800497, and PPP1R1Brs87694) and neuroimaging data (medial/lateral OFC and VS), and between neuroimaging data and alcohol misuse at 14 and 16 years. In addition, interactions terms (medial OFC × VS and lateral OFC × VS) were derived from the product of the medial/lateral OFC and VS standardized scores in order to examine whether the interaction between the two reward regions contributes to the prediction of alcohol misuse scores. Type 1 errors were statistically controlled following Benjamini and Hochberg (Reference Benjamini and Hochberg1995) with a corrected significance level of α = 0.05. Sex, age, and imaging site (eight sites) were included in each regression model as nuisance variables using a stepwise approach.
Second, a SEM path model in Mplus was conducted, in which: (1) the robustness of these gene–brain associations could be tested once all associations were entered simultaneously in one model, and the effect of sex, age, and imaging site (as a cluster variable) was controlled for; and (2) indirect effects from genes to substance use behaviors could be tested using the product of coefficients method. Full information maximum likelihood was used to account for missing data. The SEM model was fit using a complex random effects design to control for sex, age, and site, and robust maximum likelihood estimation (MLR), which is robust to non-normality. Model fit was assessed with the χ2 and Comparative Fit Indices (CFI), the Standardized Root Mean Square Residual (SRMR) and the Root Mean Square Error of Approximation (RMSEA). Hu and Bentler (Reference Hu and Bentler1999) suggest the following guidelines for interpreting Goodness-of-Fit Indices: SRMR and values close to or below 0.08, RMSEA values close to or below 0.06 and CFI close to or above 0.90 indicate acceptable model fit. To help interpret the interaction effects, these were plotted based on procedures by Aiken and West (Reference Aiken and West1991), Dawson (Reference Dawson2013) and Dawson and Richter (Reference Dawson and Richter2006).
Results
Univariate results
Gene–brain associations
SNP (DRD1rs686, DRD2rs12364283, ANKK1rs1800497, and PPP1R1Brs87694), and ROI (VS, medial and lateral OFC) associations were assessed using univariate regression models, while controlling for sex, age and imaging site (corrected for multiple comparisons, B-H, p < 0.0125). All regression results are presented in online Supplementary Table S2. This analysis yielded two significant associations. First, DRD1rs686 reliably predicted medial OFC BOLD signal (β = −0.08, t = −2.7, p = 0.008) to the large reward anticipation cue (F (10, 1230) = 6.5, p < 0.001, r 2 = 0.05,), indicating that increasing the number of G allele was associated with a stronger medial OFC BOLD response to the large reward anticipation cue (see Fig. 2, middle panel). It is also worth noting that DRD1rs686 also predicted medial OFC BOLD response to small reward anticipation, (β = −0.07, t = −2.4, p = 0.014), but this relationship did not survive our correction for multiple-comparisons. Second, ANKK1rs1800497 significantly predicted lateral OFC BOLD, (β = −0.09, t = −3.1, p = 0.002) response to the large reward anticipation cue (F (10, 1227) = 2.9, p < 0.001, r 2 = 0.03), indicating that increasing the number of A2 alleles was associated with larger decreases in lateral OFC BOLD signaling during large reward anticipation (see Fig. 2, bottom panel). It is also worth noting that these SNP→ROI relationships remained significant [ANKK1rs1800497 (β = −0.11, t = −3.1, p = 0.002); DRD1rs686 (β = −0.08, t = −2.3, p = 0.01)] when AUDIT Zone 0 participants (i.e. reported never using alcohol) were the only participants included in the regression analysis, suggesting that this genetic influence on reward activity precedes alcohol use at age 14.

Fig. 2. Gene-dose effects. DRD1 (left panel) and ANKK1 (right panel) gene-dose effects on small (clear columns) and large (dashed columns) reward anticipation cues for ventral striatum (top panel, green bars), medial OFC (middle panel, blue bars), and lateral OFC (bottom, red bars). Associated ROIs are displayed in right box. Error bars indicate standard errors of the means. OFC, orbital frontal cortex.
Brain–AUDIT associations
The relationship between reward anticipation (large and small) and AUDIT scores (Time 1 and Time 2) were assessed using univariate regression models (corrected for multiple comparisons, B-H, p < 0.0125). All regression results are presented in online Supplementary Table S3. While the ROIs did not uniquely predict AUDIT scores at either time point, this analysis demonstrated that the interaction between medial OFC and VS (β = 0.09, t = 2.98, p < 0.005; F (10, 1079) = 5.3, p < 0.001, r 2 = 0.05) and lateral OFC and VS (β = 0.08, t = 2.6, p < 0.01; F (10, 1079) = 5.3, p < 0.001, r 2 = 0.05) during high reward anticipation uniquely predicted alcohol misuse at 14 years of age. No other associations were observed (p > 0.1). The finding suggests that when both the medial OFC and VS are highly active or inactive (i.e. synergistic), individuals displayed higher levels of Audit scores at 14 years of age (Fig. 3c). It is interesting to note that the rs686 SNP of the DRD1 gene reliably predicted both medial OFC and VS interaction (β = −0.10, t = −3.4, p < 0.001) for the large reward anticipation condition (F (10, 1230) = 4.3, p < 0.001, r 2 = 0.03)Footnote 2, and AUDIT scores (β = 0.07, t = 2.7, p = 0.008) at Time 2, (F (10, 1385) = 4.3, p < 0.001, r 2 = 0.03).

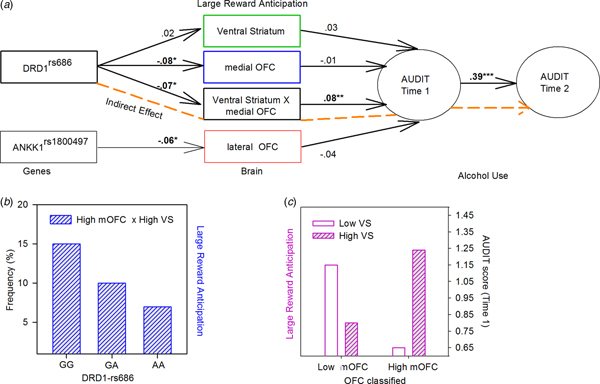
Fig. 3. Results of the SEM (a) Significant direct and indirect paths between gene, brain, and alcohol misuse. Paths that are part of significant indirect effects are highlighted in dashed (orange), other direct effects are shown in black. *p < 0.05, **p < 0.005, ***p < 0.001 (two-tailed). (b) DRD1 genotypes plotted by individuals classified as high medial OFC and high VS. (c) Audit scores at 14 years plotted by groups classified as high and low medial OFC and VS activation during large reward anticipation. VS, ventral striatum; OFC, orbital frontal cortex.
SEM results
In the hypothesized model, all brain variables with genetic predictors were modeled to predict alcohol misuse at 14 years of age, which in turn predicted alcohol misuse at 16 years of age. Results from the SEM analysis showed that this model fit the data very well, χ2 (21, 2052) = 29.69; CFI = 0.97; TLI = 0.95; RMSEA = 0.014 (90% CI 0.00–0.025); SMRM = 0.018. The model indicated that the medial OFC × VS interaction term calculated for the large reward condition predicted alcohol use at 14 years of age (β = 0.08, t = 2.9, p < 0.01), which in turn significantly predicted alcohol use at 16 years (β = 0.39, t = 13.57, p < 0.001). In order to better understand the interaction effects, a χ2 Test of Independence was conducted and indicated that the DRD1rs686 genotypes differ in the medial OFC × VS interaction, χ2 (4, 1396) = 12.85, p = 0.012, namely, GG carriers, more than GA and AA carriers, were classified as high medial OFC and high VS (15, 10, and 7% respectively) (Fig. 3b). Furthermore, the interaction effect on alcohol use at 14 years was plotted (see Fig. 3c), which indicated that when both the medial OFC and VS are highly active or inactive (i.e. synergistic), individuals displayed higher levels of Audit scores at 14 years of age. Finally, two significant indirect effects/paths from genes to alcohol misuse were identified: from rs686 SNP of the DRD1, through the medial OFC × VS interaction, to alcohol misuse at 14 years (ab = −0.006, s.e. = 0.003, 95% CI −0.013 to −0.01), and then on to alcohol misuse at 16 years (abc = −0.002, s.e. = 0.001, 95% CI −0.005 to −0.001) (Fig. 3a, orange path).
Discussion
Human neuroimaging studies confirm that the reward function of the mesocorticolimbic system is altered in substance use disorders (Volkow et al., Reference Volkow, Wang, Fowler, Tomasi and Telang2011, Reference Volkow, Wang, Fowler and Tomasi2012). However, these data cannot distinguish whether the abnormalities observed in adults are induced by drug exposure or represent a pre-existing condition that predisposes individuals to drug addiction, or a combination of both (Schoenbaum and Shaham, Reference Schoenbaum and Shaham2008; Schneider et al., Reference Schneider, Peters, Bromberg, Brassen, Miedl, Banaschewski, Barker, Conrod, Flor, Garavan, Heinz, Ittermann, Lathrop, Loth, Mann, Martinot, Nees, Paus, Rietschel, Robbins, Smolka, Spanagel, Ströhle, Struve, Schumann and Büchel2012). In the present study, we attempted to resolve this issue by examining the relationship between dopaminergic functional polymorphisms, VS and OFC reward functioning, and alcohol use behavior in early adolescence.
Foremost, we found a novel association between the DRD1rs686 (Huang and Li, Reference Huang and Li2009) and medial OFC activation during reward anticipation: reducing DRD1 expression (increasing G alleles) (Huang and Li, Reference Huang and Li2009) predicted an increase in medial OFC response (but not lateral OFC or VS) to reward-predicting cues. This finding appears consistent with a plethora of evidence highlighting the role of D1 receptors and medial OFC in reward-related learning (Elliott et al., Reference Elliott, Dolan and Frith2000, Reference Elliott, Agnew and Deakin2008; Hikosaka and Watanabe, Reference Hikosaka and Watanabe2000; Durstewitz and Seamans, Reference Durstewitz and Seamans2002; Cetin et al., Reference Cetin, Freudenberg, Fuchtemeier and Koch2004; Frank and Claus, Reference Frank and Claus2006). Further, D1 density differs quantitatively between sub-compartments of the frontal cortex with the highest expression in the medial OFC (Hurd et al., Reference Hurd, Suzuki and Sedvall2001). Our findings suggest that a reduction in DRD1rs686 expression may allow a greater proportion of D1 housing medial OFC neurons to become stimulated by dopaminergic reward signals, thereby intensifying its hemodynamic response. Although suggestive, this idea aligns with the proposal that the intensity of a reward response depends on the absolute number of interactions between dopamine and its post-synaptic D1 (or D2) receptors (Cox et al., Reference Cox, Frank, Larcher, Fellows, Clark, Leyton and Dagher2015, pg.99), and further, with evidence demonstrating that when D1 receptors are more highly activated in OFC, behaviors become more focused, and reward associations learned more rapidly (Garske et al., Reference Garske, Lawyer, Peterson and Illig2013). Taken together, this novel finding revealed that variation in expression DRD1rs686 can modulate the reward response of the medial OFC.
Perhaps more intriguing was the SEM findings, which point to a specific molecular pathway by which DRD1rs686 modulated the balance of activity between medial OFC and VS during reward anticipation, and this specific balance of activity predicted the level of problematic alcohol use behaviors early in adolescence. Consistent with anatomical, functional, and computational evidence highlighting the interplay between medial OFC and VS during learning (Pujara and Koenigs, Reference Pujara and Koenigs2014), a synergistic (hypo or hyper) response between medial OFC and VS during reward anticipation predicted elevated levels of problematic alcohol use behaviors. Such a synergistic relationship of activity between medial OFC and VS are interesting in light of known differential developmental trajectories for these regions in relation to reward processing and to increased risky behavior during adolescents (Galvan et al., Reference Galvan, Hare, Parra, Penn, Voss, Glover and Casey2006). In particular, differential recruitment of frontostriatal regions are typically interpreted in terms of immature prefrontal regions or an imbalance between prefrontal and subcortical regions (Galvan et al., Reference Galvan, Hare, Parra, Penn, Voss, Glover and Casey2006), a developmental pattern proposed to be exacerbated in those adolescents with a predisposition toward risk-taking (Galvan et al., Reference Galvan, Hare, Parra, Penn, Voss, Glover and Casey2006; Casey et al., Reference Casey, Jones and Hare2008; Casey, Reference Casey2015). However, our results seem to suggest that a synergistic recruitment of medial OFC and VS during reward processing may facilitate a progression towards excessive drug use behaviors in adolescents.
Notably, the relationship between a synergistic medial OFC and VS reward response and problematic alcohol use may be explained in the context of a recent dual system model of decision making, which refers to the competition between an automatic and deliberative system during learning (McClure and Bickel, Reference McClure and Bickel2014). According to this model, behaviors reflected in VS and OFC circuitry (the automatic system) develop slowly through the regular co-occurrence of stimuli and reinforcers, a process facilitated by positive (increase in dopamine activity) or negative (decrease in dopamine activity) reward prediction error (RPE) signals (Schultz, Reference Schultz2010). With sufficient experience, this learning process is thought to give rise to stereotyped or habitual (automatic) behaviors (McClure and Bickel, Reference McClure and Bickel2014). By contrast, the role of the deliberative system, comprised the dorsal lateral prefrontal/posterior parietal cortex, is to modulate behaviors by down-regulating value-related responses in the automatic behavioral system (McClure and Bickel, Reference McClure and Bickel2014).
In line with this model, we propose that an automatic system with low DRD1 expression may function at a supraoptimal reward state during positive RPE signaling, allowing behaviors to become more focused, and associations learned more rapidly (for example, see Garske et a l., Reference Garske, Lawyer, Peterson and Illig2013). Further, the dopamine-potentiation effects of addictive substances would compound this problem, resulting in an exaggerated reward response by the automatic system. Such a maladaptive process may, in turn, prevent the deliberative system to sufficiently compete in the decision-making process, failing to downregulate and implement control over high-valued drug-related stereotype, possibly explaining how early drug use can quickly spiral to problematic use. Alternatively, an automatic system with high DRD1 expression may function at a suboptimal reward state and antagonize positive RPE signaling. In turn, the automatic system may bias behaviors that are highly rewarding (e.g. following high-risk behaviors, drug use) to compensate for a chronically low ‘reward’ state (Blum et al., Reference Blum, Braverman, Holder, Lubar, Monastra, Miller, Lubar, Chen and Comings2000; Comings and Blum, Reference Comings and Blum2000). Furthermore, the deliberative system may fail to recognize the need to downregulate such high value-related responses by the automatic system since these reward responses may appear normalized. Although speculative, the association between a synergistic response between medial OFC and VS by DRD1rs686 (Huang and Li, Reference Huang and Li2009), and early onset of alcohol misuse behavior may provide initial support for such possibilities.
A challenging question is why this pattern of activation between the medial OFC and VS directly predicted AUDIT scores at 14 years of age, and mediated the effect between the DRD1rs686 and AUDIT scores at 16 years of age. Presently we can only speculate about the answer to this riddle. In regards to the former, it is important to point out that the relationship between the pattern of activation between medial OFC and VS, and AUDIT scores at 14 years of age preceded early alcohol use (see results), providing an explanation of how dopamine-related genes may predispose individuals to alcohol misuse. In regards to the latter, given the critical developmental period that the frontal and striatal brain systems go through between 14 and 16 years of age, and taking into account the impact alcohol use may have during this time period, perhaps imaging data at 16 years of age may provide better predictions of AUDIT scores at Time 2, as well as other risky behaviors. Alternatively, these findings could also be interpreted in the context of the many type I errors observed in candidate gene studies. Nevertheless, we hope that the results of this study will motivate future research on this issue.
The DRD2 gene has received the most attention as a risk candidate for the genetic transmission of substance use disorders, yet, we did not observe such an association in this adolescent sample. Instead, we observed that an increase in A2 alleles of the ANKK11800497 gene (Thompson et al., Reference Thompson, Thomas, Singleton, Piggott, Lloyd, Perry, Morris, Perry, Ferrier and Court1997) was associated with an increase in lateral OFC deactivation or suppression during reward anticipation. To note, this association is complicated by the difficulty in determining whether suppression or deactivations reflect an active process such as inhibition, a passive consequence of the redistribution of blood as activity is orchestrated within a distributed network (i.e. due to increasing medial OFC activation) or a product of the baseline (Frankenstein et al., Reference Frankenstein, Wennerberg, Richter, Bernstein, Morden, Florence and Mcintyre2002). Nevertheless, increasing A2 alleles, which have been associated with an increase in D2 density, may have strengthened the D2 inhibitory signal in lateral OFC, thereby reducing neuronal excitability for the purpose of suppressing competing behavioral responses maintained in working memory (Elliott et al., Reference Elliott, Dolan and Frith2000; Elliott and Deakin, Reference Elliott and Deakin2005). Based on these findings, perhaps D2's role in addiction is only observed in later stages of addiction (Blum et al., Reference Blum, Noble, Sheridan, Montgomery, Ritchie, Ozkaragoz, Fitch, Wood, Finley and Sadlack1993; Noble et al., Reference Noble, Syndulko, Fitch, Ritchie, Bohlman, Guth, Sheridan, Montgomery, Heinzmann and Sparkes1994; Munafo et al., Reference Munafo, Matheson and Flint2007), which might be through impaired inhibitory control by lateral OFC. For instance, in a drug-using state (elevated dopamine levels), the lateral OFC should serve to inhibit the execution of competing behaviors to promote heightened drug-seeking behavior. In an abstinent state (reduced dopamine levels), the lateral OFC may be unable to suppress drug-related behaviors that are not aligned with prosocial goals. Although speculative, how genetic variants related to D2 expression translate into a vulnerability to addiction warrants continued research.
Conclusion
Adolescence is thought to constitute a critical developmental period during which the frontal and striatal brain systems implicated in decision-making are particularly vulnerable to the addictive properties of drugs (Castellanos-Ryan et al., Reference Castellanos-Ryan, Struve, Whelan, Banaschewski, Barker, Bokde, Bromberg, Büchel, Flor, Fauth-Bühler, Frouin, Gallinat, Gowland, Heinz, Lawrence, Martinot, Nees, Paus, Pausova, Rietschel, Robbins, Smolka, Schumann, Garavan and Conrod2014; Conrod and Nikolaou, Reference Conrod and Nikolaou2016). Our study provides a potential genetic link to this vulnerability, supporting the possibility that alterations in OFC and VS signaling by DRD1rs686 render youth susceptible to the early onset of substance misuse. Specifically, a genetic profile contributing to the presence of a suboptimal or supraoptimal balance between OFC and VS may present a primary risk factor of drug-seeking behavior. Although speculative, it is possible that our findings may reflect a maladaptive U-shaped tuning of reciprocal projections between these brain regions during reward functioning (e.g. motivated behavior, working memory, and reward-related learning) and dopamine signaling (e.g. dopamine concentration, dopamine receptor availability) (Cools and D'Esposito, Reference Cools and D'Esposito2011). By moving out of the optimum level of dopaminergic stimulation (trough) towards either peak by excessive or low levels of dopamine stimulation, the mesocorticolimbic system may become hyper or hypo sensitive to rewarding events, possibly biasing the adolescent's action toward drug-related behaviors. Lastly, our results point to a regional specificity in the relationship between functional polymorphisms associated with D1 and D2 receptors and reward-related activity in the medial and lateral OFC, respectively. By identifying such a dopamine-related genetic path in adolescence, our study points to targets for intervention at the genetic, neural, and cognitive level to help vulnerable youth prevent progression to heavy drinking.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001459.
Acknowledgements
The IMAGEN study receives research funding from the European Community's Sixth Framework Programme (LSHM-CT-2007-037286) the FP7 project IMAGEMEND (Imaging Genetics for Mental Disorders) and the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Programme Grant ‘Developmental Pathways Into Adolescent Substance Abuse’ (93 558), and the Swedish funding agency FORMAS. Further support was provided by the Bundesministerium für Bildung und Forschung (BMBF grants 01GS08152 and 01EV0711), the Deutsche Forschungsgemeinschaft (DFG) Reinhart- Koselleck Award (SP 383/5-1), and DFG grants SM80/5-2, SM 80/7-1, SFB 940/1. This research was also supported by the German Ministry of Education and Research (grant 01EV0711). Dr Baker's salaries are awarded from the Canadian Institute of Health Research Post-doctoral fellowship.
Declaration of Interest
Dr Banaschewski has served as an adviser to or consultant for Eli Lilly, Hexal Pharma, Medice, Novartis, Otsuka, Oxford Outcomes, PCM Scientific, Shire, and Viforpharma and has been involved in clinical trials conducted by Eli Lilly, Shire, Viforpharma; he has received conference attendance support and conference support or received speaking fees from Eli Lilly, Medice, Novartis, and Shire. The other authors report no financial relationships with commercial interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.