Introduction
Iceland is a sub-Arctic volcanic island formed by on-going volcanic eruptions of a localised mantle plume and mid-Atlantic rift volcanism (Lawver & Muller Reference Lawver and Muller1994). Consequently, the majority of the island consists of basaltic lithologies with poorly developed andisols (Arnalds Reference Arnalds2004). The high latitude of the island and an elevated inland plateau causes the mean inland annual air temperature to be <10 °C (Einarsson Reference Einarsson1984; Striberger et al. Reference Striberger, Björck, Ingólfsson, Kjaer, Snowball and Uvo2010). The inland basalts have a typical organic carbon content of <0.15% (Kelly et al. Reference Kelly2011), comparable to regions of the Atacama Desert (Barros et al. Reference Barros, Feijóo, Salgado, Ramajo, Garcia and Hansen2008). The interaction of ice and volcanism has made Iceland a focus in Mars analogue studies (Cousins & Crawford Reference Cousins and Crawford2010).
Despite the inhospitable environmental conditions, the island hosts diverse microbiological populations. Icelandic basaltic and rhyolitic glass and minerals host a variety of bacterial taxa (Herrera et al. Reference Herrera, Cockell, Self, Blaxter, Reitner, Gernot, Drose, Thorsteinsson and Tindle2008; Cockell et al. Reference Cockell, Olsson, Knowles, Kelly, Herrera, Thorsteinsson and Marteinsson2009; Kelly et al. Reference Kelly, Cockell, Piceno, Andersen, Thorsteinsson and Marteinsson2010, Reference Kelly2011). The microbial communities of volcanic rocks are typically dominated by Acidobacteria, Cyanobacteria and Actinobacteria (Cockell et al. Reference Cockell, Olsson, Knowles, Kelly, Herrera, Thorsteinsson and Marteinsson2009; Kelly et al. Reference Kelly, Cockell, Piceno, Andersen, Thorsteinsson and Marteinsson2010, Reference Kelly2011).
However, microbial species atypical of this environment have also been detected. Potentially thermophilic taxa such as Geobacillus and Thermobacterium have been identified via microarray analysis (G2 PhyloChip) of the 16S rDNA gene amplified from the whole rock community genomic DNA (Kelly et al. Reference Kelly, Cockell, Piceno, Andersen, Thorsteinsson and Marteinsson2010, Reference Kelly2011), despite the fact that the atmospheric temperature of Iceland does not approach the range required for thermophile activity.
However, rock temperatures can exceed air temperatures. In Antarctic sandstones, for instance, temperatures have been observed to be 10–15 °C higher than local atmospheric temperature (McKay & Friedmann Reference McKay and Friedmann1985; Hall et al. Reference Hall, Lindgren and Jackson2005). Icelandic basalts have a low albedo of approximately 0.11, suggesting they will absorb solar radiation (McGreevy Reference McGreevy1985) and potentially exhibit surface and near-surface temperatures higher than air temperatures.
In this paper, we sought to address the hypothesis that Icelandic rocks could reach a sufficient temperature to support the activity of thermophilic organisms despite the fact that the macroclimatic conditions have a mean annual temperature well below the optimum temperature for thermophiles.
Methods
Temperatures of basalt
A sample of vesicular basalt of size approximately 20 × 30 × 20 cm was collected from a lava flow at 64°4.83′N, 19°32.53′W approximately 4 km north of Lanmannalaugur, Iceland. Holes were drilled into the rock from the sides at 2 cm depth from the surface, and the rock was returned to the field site. This depth was chosen to probe the endolithic habitat near the surface of the rock where water and potentially carbon from phototrophs are available. Thermistors (TMC20-HD), connected to temperature logging probes (HOBO U12 4 external dataloggers, Onset Computing, Pocasset, MA, USA), were inserted into the rock holes at 2 cm depth. The temperature in the basalt was measured from 4 July 2009 to 27 June 2010 every hour. At the same intervals a separate thermistor was used to measure air temperature 10 cm above the rock.
Similar measurements were made in an outcrop of obsidian. Obsidian is a silica-rich volcanic glass which is resistant to weathering and oxidation causing it to have a dark, almost black colouration (Herrera et al. Reference Herrera, Cockell, Self, Blaxter, Reitner, Thorsteinsson, Arp, Drose and Tindle2009), giving it one of the lowest albedos of igneous habitats. Measurements at 2 cm depth in the outcrop (64°2.01′N, 19°7.75′W) were obtained from 9 June 2007 to 11 June 2008.
From 1 June 2007 to 30 June 2008 relative humidity was measured at the obsidian outcrop using a HOBO U23 Relative Humidity data logger (Onset Computing, Pocasset, MA, USA) at 30 min intervals.
During June 2008, a period of warm weather prompted us to measure temperatures in more detail in the obsidian outcrop. Measurements were taken at the surface of the outcrop every 5 min during five consecutive days (11–16 June 2008).
Samples used to isolate thermophiles
Vesicular basalt (designated here, VBas1) was aseptically collected in June 2008 from the same lava flow at which temperatures were measured. The flow was produced approximately 80–800 thousand years ago (Crovisier et al. Reference Crovisier, Advocat and Dussossoy2003). Basaltic tephra produced from the April 2010 eruption of the Eyjafjallajökull at 63°38.22′N, 19°26.97′W was also collected into aseptic bags (Whirlpak, Fisher Scientific, Loughborough, UK) approximately 14 weeks after the eruption. Samples were maintained at ambient temperatures. X-ray florescence spectrometry (XRF, Applied Research Laboratories 8420 + dual goniometer, Thermo Scientific, Waltham, MA, USA) was used to characterise major elements present in the basalt sample. Heavy metal concentrations were measured by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500 s, Berkshire, UK).
Culture and isolation of thermophilic organisms
Thermophilic organisms were isolated using complex organic media incubated at temperatures above 50 °C. Thermophilic enrichments were prepared using tryptone soya broth (TSB) (Oxoid, Basingstoke, UK) produced to manufacturer's instructions. Solid medium was produced with the addition of 3% wt/vol. agar (Oxoid Agar No. 1, Basingstoke, UK). Media was adjusted to pH 7.4 with phosphate buffer and then autoclaved (20 min at 121 °C). For microbial enrichments, 50 ml of TSB was inoculated with 1 g of crushed sample (approximate grain size 1 mm) and then incubated at 50 °C with three further identical inoculations incubated at 70 °C. This was performed to prevent heat damage to initially dormant cells (Beaman et al. Reference Beaman, Pankratz and Gerhardt1988). To isolate thermophiles, enrichments were streaked onto TSB agar plates and incubated at 70 °C repeatedly until single isolates were obtained. Isolates were stored in 10 wt.% glycerol at −80 °C.
Minimum and optimum growth conditions of isolates
The minimum and optimum growth temperature range was determined for isolates in order to assess the potential activity of organisms in the natural environment. Specific growth rates were measured over the temperature range of 30–90 °C using two degree increments at temperatures lower than 40 °C and 10 °C increments at higher temperatures. Isolates were revived by gradually increasing the incubation temperatures to avoid heat shocking the cells (Beaman et al. Reference Beaman, Pankratz and Gerhardt1988). For growth analysis, 500 μl of mid-log phase culture (grown at 60 °C) was inoculated into 50 ml of pre-warmed TSB media which was incubated at the required temperature in a rotary incubator. To monitor growth, 1 ml of culture was aseptically removed as required and the optical density at 650 nm was measured with a Helios spectrophotometer (Thermo Scientific, Cambridge, UK).
At temperatures below 40 °C, inhibitors in complex media are thought to limit thermophilic growth (Claes Reference Claes1968). Therefore, at temperatures below 40 °C, growth analysis was carried out in a defined minimal salts medium (MSM) containing 1 mM of glucose as a carbon source. MSM consisted of, per litre, 0.04 g K2HPO4, 0.075 g MgSO4·7H2O, 0.036 g CaCl2·2H2O, 0.02 g Na2CO3, 0.05 g EDTA and 1 ml l−1 of A5 trace metal solution (containing, per litre, 2.86 g H3BO3, 1.81 g MnCl2·4H2O, 0.22 g ZnSO4·7H2O, 0.39 g Na2MoO4·2H2O, 0.08 g CuSO4·5H2O, 0.05 g Co(NO3)2·6H2O).
Growth at low temperatures was monitored by cell counts since optical density measurements did not give reliable results. One millilitre of culture was filtered through a 0.2 μm polycarbonate filter disc (Whatman, Fisher Scientific, Cambridge, UK) using a vacuum pump. Cells were stained with SYBR Green (Invitrogen, Paisley, UK) according to the manufacturer's instructions and observed under a UV fluorescence microscope (Lecia, DMRP, Wetzlar, Germany). Stained cells were observed using an excitation waveband of 450–490 nm (Leica filter cube I3) and an emission long band cut-off filter of >515 nm. Counts were performed for 50 random fields of view with cell numbers being calculated per ml of media.
In order to determine whether autochthonous nutrients can support thermophilic growth, 50 ml of MSM was supplemented with 1 g of sterile (autoclaved) powdered basalt sample as the sole organic nutrient source. This medium was inoculated with 500 μl of thermophilic isolate HeT36. This medium was incubated at 60 °C for 6 days. Protein concentrations were monitored as described by Markwell et al. (Reference Markwell, Haas, Bieber and Tolbert1979). Protein concentrations were measured as a proxy for biomass since cell attachment to rocks made both cell counts and optical density unreliable.
Identification of isolates
Organisms were identified using 16S rDNA gene PCR amplification and sequencing. Genomic DNA was extracted from cell pellets using the FastDNA SPIN kit for soil (Qbiogene, USA) according to the manufacturer's instructions. The 16S rDNA gene was amplified using primers targeting the V1–V9 hypervariable regions in the following pairs pA-com2 and com1-pH (Bruce et al. Reference Bruce, Hiorns, Hobman, Osborn, Strike and Ritchie1992; Schwieger & Tebbe Reference Schwieger and Tebbe1998). This created an approximate 400 bp overlap used to assemble the whole 16S rRNA gene. The PCR protocol was as follows: 1 μM forward primer, 1 μM reverse primer, 1X Buffer (200 mM Tris–HCl (pH 8.4)), 500 mM KCl (Invitrogen Corporation, Paisley, UK), 1.5 mM MgCl, 200 μM dNTP (New England Biolabs, USA), 2.5 U Taq (Invitrogen, Paisley, UK). The thermal cycler (G-Storm GS1 thermal cycler, GeneTechologies, Ltd, Essex, UK) protocol was as follows: 5 min 95 °C, 30 cycles of (30 s 94 °C, 30 s 56°C, 1 min 72 °C), 3 min 72 °C. Products were cleaned and purified (Illustra™ GFX™, GE Healthcare, Buckinghamshire, UK). Isolates and clones were sequenced at Molecular Cloning Laboratories (MCLAB, San Francisco, USA). Clone libraries were produced using the TOPO TA Cloning kit for Sequencing (Invitrogen, Paisley, UK) according to the manufacturer's instructions.
16S rDNA gene sequences were deposited in GenBank (accession numbers JN087511–JN087517).
Weathering rates of an isolate
Although thermophiles might potentially be active, we also sought to determine whether they were capable of carrying out any active geochemical processes at the upper temperatures measured in the field site near their minimum growth temperatures. In order to assess whether thermophiles could be geochemically active, basalt dissolution rates in the presence of thermophilic isolate HeT36 were determined using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) to measure the changes in elemental concentrations in the medium. To measure dissolution rates, the thermophilic isolate was inoculated into simple phosphate buffer medium, consisting of 3.2 mM of KH2PO4 and 6.7 mM of K2HPO4 giving a pH of 7. Ten grams of basalt powder of particle size range 50–125 μm was added, produced using a disc mill (TEMA, Whatford Halse, UK) and a series of mesh filters that were previously autoclaved (121 °C for 30 min). Organics for the organisms were supplied as autochthonous organics from the rocks. Flasks were inoculated with the thermophilic isolate washed in the buffered medium. The inoculant was washed four times by centrifugation and re-suspension into MSM. The experiment was performed at 40 °C, within the range observed in the field and at 60 °C as a control to show that the isolate could induce enhanced elemental release at conditions nearer its optimum growth temperature. The pH and protein concentration of the flasks were measured by removal of a 1 ml aliquot of medium at time points throughout the experiment. Protein concentrations were measured as a proxy for biomass since cell attachment to rocks made both cell counts and optical density unreliable and protein concentrations were monitored as described previously. The pH was measured using a Hydrus 300 electronic pH meter (Fisherbrand, Leicester, UK). After 45 days of incubation, 5 ml of media was removed and briefly centrifuged to remove suspended basalt particulates. Elemental release rates from the rocks were measured by ICP–AES (Teledyne Leeman, Hudson, USA) using the methods of Wu et al. (Reference Wu, Jacobson, Chen and Hausner2007) and Olsson-Francis et al. (Reference Olsson-Francis, Simpson, Wolff-Boenisch and Cockell2012). The ICP–AES data were corrected for the decrease in fluid volume and the loss of elemental mass during sampling using the following equation:
 $$C_{\,j,i}^* = \displaystyle{{C_{\,j,i}[V_{\rm o} - (\,j - 1)V_{\rm s}] + \sum\nolimits_{h = 1}^{\,j - 1} {C_{h,i}V_{\rm s}}} \over {V_{\rm o}}},$$
$$C_{\,j,i}^* = \displaystyle{{C_{\,j,i}[V_{\rm o} - (\,j - 1)V_{\rm s}] + \sum\nolimits_{h = 1}^{\,j - 1} {C_{h,i}V_{\rm s}}} \over {V_{\rm o}}},$$
C
j,i
* is the corrected elemental concentration value of element i in the j sample, C
j,i
is the original ICP–AES data, V
o is the initial fluid volume, V
s is the sample volume, and
![]() $\sum\nolimits_{h = 1}^{j - 1} {C_{h,i}V_{\rm s}} $
accounts for the mass of element i extracted during the sampling (Wu et al. Reference Wu, Jacobson, Chen and Hausner2007). The experiment was run in triplicate and sterile control experiments were run in which no inoculant was added.
$\sum\nolimits_{h = 1}^{j - 1} {C_{h,i}V_{\rm s}} $
accounts for the mass of element i extracted during the sampling (Wu et al. Reference Wu, Jacobson, Chen and Hausner2007). The experiment was run in triplicate and sterile control experiments were run in which no inoculant was added.
Results
Composition, temperature profile of Icelandic rocks and relative humidity
The major element composition of the basalts used in this experiment is shown in Table 1. Heavy metal concentrations were 123.55, 122.64, 84.70 and 73.05 ppm for Cu, Cr, Zn and Ni, respectively.
Table 1. Major element chemical composition of basalt used in the experiments

a Loss on ignition.
The temperature at 2 cm depth within Icelandic crystalline basalt and obsidian (silica-rich glass) was logged over the period of a year (Fig. 1(a)). The annual mean temperature over that period in basalt was 4.2 °C with a minimum of −12.6 °C. In obsidian, the values were 2.1 and −20.1 °C, respectively. In basalt, we observed five excursions above the minimum growth temperature of the thermophile isolates examined here (36 °C) and one in obsidian over the year-long measurement period. By contrast, the mean air temperature above the basalt sample was 3.4 °C with a minimum of −11.7 °C. The air temperature during the year-long measurement period never exceeded 18.3 °C. Figure 1(b) shows the surface temperature profile of the Icelandic obsidian for 5 days during June 2008, immediately after the year-long measurement period. The peak temperature during this time was 44.5 °C. On all 5 days of measurement, there were midday temperature excursions above the minimum growth temperature of the thermophile isolates examined here (36 °C). On June 12, this excursion lasted continuously for 3 h 24 min and on 13 and 14 June, the excursions were intermittent over a period of ~3.5 h.

Fig. 1. Temperature profiles of Icelandic rocks and relative humidity. (a) Temperature at 2 cm depth for basalt [top] (4 July 2009–27 June 2010) and obsidian [middle] for 1 year (9 June 2007–11 June 2008). The horizontal dotted line show the minimum growth temperature for growth of thermophilic isolates obtained in this study. The relative humidity from 9 June 2007 to 27 June 2008 is also shown [bottom]. (b) The temperature profile of the surface of obsidian during 5 days in June (11–16) 2008.
The relative humidity at the obsidian outcrop is shown in Fig. 1(a). The mean relative humidity over the whole measurement period was 91.4%. During June–August 2007, when temperatures reach their highest and there is no or very limited snow cover, the average relative humidity was 83.0%.
Thermophiles identified
Seven thermophilic isolates were obtained, each sharing 16S rDNA gene sequence homology to known thermophilic Geobacillus species. The minimum and maximum growth temperatures of these organisms are shown in Table 2. Growth below 40 °C was observed for isolates HeT24, Het22, HeT19, HeT36 and Het37 using the defined MSM supplemented with glucose. Growth was also observed in MSM incubations supplemented with 1 g of sterile basalt rock as the sole carbon source at 60 °C. This medium supported growth of isolate HeT36 for 6 days with a maximum specific growth rate of 0.128 day−1 at 60 °C (data not shown) demonstrating the presence of autochthonous organics capable of supporting the growth of these thermophiles.
Table 2. Thermophilic isolates. The 16S rDNA GenBank identification, closest sequence match, minimum and optimum growth temperatures of thermophilic organisms isolated from Icelandic basalt rock. ‘VBas’ refers to isolates from Hekla basalt. ‘Eyj’ refers to isolate obtained from ash at the Eyjafjallajökull

Geochemical activity of isolate
Rock weathering is an influential component of the global carbonate-silicate cycle (Dessert et al. Reference Dessert, Dupré, Gaillardet, François and Allègre2003). Given the biogeochemical importance of this process, one parameter with which to assess the potential involvement of organisms in biogeochemistry is to examine their ability to enhance rock elemental release rates compared to an abiotic control. Flasks containing powdered basalt at pH 7 in phosphate buffer were produced in triplicate and incubated with a thermophilic isolate. Flasks with the same conditions, but no organism (abiotic controls) were included. Although the flasks were buffered, at 60 °C the organisms still reduced the pH of the medium from pH 7 to approximately 4.8 and at 40 °C from pH 7 to approximately 6.1 (Fig. 2(a)). This is likely to be the result of the formation of acidic metabolic products caused by metabolism of the thermophile. Growth was confirmed by showing an increasing in the protein concentration in the flasks (concentrations of 0.47 mg ml−1 and 0.24 mg ml−1 for the 60 and 40 °C flasks, respectively, after 45 days), as shown in Fig. 2(b). The maximum specific growth rate for the 60 °C flasks was 0.43 and 0.24 day−1 for 40 °C flasks.
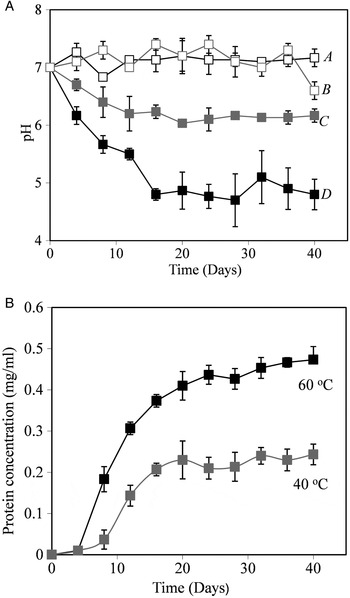
Fig. 2. Rock weathering by a thermophilic isolate. The pH and protein concentration in flasks. (a) pH changes. A, sterile control flask 40 °C; B, flask containing a thermophilic isolate at 40 °C; C, sterile control flask 60 °C; D, flask containing a thermophilic isolate at 60 °C. (b) Protein concentration of flasks containing the thermophilic isolate, no growth was observed in sterile control flasks.
Using ICP–AES, the concentration of Si, Ca, K, Mg and Na in flasks was measured after 45 days of incubation. Element concentrations are shown in Table 3. Element release rates in biological flasks were typically higher than in sterile flasks. Elemental release rates were found to be significantly higher (P < 0.05, Student's t-test) for Ca, Mg and Si in the 60 °C flasks, and for Si and Na in the 40 °C flasks.
Table 3. Elemental release induced by thermophilic isolates. The concentration (μM) of selected elements released into solution from powdered basalt after 45 days of incubation. Biological flasks contained a thermophilic isolate, whereas control flasks remained sterile. The experiment was run in triplicate. The standard deviation of replicate flasks is given in brackets. Student's t-test was performed to determine significance differences between biological flasks and sterile controls

Discussion
Low air temperatures in Icelandic habitats, and indeed any cold environment on the Earth, would suggest that these are not habitats suitable for thermophiles, but instead, environments more suitable for mesophilic organisms (growth temperature optimum between 15 and 45 °C) or psychrophilic/tolerant organisms (optimum temperature or capacity for growth <15 °C). However, we have previously observed 16S rDNA sequences indicating the presence of thermophiles such as Geobacillus spp. in environmental genomic studies of Icelandic volcanic rocks and glasses (Kelly et al. Reference Kelly, Cockell, Piceno, Andersen, Thorsteinsson and Marteinsson2010, Reference Kelly2011). Thermophiles have previously been observed in environments where conditions are generally not suitable for their propagation. For example, the spores of thermophilic sulphate reducers have been recorded in Aarhus Bay, Denmark, where water temperatures are too low to support thermophily. Their presence there is generally attributed to dispersion from hydrothermal vents and other oceanic habitats suitable for the growth of thermophiles (de Rezende et al. Reference de Rezende, Kjeldsen, Hubert, Finster, Loy and Jørgensen2013). Thermophiles in temperate soils have previously been detected (Isaksen et al. Reference Isaksen, Bak and Jørgensen1994; Marchant et al. Reference Marchant, Banat, Rahman and Berzano2002, Reference Marchant, Franzetti, Pavlostathis, Tas, Erdbrugger, Unyayar, Mazmanci and Banat2008; Banat et al. Reference Banat, Marchant and Rahman2004). These organisms are thought to be deposited into these cool environments by continuous aeolian transfer (Marchant et al. Reference Marchant, Franzetti, Pavlostathis, Tas, Erdbrugger, Unyayar, Mazmanci and Banat2008). For instance, Marchant et al. (Reference Marchant, Franzetti, Pavlostathis, Tas, Erdbrugger, Unyayar, Mazmanci and Banat2008) estimated a thermophile input rate of 1.4 × 105 cells m−2 yr−1 for Irish soils and suggested this input maintains a viable population of thermophiles in this cold environment.
Similarly, one hypothesis for the presence of thermophile DNA sequences in Icelandic volcanic rocks is that they represent the DNA from thermophiles dispersed from Icelandic hot springs and other continental environments on the island where thermophiles are known to grow, or they are atmospheric contamination from outside Iceland. However, regardless of their origin, a separate question is whether any environments into which these organisms are delivered could allow for their growth.
Motivated by a desire to understand the relationship between thermophiles and the rock environment, we examined the physical environment of the rock and the physiology of the thermophiles that they contain to address the question of whether they are passive contaminants or whether they could potentially be active members of the rock community, even if only transiently.
In this study, we isolated seven thermophilic bacteria of the genus Geobacillus. Several of these organisms had a minimum growth temperature of 36 °C.
Temperatures experienced in crystalline basalt and obsidian glass were examined. The former material is a common igneous material on the Earth, the latter is a silica-rich igneous material that because of its slow weathering and oxidation rate, is black coloured and therefore has low albedo. We found that on several occasions the thermal conductivity of the rocks (basalts have a conductivity typically approximately two to three orders of magnitude lower than typical metals; Robertson & Peck Reference Robertson and Peck1974) was sufficient to allow for temperature excursions above 36 °C. These excursions are likely to be caused by the low albedo of the rock (McGreevy Reference McGreevy1985). Our year-long measurements were made at 2 cm depth in the rocks. However, we observed surface temperatures on obsidian that exceeded 36 °C for several hours on consecutive days in June, reaching a peak value of 44.5 °C, suggesting that in the near-surface environment of rocks, temperatures during the summer months offer conditions suitable for the growth of thermophiles.
These data demonstrate that although the macroclimatic temperatures are always below the temperature range classically defined for thermophily (optimum growth temperature >45 °C), the microclimate within and on the rock provides an environment in which Geobacillus spp. could potentially grow.
The thermophiles identified in this study are obligate heterotrophs and thus require a source of organic carbon for growth. The organic carbon concentration of basalt glass located at 64°4.83′N, 19°32.53′W was approximately 0.15 wt% (Kelly et al. Reference Kelly2011). However, micro-localization and concentration of organic matter in Icelandic silicate rock pore spaces, particularly in rocks that contain phototrophs, was described by Herrera et al. (Reference Herrera, Cockell, Self, Blaxter, Reitner, Gernot, Drose, Thorsteinsson and Tindle2008) and is one example of a potential source of organics for heterotrophic thermophiles. In this study, it was also observed that 1 g of powdered basalt in 50 ml of defined salts media was sufficient to support the growth of thermophilic isolate HeT36, showing that autochthonous organics within the rocks can support growth of a thermophile.
We investigated the influence of active Geobacillus on the dissolution rate of basalt. In the presence of Geobacillus isolate HeT36, the average elemental release rate of the ions measured at 40 °C from powdered basalt was 25% greater when compared to sterile controls. At 60 °C the release rate was 43.2% greater than sterile controls showing that when active, the thermophiles can also contribute to biogeochemical processes.
Geobacillus are spore-formers (Marchant et al. Reference Marchant, Franzetti, Pavlostathis, Tas, Erdbrugger, Unyayar, Mazmanci and Banat2008). The germination of Bacillus spores can occur within minutes (Vary & Halvorson Reference Vary and Halvorson1965) and we observed high-temperature excursions lasting hours suggesting the potential for reproduction. Nevertheless, temperature excursions would be more likely to be advantageous for vegetative cells that can respond almost immediately to a short suitable growth window. Organisms also require water. The high relative humidities observed on the basalt outcrop are caused by damp Icelandic air and the frequent sustained or intermittent rain episodes experienced in this region of Southern Iceland. Although during high-temperature excursions the surface of the rock is likely to be dried from evaporation, the vesicular interiors of rocks have been observed to trap water for several days after rain events in the Arctic (Cockell et al. Reference Cockell, Osinski, Voytek, Osinski and Pierazzo2013). This suggests the possibility of high-temperature excursions occurring concomitantly with the presence of liquid water inside rocks if the period between rain and high temperatures is sufficiently short.
Our data suggest two implications for life in extreme rocky environments. First, as rock temperatures vary diurnally and seasonally, so different taxa adapted to different temperature may become active within the rock environment, suggesting the possibility of temperature-induced niche differentiation (Fig. 1). Second, if thermophiles are active in the rock environment, then warm surface rocks might represent a widespread habitat for them in addition to the more traditionally studied hot spring and caldera environments (Takacs-Vesbach et al. Reference Takacs-Vesbach, Mitchell, Jackson-Weaver and Reysenbach2008), provided liquid water is available. This suggests a more widespread biogeography of thermophiles in continental environments, for example in rocky cold and hot deserts, than previously appreciated.
In recent decades, surface air temperatures in Arctic regions have increased at twice the rate of the global average (McBean et al. Reference McBean, Alekseev, Chen, Førland, Fyfe, Groisman, King, Melling, Vose and Whitfield2005). Rock temperatures increase with increasing atmospheric temperatures as observed in long-term records of air and subsurface temperatures at decimetre scales in Taiwan (Chen et al. Reference Chen, Wang, Chen, Sun, Liu, Yeh, Yen, Chang and Taniguchi2011) and in records of much deeper boreholes found globally (Pollack & Huang Reference Pollack and Huang2000). We might predict that climatic warming could increase the potential for the growth of thermophilic organisms in rock microenvironments.
These data have implications for planetary habitability. They provide a significant example of how the measurement of temperatures over regional scales on a planet or even on a whole planetary scale cannot in itself be used to predict the available niches for life. Microenvironments in rocks are known to provide protection from a number of physical extremes (McKay & Friedmann Reference McKay and Friedmann1985; Hall et al. Reference Hall, Lindgren and Jackson2005). The measurement or modelling of environmental conditions at the scale of microorganisms is essential for assessing the range of microbial physiologies that might be supported by such environments.
Acknowledgements
We thank the STFC for providing a studentship to PW for this work. This work was carried out in partial fulfilment of the degree of Doctor of Philosophy by PW. This work was made possible with support from the UK Science and Technology Facilities Council (STFC; Grant No. ST/1001964/1).







