INTRODUCTION
The Gulf of Mexico is a marginal basin of the Atlantic Ocean and possesses a high number of unusual bathyal habitats ranging in size from large hydrocarbon seeps to relatively small wood falls. Cold hydrocarbon seeps often co-occur with hypersaline brines caused by exposure of the Louann salt layer (Salvador, Reference Salvador1987; MacDonald et al., Reference MacDonald, Reilly, Guinasso, Brooks, Carney, Bryant and Bright1990). Some brine flows fill bottom depressions to form pools, which are characterized by distinct microbial and macrofaunal assemblages that resemble the fauna of hydrothermal vents (Paull et al., Reference Paull, Hecker, Commeau, Freeman-Lynde, Neumann, Corso, Golubic, Hook, Sikes and Curray1984; Kennicutt et al., Reference Kennicutt, Brooks, Bidigare, Fay, Wade and MacDonald1985, Reference Kennicutt, Brooks, Bidigare and Denoux1988; Cordes et al., Reference Cordes, Carney, Hourdez, Carney, Brooks and Fisher2007). The cold seep known as Brine Pool NR-1 is filled with brine containing large amounts of methane gas and is completely surrounded by dense beds of Bathymodiolus childressi (MacDonald et al., Reference MacDonald, Reilly, Guinasso, Brooks, Carney, Bryant and Bright1990). The mussels harbour methanotrophic symbionts (Childress et al., Reference Childress, Fisher, Brooks, Kennicutt, Bidigar and Anderson1986; Cary et al., Reference Cary, Fisher and Felbeck1988) and have been shown by stable isotope analyses to be a food source for vertebrate and invertebrate predators (MacAvoy et al., Reference MacAvoy, Macko and Carney2003). Other taxa associated with Brine Pool NR-1 include chemosynthetic tube worms, several species of crustaceans, and demersal fish (MacDonald et al., Reference MacDonald, Reilly, Guinasso, Brooks, Carney, Bryant and Bright1990).
Wood falls provide periodic pulses of organic matter to the deep sea, and can sustain distinct communities that resemble assemblages associated with whale falls, hydrothermal vents, and cold seeps (Van Dover, Reference Van Dover2000). The Mississippi River serves as a ready source of wood in the northern Gulf of Mexico. Trophic and ecological relationships among animals on wood falls have been described and include distinct opportunistic wood-borers, fouling organisms and predators (Turner, Reference Turner1978; Pailleret et al., Reference Pailleret, Haga, Petit, Prive-Gill, Saedlou, Gaill and Zbinden2007). To date, the only flatworms reported from wood falls are an unidentified predatory turbellarian (Turner, Reference Turner1978) and two recently described species of polyclads, Anocellidus profundus and Oligocladus voightae from the Cascadia Basin and the Escanaba Trough in the North Pacific Ocean at depths of 2639 to 3232 m (Quiroga et al., Reference Quiroga, Bolaños and Litvaitis2006). Lastly, in Van Dover et al. (Reference Van Dover, Humphris, Fornari, Cavanaugh, Collier, Goffredi, Hashimoto, Lilley, Reysenbach, Shank, VonDamm, Banta, Gallant, Götz, Green, Hall, Harmer, Hurtado, Johnson, McKiness, Meredith, Olson, Pan, Turnipseed, Won, Young and Vrijenhoek2001), Figure 2E shows deep-sea flatworms at hydrothermal vents of the Indian Ocean, and although no positive identifications are provided, the flatworm specimens are, in all likelihood, polyclads.
Similarly, the polyclad fauna from bathyal environments (200–2000 m) still remains largely unknown. Faubel (Reference Faubel1984) mentions Stygolepta hjalmari from 603 m depth off the coast of Mauritania. Other polyclads from similar depths include Plehnia arctica, collected from 1275 m at Jan Mayen Island in the Arctic Ocean (Bock, Reference Bock1913) and Discoprosthides patagoniensis, reported from the bathyal zone (no specific depth given) off Argentina (Faubel, Reference Faubel1983). Stylochus crassus has been found in samples collected at 2000 m deep off the coast of New England, USA; however, the species is presumed to live in association with floating algae rather than on the sea floor (Verrill, Reference Verrill1892). Here we add two new species collected from a hypersaline cold seep and from a natural wood fall in the northern Gulf of Mexico to the known polyclad fauna of bathyal environments.
MATERIALS AND METHODS
All specimens were collected by Dr Janet R. Voight of the Field Museum (FMNH), Chicago Illinois, USA. Once located, the wood fall was picked up with the manipulator arm of the crewed submersible, ‘Johnson SeaLink’, and placed in a lidded box for recovery. The sample from Brine Pool NR-1 was collected among mussels with a scoop integral to the ‘Johnson SeaLink’ and transferred to Plexiglas containers for recovery. Specimens examined here were fixed in 8% formalin in seawater and stored in 70% ethanol. Fixed animals were photographed, and body measurements were taken (measurements given as length mm × width mm). Animals were processed following the protocol of Quiroga et al. (Reference Quiroga, Bolaños and Litvaitis2006), and diagrammatic reconstructions of the reproductive system were derived from sectioned material and whole mounts. Taxonomic identifications follow the classification of Faubel (Reference Faubel1983, Reference Faubel1984).
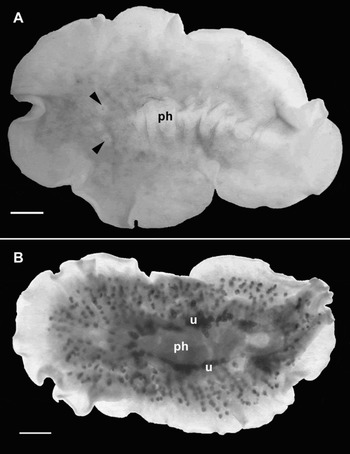
Fig. 1. Didangia carneyi, sp. nov. (A) Dorsal view of preserved specimen showing two clusters of tentacular eyes (arrow heads) and pharynx (ph); (B) ventral view of cleared whole mount, showing uteri (u) and pharynx (ph). Scale bars = 1 mm.

Fig. 2. Didangia carneyi, sp. nov. (A) Dorsal view of tentacular eye clusters (arrowheads) and two rows of cerebral eyes (ce) extending anterior. Scale bar = 250 µm; (B) male copulatory organ showing two accessory prostatic vesicles (apv) and stylet (s) entering the male atrium (ma); note female pore (fp) opening close to the male atrium. Scale bar = 50 µm.

Fig. 3. Didangia carneyi, sp. nov. (A) Sagittal section through male and female reproductive systems, showing seminal vesicle (sv), ejaculatory duct (ed), prostatic vesicle (pv), accessory prostatic vesicles (apv), prostatic duct (pd), stylet (s), female pore (fp), vagina (v) and cement glands (cg); (B) sagittal section through prostatic vesicle, showing large glandular cells (gc) releasing secretions into prostatic vesicle (pv) or the male atrium (ma). Scale bars = 250 µm.

Fig. 4. Didangia carneyi, sp. nov. Diagrammatic reconstruction of the male and female reproductive systems; apv, accessory prostatic vesicle; cg, cement glands; ed, ejaculatory duct; fp, female pore; gc, gland cells; ma, male atrium; mp, male pore; ov, oviduct; pd, prostatic duct; pv, prostatic vesicle; s, stylet; sdb, spermiducal bulbs; sv, seminal vesicle; v, vagina. Scale bar = 250 µm.
TYPE MATERIAL
Holotype: one mature specimen (9 mm × 6 mm) as sagittal sections of reproductive structures, remainder of specimen as whole mount (FMNH 13968, 5 slides) (USA: Louisiana, Gulf of Mexico from a wild wood fall at Station VOIJ-JSLII-3531, JSL Dive number 3531; coordinates: 27°44.09′N 91°14.49′W, water depth: 610 m). Collected 19 August 2006.
Paratype: one mature specimen (11 mm × 5 mm) as sagittal sections of the reproductive structures, remainder of specimen as whole mount (FMNH 13969, 4 slides). Collected with the holotype.
OTHER MATERIAL (ALL COLLECTED WITH THE HOLOTYPE)
— one mature specimen (7 mm × 3.5 mm), as sagittal sections of the reproductive structures, remainder of specimen as whole mount (FMNH 13775E, 3 slides);
— two juveniles (4 mm × 2.5 mm; 6 mm × 3 mm), as whole mounts (FMNH 13775F, 2 slides);
— one mature specimen (5 mm × 3 mm), as sagittal sections of the reproductive structures (FMNH 13775A, 4 slides);
— one mature specimen (8 mm × 5 mm), as whole mount (FMNH 13775C, 1 slide);
— one mature specimen (10 mm × 7 mm), as sagittal sections of the reproductive structures, remainder of specimen as whole mount (FMNH 13774C, 6 slides);
— one mature specimen (7 mm × 6 mm) as whole mount (FMNH 13774A, 1 slide);
— one mature specimen (9 mm × 5 mm) as whole mount (FMNH 13774D, 1 slide);
— one juvenile specimen (6 mm × 4 mm), as sagittal sections of the reproductive structures (FMNH 13774F, 6 slides); and
— an additional 16 specimens were examined.
ETYMOLOGY
Named in honour of Dr Robert Carney, Louisiana State University, chief scientist of the cruise on which the type specimens were collected.
DISTRIBUTION AND HABITAT
To date, known from the type locality; on and in a wild wood fall.
DIAGNOSIS
Prostatic vesicle interpolated and provided with two accessory prostatic vesicles, each bearing a stylet. The stylets merge to form a single copulatory organ. Large, glandular cells surround the male atrium. Female complex opens into the male atrium or very close to it. Cerebral eyes are in two rows. Tentacular eyes present.
DESCRIPTION
Colour: in preserved specimens, the dorsal surface is whitish with brown to pink pigment, more concentrated in the region surrounding the pharynx. Numerous brown dots are disposed radially from the region of the pharynx toward the margin, corresponding to the ovaries and testes (Figure 1A). Ventral surface is cream-whitish, the uteri are visible as two dark lines located on either side of the pharynx (Figure 1B). Probably the species is very pale pink when alive (J. Voight, personal communication).
Form: ovoid, somewhat tapered at the posterior end in some specimens. Length to width ratio of most specimens is from 1.4 to 2.2.
Tentacles: no evidence of tentacles.
Eyes: two rounded clusters of tentacular eyes present on either side of the brain, they are separated by about 1 mm; each cluster contains 4 to 7 eyes. The cerebral eyes form two rows anterior to the brain, each containing 3 to 5 eyes (Figure 2A). Marginal eyes are lacking.
Digestive system: a long ruffled pharynx, with about 7 pairs of folds, is positioned centrally (Figure 1A) and occupies about 30% of the total body length. The mouth is located at the posterior half of the pharynx. The main intestine branches toward the margin forming a highly anastomosed digestive system. A median intestinal frontal branch is present extending dorsally over the bilobed brain.
Epidermis and body wall: a simple epithelium encloses the entire body (Figure 3). Although cilia are present, they are not easily seen in histological sections. The thickness of the epidermis is 15 µm both on the ventral and the dorsal surfaces. Rhabdites are scarce and not very conspicuous either ventrally or dorsally. Commonly, polyclad rhabdites stain intensely pink, however, in this species they do not. Cyanophilous glands of a rounded shape are abundant throughout the entire epidermis. The dorsal epidermis carries only clusters of small rounded black pigment spots that are well-spaced and not very numerous. The basement membrane is difficult to distinguish from the base of the epidermis. The body wall is thicker ventrally (50 µm) with clear differentiation between circular, longitudinal and diagonal muscle layers (Figure 3B). In contrast, the dorsal body wall musculature is much thinner (20 µm) and the muscle layers are not well organized. Only a few weakly developed diagonal fibres and a layer of longitudinal muscles are evident. Beneath the epidermis and muscles of the dorsal surface, concentrated brown pigment granules form a layer that runs parallel to the muscles and mix with some muscle fibres. No pigment was observed in the parenchyma.
Gonopores: are located at the distal posterior half of the body. The female gonopore is extremely close to the male gonopore (Figure 2B) making it difficult to distinguish them in whole mount preparations. The uteri appear as two dark rows on each side of the pharynx (Figure 1B).
Male reproductive system: the male copulatory apparatus is characteristic for the genus. It is composed of two spermiducal bulbs connected ventro-anteriorly to the seminal vesicle. The seminal vesicle is a large oval organ (400 µm × 200 µm) positioned perpendicular to the body wall (Figures 3A & 4). The interpolated prostatic vesicle is a complex organ lined by a smooth glandular epithelium. Two accessory prostatic vesicles connect to the prostatic vesicle proper at its distal end (Figures 2B, 3A & 4). Each accessory prostatic vesicle bears a cuticular stylet (~175 µm) which fuse to emerge as a single copulatory organ (Figures 2B, 3A & 4). Surrounding the male atrium and the prostatic vesicle are several large glandular cells (Figures 2B, 3B & 4). Each cell has a neck that penetrates the musculature of the prostatic vesicle wall to release its glandular content into the prostatic vesicle lumen or into the male atrium (Figure 3B & 4). The deep and muscular atrium houses the copulatory organ (composed of fused cuticular stylets) and the distal portions of the prostatic organs (Figures 3A & 4). Testes are located ventrally in the posterior part of the body.
Female reproductive system: in most of the specimens, the female pore opens into or very close to the male atrium (Figures 2B, 3A & 4). The simple female apparatus consists of a very short vagina that parallels the body wall (Figures 3A & 4). The glandular cells of the cement glands surround the entire vagina (Figure 3A). Lang's vesicle is absent. The oviducts join the vagina dorsally, extend anteriorly, and connect to the uteri on each side of the pharynx (Figure 4).
TAXONOMIC REMARKS
A male copulatory apparatus located anterior to the male pore and directed posteriorly, a prostatic vesicle with a smooth glandular lining and no projection of the ejaculatory duct into its lumen, a ruffled pharynx, and two accessory prostatic vesicles all place the currently described species in the family Didangiidae (Faubel, Reference Faubel1983). Didangiidae contains only Didangia mactanensis (Faubel, Reference Faubel1983), and the family is defined based on characters of that species. A request to the Zoological Museum at the University of Hamburg, Germany for the original type material of D. mactanensis revealed that the type cannot be found (P. Stiewe, collections manager, personal communication).
The most conspicuous character of the family Didangiidae is the presence of a prostatic vesicle with smooth glandular lining and composed of two accessory prostatic vesicles. Didangia carneyi differs from the definition of the family, and from D. mactanensis, by the apparent presence of a common pore or gonopore, tentacular eyes and spermiducal bulbs. Yet, the most important character differentiating this new species is the orientation of the two accessory prostatic vesicles. In D. mactanensis, the accessory prostatic vesicles are situated laterally on both sides of the prostatic vesicle (perpendicular to the long axis of the body), whereas in D. carneyi, one accessory vesicle is located anterior to the prostatic vesicle and the other posterior to it (parallel to the long axis). The common gonopore, presence of tentacular eyes and spermiducal bulbs and the orientation of the accessory prostatic vesicles support the recognition of a new species but are not considered to be sufficient basis to erect a new family or genus. Among polyclads in general, only the cotylean family Prosthiostomidae and the acotylean Didangiidae have two accessory prostatic vesicles. In prosthiostomids though, multiple cuticular elements forming a copulatory organ are not found, and of course, by possessing a sucker, the family clearly belongs to the Cotylea.
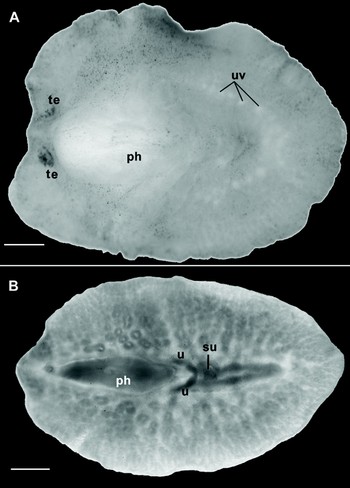
Fig. 5. Oligocladus bathymodiensis, sp. nov. (A) Dorsal view of preserved specimen, showing pharynx (ph), uterine vesicles (uv) and tentacular eyes (te); (B) ventral view of cleared whole mount, showing pharynx (ph), sucker (su) and uteri (u). Scale bars = 1 mm.

Fig. 6. Oligocladus bathymodiensis, sp. nov. (A) Anterior end of preserved specimen showing pseudotentacles and cerebral eyes. Scale bar = 100 µm; (B) sagittal section showing tentacular eyes (te). Scale bar = 250 µm.

Fig. 7. Oligocladus bathymodiensis, sp. nov. (A) Sagittal section showing mouth opening (m) with protruded pharyngeal tissue (pt) and brain (br) located posterior to the mouth; (B) sagittal section with sucker (su) and ventrally opening anus (a); (C) sagittal section through the reproductive systems; cg, cement glands; ed, ejaculatory duct; fp, female pore; ma, male atrium; pv, prostatic vesicle; s, stylet; sv, seminal vesicle; v, vagina. Scale bars = 250 µm.

Fig. 8. Oligocladus bathymodiensis, sp. nov. Diagrammatic reconstruction of the male and female reproductive systems; cg, cement glands; ed, ejaculatory duct; fp, female pore; ma, male atrium; mp, male pore; pv, prostatic vesicle; s, stylet; sv, seminal vesicle; v, vagina. Scale bar = 250 µm.
TYPE MATERIAL
Holotype: one mature specimen (8 mm × 6 mm) as sagittal sections of the reproductive structures (FMNH 13970, 7 slides) (USA: Lousiana, Gulf of Mexico, crewed submersible ‘Johnson Sea-Link II’ from the Brine Pool NR-1 at Station VOIJ-JSLII-3521, coordinates: 27°00′28″ N 91°16′45″W, water depth: 650 m). Collected 14 August 2006.
Paratype: one mature specimen (8 mm × 5 mm) as whole mount (FMNH 13971, 1 slide); collected with the holotype.
OTHER MATERIAL
— one mature specimen (8.5 mm × 5 mm) as sagittal sections of the reproductive structures (FMNH 13776A, 6 slides); collected with the holotype;
— one juvenile (3.5 mm × 2 mm) as whole mount (FMNH 13776D, 1 slide); collected with the holotype;
— one mature specimen (8 mm × 5 mm) as whole mount (FMNH 13972, 1 slide); collected with the holotype of Didangia carneyi (USA: Louisiana, Gulf of Mexico from a wild wood fall at Station VOIJ-JSLII-3531, JSL Dive number 3531; coordinates: 27°44.09′N 91°14.49′W, water depth: 610 m). Collected 19 August 2006.
ETYMOLOGY
Named for the deep-sea mussel Bathymodiolus childressi, Gustafson, 1998 with which the holotype and many of the paratypes of this flatworm species were associated.
DISTRIBUTION AND HABITAT
To date known from the type locality, a brine pool (NR-1) at 27°00′28″ N 91°16′45″W and from a wild wood fall at 27°44′09″N 91°14′49″W, Louisiana, Gulf of Mexico, USA.
DIAGNOSIS
Mouth anterior to the brain. A few cerebral eyes in two close clusters. Other minute eyes scattered over the pseudotentacles. About four pairs of main intestinal branches radiating from the main median intestinal branch. Ventral anal pore located posterior at the end of the main median branch of the intestine.
DESCRIPTION
Colour: in preserved animals, the ventral and the dorsal surfaces are whitish with sandy brown pigment distributed irregularly in the epidermis and concentrated at the tip of the tentacles. In some specimens, conspicuous white dots on both sides behind the pharynx are visible, corresponding to the uterine vesicles (Figure 5A).
Form: overall shape is oval in most specimens, somewhat tapered at the posterior end (Figure 5B). Length to width ratios in all material examined were between 1.3 and 1.75. The dorsal surface is raised where the anterior tubular pharynx is located (Figure 5A). The pharynx occupies about 30% of the total body length. A prominent sucker is located in the centre of the ventral surface posterior to the pharynx (Figures 5B & 7B).
Tentacles: a pair of conspicuous pseudotentacles is present; widely separated (~1 mm), long and blunt (Figure 6) and formed by the elongation and folding of the anterior margin. Their shape is typical of that of the family Euryleptidae.
Eyes: superficially this species appears to have only a pair of cerebral eyes (Figure 6A). However, histological sections and cleared whole mounts revealed other small eyes immersed in the epidermis and the parenchyma and scattered in the cerebral and frontal regions, with a few scattered eyes on the pseudotentacles as well (Figure 6B).
Digestive system: the mouth is a small ventral opening located anterior to the brain (Figure 7A). However, sometimes when the pharynx is extended, the mouth has a wide aperture. The cylindrical pharynx is typical for the family and is directed anteriorly. It connects to a wide median intestine that extends posteriorly, ending in a ventral aperture considered to be an anus (Figure 7B). Four pairs of intestinal ramifications extend from the median intestinal branch and anastomose towards the margin (Figure 5B).
Epidermis and body wall: a simple multi-ciliated epithelium surrounds the entire body. The cilia, readily visible in histological sections, are denser and longer on the dorsal surface. Although rhabdites are evident on both surfaces, they are arranged in dense packets in the dorsal epithelium and individually in the ventral epidermis. Also, the rhabdites are longer and more numerous dorsally than ventrally, making the dorsal epidermis taller (40 µm). Cyanophilous glands are present basally in the epidermis. There is not a significant difference in the thickness of the dorsal and ventral body wall musculature (15 µm). Muscle layers are not conspicuous and only a longitudinal layer is clearly visible.
Gonopores: are located in the anterior half of the body, ventral to the pharynx. The male gonopore is anterior to the female pore.
Male reproductive system: small male system located posterior to the male pore and directed anteriorly. The elongated free prostatic vesicle (~500 µm × 200 µm) is positioned dorsally to the ejaculatory duct. The rounded seminal vesicle (450 µm × 600 µm) connects with the penis stylet through a wide ejaculatory duct (Figures 7C & 8). The male atrium is deep in relation to the size of the whole male complex and houses a conical penis papilla that bears a cuticular stylet (Figures 7C & 8). The testes are ventral.
Female reproductive system: the female apparatus is very simple, with a pore leading into a short vagina (Figures 7C & 8). No cement pouches are evident, but cement glands are present. The female gonopore is positioned about 1.4 mm from the male gonopore (Figure 7C). About 9 pairs of uterine vesicles are present posterior to the pharynx (Figure 5A).
TAXONOMIC REMARKS
All species in the genus Oligocladus are characterized by the mouth positioned anterior to the brain (Hadenfeldt, Reference Hadenfeldt1929). In some of the examined specimens of O. bathymodiensis, the mouth is positioned either just ventral or slightly posterior to the brain. This is due to a fixation artefact. Upon fixation, specimens will contract to some extent and in most euryleptids this contraction results in a curvature of the strongly muscular pharynx and hence of the entire anterior end. This in turn, is responsible for the artificial positioning of the mouth either slightly posterior or ventral to the brain.
The main characteristics that distinguish Oligocladus bathymodiensis from its congeners, and especially from O. voightae, include the complete absence of an anterior median intestinal branch; this branch is present in O. voightae and is trifurcated in O. sanguinolentus. Secondly, a distinctive ventral position of the anus separates O. bathymodiensis from other Oligocladus species. An anus is a unique characteristic of the genus, however to date, in all known species it has been shown to open dorsally. A third unique characteristic is the presence of paired cerebral eyes, which actually consist of two distinct clusters of eyespots. Oligocladus voightae completely lacks cerebral eyes and O. sanguinolentus has two very well differentiated clusters of cerebral eyes. Finally, an auxiliary sperm storage vesicle connected to the seminal vesicle continues to be an exceptional character of O. voightae and is lacking in O. bathymodiensis.
DISCUSSION
Members of the polyclad family Euryleptidae generally are quite common in shallow tropical waters, where they contribute to the colourful fauna of coral reefs (Newman & Cannon, Reference Newman and Cannon1994, Reference Newman and Cannon2000, Reference Newman and Cannon2002). However, Oligocladus bathymodiensis is now the third euryleptid species described from deeper waters, the other two being O. voightae Quiroga et al. Reference Quiroga, Bolaños and Litvaitis2006 and Stygolepta hjalmari Faubel, Reference Faubel1984. Two additional euryleptid specimens have been found in a trawl sample collected off the Pacific coast of Guatemala from about 110 m. Again, the worms were associated with wood-boring clams. Positive identification to species was not possible because of the conditions of the specimens. The two specimens are in the Benthic Invertebrate Collection at the Scripps Institution of Oceanography in La Jolla, California (Catalogue Number Pt 49). Hence, it appears that euryleptids may actually constitute a large component of the polyclad fauna of deeper waters.
A literature-based survey of the polyclad fauna of the Gulf of Mexico revealed 26 species, of which 4 species are endemic (Hooge & Newman, in press). The distribution of the other 22 species extends well into the Caribbean and in some cases even into the north-western Atlantic. Sampling in these early studies however was biased towards the intertidal to shallow subtidal zones and regionally confined to Florida and Texas (Pearse, Reference Pearse1938; Hyman, Reference Hyman1940, Reference Hyman1954, Reference Hyman1955a, Reference Hymanb). This is the first report of two species of polyclads from depths greater than 600 m in the Gulf of Mexico.
Several species of acotylean polyclads are commonly referred to as ‘oyster wafers’ or ‘oyster leeches’, although they clearly are not annelids. This label is due to their voracious feeding on juvenile oyster spat and other commercially important bivalve species (Provenzano, Reference Provenzano1961; Webster & Medford, Reference Webster and Medford1961; Christensen, Reference Christensen1973; Chintala and Kennedy, Reference Chintala and Kennedy1993; Newman et al., Reference Newman, Cannon and Govan1993; Newell et al., Reference Newell, Alspach, Kennedy and Jacobs2000). Stylochids (Stylochus frontalis, S. ellipticus and S. oculiferus) are common in the Gulf of Mexico and either have been shown to prey directly on oysters or have been found in association with oyster shells and are thus suspected bivalve predators. It is interesting to note that at least one of the newly described species, Oligocladus bathymodiensis was found associated with beds of the mussel, Bathymodiolus childressi. Specimens of D. carneyi were collected from a wood-fall heavily bored by both teredinids and species of Xylophagainae. It is thought highly likely that the mussels and wood-boring bivalves represent a food source for the worms. A similar relationship has been described for two other deep-sea polyclads (Quiroga et al., Reference Quiroga, Bolaños and Litvaitis2006), which were only found on wood heavily colonized by wood-boring clams of Xylophaga (Voight, Reference Voight2007). Thus, the present study contributes not only to our knowledge of polyclad biodiversity but also increases our understanding of the ecology of deep-sea fauna.
ACKNOWLEDGEMENTS
We thank Dr Janet Voight (Field Museum of Natural History, Chicago, Illinois, USA) and Dr Robert Carney (Louisiana State University, USA) for providing us with the specimens collected with support of NSF DEB-0103690 0103690 and NOAA Ocean Exploration grant NA06OAR4600096. This work was supported by NSF grant DEB-0412932, and is Scientific Contribution No. 2355 from the New Hampshire Agricultural Experiment Station.










